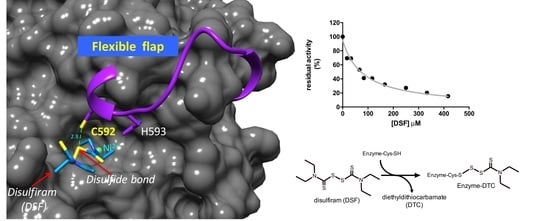
Inhibition of Urease by Disulfiram, an FDA-Approved Thiol Reagent Used in Humans
Abstract
:1. Introduction
2. Results
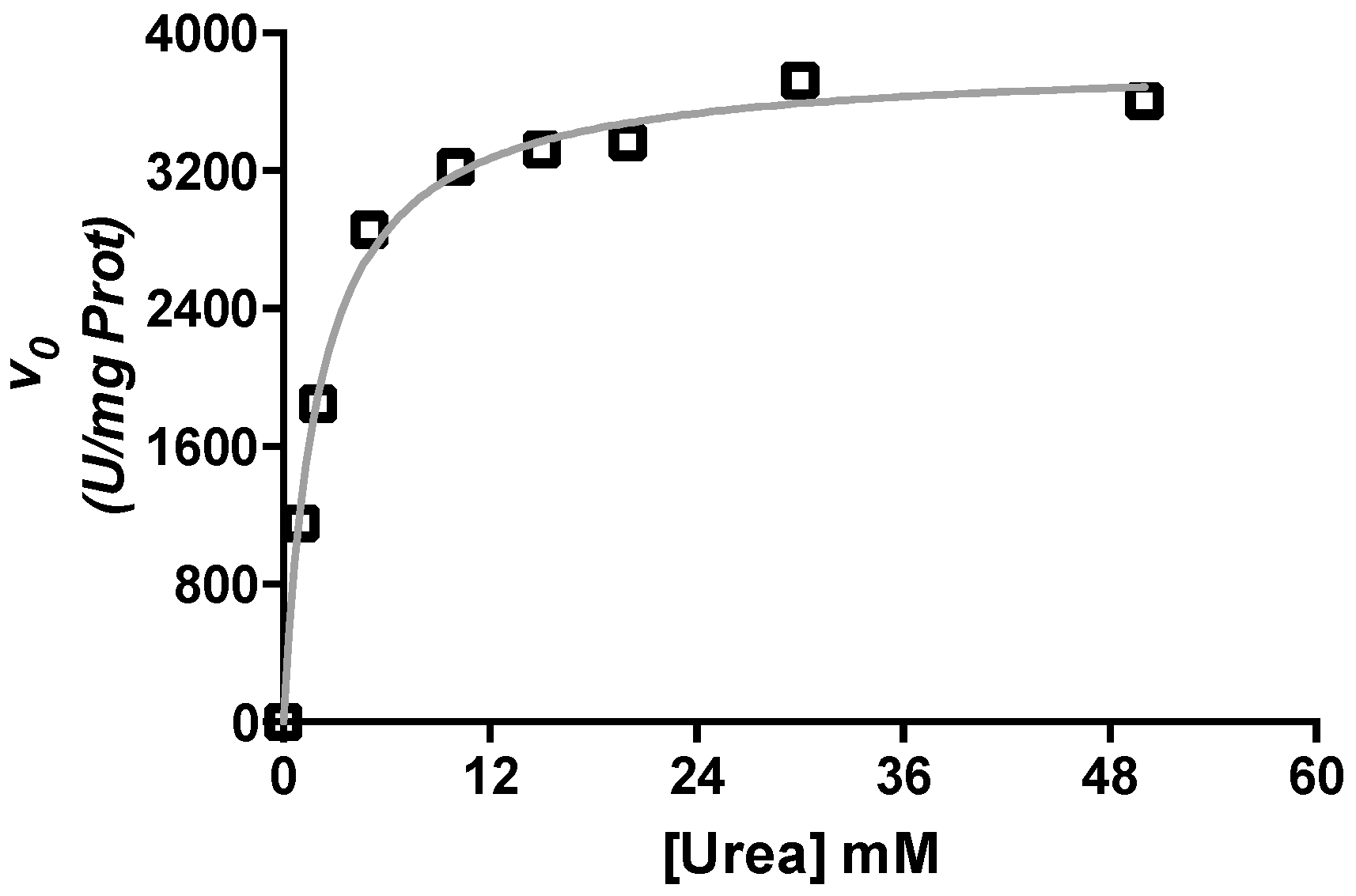
2.1. Kinetic Characterization of Urease from C. vulgaris Seeds
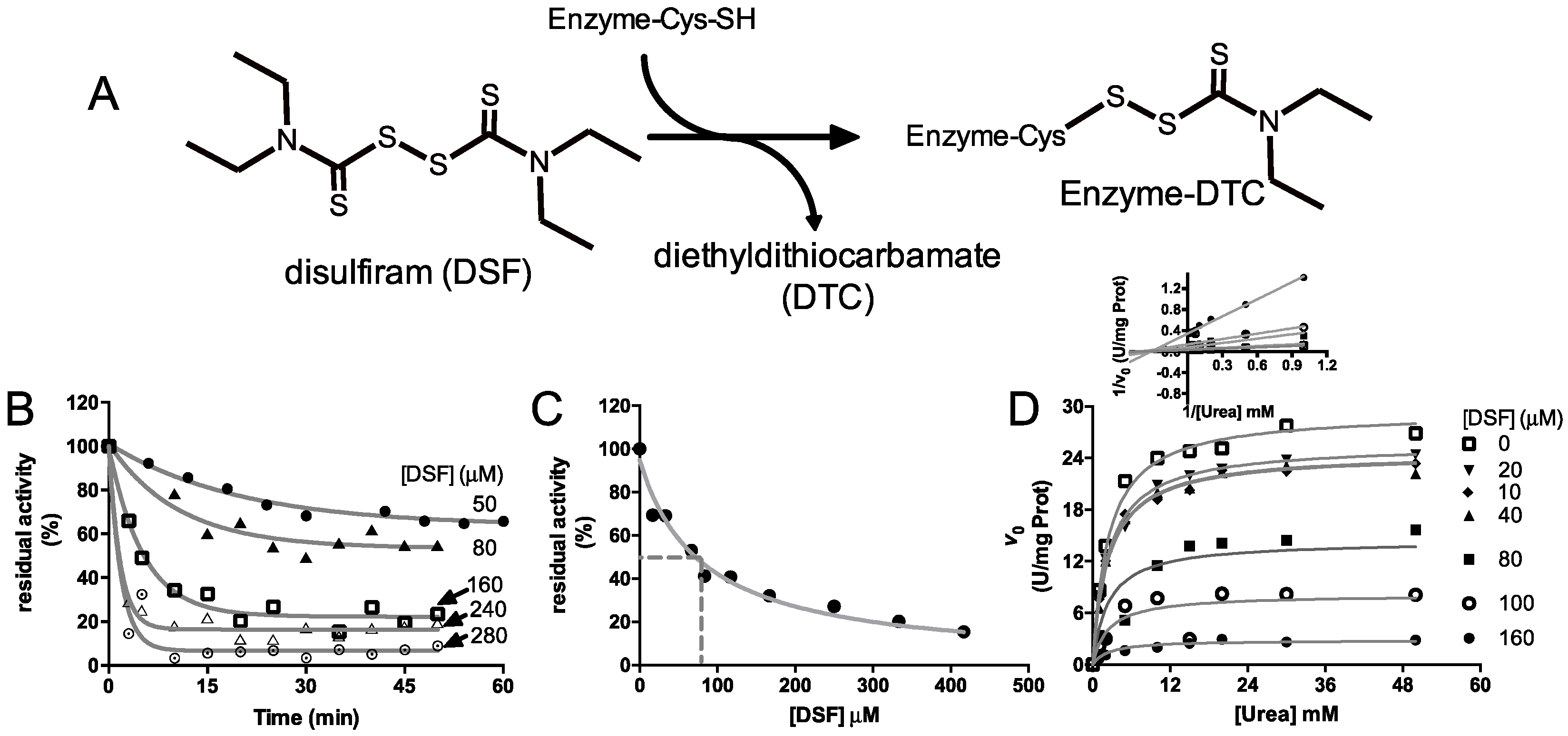
2.2. Kinetic Characterization of DSF Inhibition over Citrullus vulgaris Urease
2.3. Inhibition of C. vulgaris Urease by Other Thiol Reactive Compounds
2.4. Binding of DSF to Citrullus vulgaris Urease Revealed by Fluorescence Auenching Experiments
2.5. Binding of DSF to Citrullus vulgaris Urease Revealed by Docking Experiments
3. Discussion
3.1. Potential Use of Disulfiram to Control Urease Activity
3.2. Proposed Mechanism of Urease Inhibition by Disulfiram
4. Materials and Methods
4.1. Urease Preparation
4.2. Enzyme Activity Assay and Kinetic Characterization
4.3. Inhibition Kinetic Characterization
4.4. Binding Equilibrium Experiments
4.5. DSF Docking
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dixon, N.E.; Gazzola, T.C.; blakeley, R.L.; Zermer, B. Letter: Jack bean urease (EC 3.5.1.5). A metalloenzyme. A simple biological role for nickel? J. Am. Chem. Soc. 1975, 97, 4131–4133. [Google Scholar] [CrossRef] [PubMed]
- Hausinger, R.P. Nickel utilization by microorganisms. Microbiol. Rev. 1987, 51, 22–42. [Google Scholar] [PubMed]
- Rutherford, J.C. The emerging role of urease as a general microbial virulence factor. PLoS Pathog. 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Hasan, H.A.H. Ureolytic microorganisms and soil fertility: A review. Commun. Soil Sci. Plant. Anal. 2000, 31, 2565–2589. [Google Scholar] [CrossRef]
- Scheurer, M.; Brauch, H.-J.; Schmidt, C.K.; Sacher, F.; Zerulla, W.; Barth, T.; Dressel, J.; Erhardt, K.; Locquenghien, K.H.; Pasda, G. Occurrence and fate of nitrification and urease inhibitors in the aquatic environment. Environ. Sci. Process. Impacts 2016, 34, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Jabri, E.; Carr, M.B.; Hausinger, R.P.; Karplus, P.A. The crystal structure of urease from Klebsiella aerogenes. Science 1995, 268, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Karplus, P.A.; Pearson, M.A.; Hausinger, R.P. 70 Years of crystalline urease: What have we learned? Acc. Chem. Res. 1997, 30, 330–337. [Google Scholar] [CrossRef]
- Rabinkov, A.; Miron, T.; Konstantinovski, L.; Wilchek, M.; Mirelman, D.; Weiner, L. The mode of action of allicin: trapping of radicals and interaction with thiol containing proteins. Biochim. Biophys. Acta-Gen. Subj. 1998, 1379, 233–244. [Google Scholar] [CrossRef]
- Juszkiewicz, A.; Zaborska, A.; Łaptaś, A.; Olech, Z. A study of the inhibition of jack bean urease by garlic extract. Food Chem. 2004, 85, 553–558. [Google Scholar] [CrossRef]
- Benini, S.; Rypniewski, W.R.; Wilson, K.S.; Miletti, S.; Ciurli, S.; Mangani, S. A new proposal for urease mechanism based on the crystal structures of the native and inhibited enzyme from Bacillus pasteurii: Why urea hydrolysis costs two nickels. Structure 1999, 7, 205–216. [Google Scholar] [CrossRef]
- Ha, N.C.; Oh, S.T.; Sung, J.Y.; Cha, K.A.; Lee, M.H.; Oh, B.H. Supramolecular assembly and acid resistance of Helicobacter pylori urease. Nat. Struct. Biol. 2001, 8, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Zaborska, W.; Krajewska, B.; Kot, M.; Karcz, W. Quinone-induced inhibition of urease: Elucidation of its mechanisms by probing thiol groups of the enzyme. Bioorg. Chem. 2007, 35, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Follmer, C. Insights into the role and structure of plant ureases. Phytochemistry 2008, 69, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, B. Ureases I. Functional, catalytic and kinetic properties: A review. J. Mol. Catal. B Enzym. 2009, 59, 9–21. [Google Scholar] [CrossRef]
- Krajewska, B.; van Eldik, R.; Brindell, M. Temperature- and pressure-dependent stopped-flow kinetic studies of jack bean urease. Implications for the catalytic mechanism. JBIC J. Biol. Inorg. Chem. 2012, 17, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, A.; Durairajpandian, V.; Elumalai, S.; Mathivanan, N.; Munirajan, A.K.; Ponnuraj, K. Structural and functional studies on urease from pigeon pea (Cajanus cajan). Int. J. Biol. Macromol. 2013, 58, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Benini, S.; Kosikowska, P.; Cianci, M.; Mazzei, L.; Vara, A.G.; Berlicki, Ł.; Ciurli, S. The crystal structure of Sporosarcina pasteurii urease in a complex with citrate provides new hints for inhibitor design. J. Biol. Inorg. Chem. 2013, 18, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Maroney, M.J.; Ciurli, S. Nonredox nickel enzymes. Chem. Rev. 2014, 114, 4206–4228. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, B. A combined temperature-pH study of urease kinetics. Assigning pKa values to ionizable groups of the active site involved in the catalytic reaction. J. Mol. Catal. B Enzym. 2016, 124, 70–76. [Google Scholar] [CrossRef]
- Mobley, H.L.; Island, M.D.; Hausinger, R.P. Molecular biology of microbial ureases. Microbiol. Rev. 1995, 59, 451–480. [Google Scholar] [PubMed]
- Park, I.S.; Hausinger, R.P. Site-directed mutagenesis of Klebsiella aerogenes urease: Identification of histidine residues that appear to function in nickel ligation, substrate binding, and catalysis. Protein Sci. 1993, 2, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.P.; Miller, B.R.; Roitberg, A.E.; Merz, K.M. Wide-open flaps are key to urease activity. J. Am. Chem. Soc. 2012, 134, 9934–9937. [Google Scholar] [CrossRef] [PubMed]
- Minkara, M.S.; Ucisik, M.N.; Weaver, M.N.; Merz, K.M. Molecular dynamics study of helicobacter pylori urease. J. Chem. Theory Comput. 2014, 10, 1852–1862. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Su, J.; Wu, D.; Yu, X.; Su, Z.; He, J.; Wu, X.; Kong, S.; Lai, X.; Lin, J.; et al. Kinetics and mechanism study of competitive inhibition of jack-bean urease by baicalin. Sci. World J. 2013, 2013, 879501. [Google Scholar] [CrossRef] [PubMed]
- Macomber, L.; Minkara, M.S.; Hausinger, R.P.; Merz, K.M., Jr. Reduction of urease activity by interaction with the flap covering the active site. J. Chem. Inf. Model. 2015, 55, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.R.; Hausinger, R.P. Site-directed mutagenesis of the active site cysteine in Klebsiella aerogenes urease. J. Biol. Chem. 1992, 267, 20024–20027. [Google Scholar] [PubMed]
- Amtul, Z.; Kausar, N.; Follmer, C.; Rozmahel, R.F.; Atta-Ur-Rahman; Kazmi, S.A.; Shekhani, M.S.; Eriksen, J.L.; Khan, K.M.; Choudhary, M.I. Cysteine based novel noncompetitive inhibitors of urease(s)-Distinctive inhibition susceptibility of microbial and plant ureases. Bioorg. Med. Chem. 2006, 14, 6737–6744. [Google Scholar] [CrossRef] [PubMed]
- Modolo, L.V.; de Souza, A.X.; Horta, L.P.; Araujo, D.P.; de Fátima, Â. An overview on the potential of natural products as ureases inhibitors: A review. J. Adv. Res. 2015, 6, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Stephenson, K.K.; Wade, K.L.; Talalay, P. Urease from Helicobacter pylori is inactivated by sulforaphane and other isothiocyanates. Biochem. Biophys. Res. Commun. 2013, 435, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-D.; Zheng, R.-B.; Xie, J.-H.; Su, J.-Y.; Huang, X.-Q.; Wang, Y.-H.; Zheng, Y.-F.; Mo, Z.-Z.; Wu, X.-L.; Wu, D.-W.; et al. Biological evaluation and molecular docking of baicalin and scutellarin as Helicobacter pylori urease inhibitors. J. Ethnopharmacol. 2015, 162, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-W.; Yu, X.-D.; Xie, J.-H.; Su, Z.-Q.; Su, J.-Y.; Tan, L.-R.; Huang, X.-Q.; Chen, J.-N.; Su, Z.-R. Inactivation of jack bean urease by scutellarin: elucidation of inhibitory efficacy, kinetics and mechanism. Fitoterapia 2013, 91, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Upadhyay, L.S.B. Effect of thiols on the activity of urease from dehusked seeds of watermelon (Citrullus vulgaris). Plant. Sci. 2003, 164, 189–194. [Google Scholar] [CrossRef]
- Quastel, J.H. The action of polyhydric phenols on urease; the influence of thiol compounds. Biochem. J. 1933, 27, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Cichero, E.; D’Ursi, P.; Moscatelli, M.; Bruno, O.; Orro, A.; Rotolo, C.; Milanesi, L.; Fossa, P. Homology modeling, docking studies and molecular dynamic simulations using graphical processing unit architecture to probe the Type-11 phosphodiesterase catalytic site: A computational approach for the rational design of selective inhibitors. Chem. Biol. Drug Des. 2013, 82, 718–731. [Google Scholar] [CrossRef] [PubMed]
- Kaserer, T.; Obermoser, V.; Weninger, A.; Gust, R.; Schuster, D. Evaluation of selected 3D virtual screening tools for the prospective identification of peroxisome proliferator-activated receptor (PPAR) γ partial agonists. Eur. J. Med. Chem. 2016, 124, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Cichero, E.; Espinoza, S.; Franchini, S.; Guariento, S.; Brasili, L.; Gainetdinov, R.R.; Fossa, P. Further insights into the pharmacology of the human trace amine-associated receptors: Discovery of novel ligands for TAAR1 by a virtual screening approach. Chem. Biol. Drug Des. 2014, 84, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Shushan, G. Isolation, purification and partial characterisation of urease from seeds of water melon (Citrullus vulgaris). J. Plant. Biochem. Biotechnol. 1997, 6, 45–47. [Google Scholar] [CrossRef]
- Zaldívar-Machorro, V.J.; López-Ortiz, M.; Demare, P.; Regla, I.; Muñoz-Clares, R.A. The disulfiram metabolites S-methyl-N,N-diethyldithiocarbamoyl sulfoxide and S-methyl-N,N-diethylthiocarbamoyl sulfone irreversibly inactivate betaine aldehyde dehydrogenase from Pseudomonas aeruginosa, both in vitro and in situ, and arrest bacterial grow. Biochimie 2011, 93, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Galkin, A.; Kulakova, L.; Lim, K.; Chen, C.Z.; Zheng, W.; Turko, I.V.; Herzberg, O. Structural basis for inactivation of Giardia lamblia carbamate kinase by disulfiram. J. Biol. Chem. 2014, 289, 10502–10509. [Google Scholar] [CrossRef] [PubMed]
- Todd, M.J.; Hausinger, R.P. Reactivity of the essential thiol of Klebsiella aerogenes urease: Effect of pH and ligands on thiol modification. J. Biol. Chem. 1991, 266, 10260–10267. [Google Scholar] [PubMed]
- Perveen, S.; El-Shafae, A.M.; Al-Taweel, A.; Fawzy, G.A.; Malik, A.; Afza, N.; Latif, M.; Iqbal, L. Antioxidant and urease inhibitory C-glycosylflavonoids from Celtis africana. J. Asian Nat. Prod. Res. 2011, 13, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Ngan, L.T.M.; Moon, J.-K.; Shibamoto, T.; Ahn, Y.-J. Growth-inhibiting, bactericidal, and urease inhibitory effects of Paeonia lactiflora root constituents and related compounds on antibiotic-susceptible and -resistant strains of Helicobacter pylori. J. Agric. Food Chem. 2012, 60, 9062–9073. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.G.; Meshram, R.J.; Gond, D.S.; Gacche, R.N. Inhibition of growth of Helicobacter pylori and its urease by coumarin derivatives: Molecular docking analysis. J. Pharm. Res. 2013, 7, 705–711. [Google Scholar] [CrossRef]
- Ahmad, V.; Hussain, J.; Hussain, H.; Jassbi, A. First natural urease inhibitor from Euphorbia decipiens. Chem. Pharm. Bull. (Tokyo) 2003, 51, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Arfan, M.; Ali, M.; Ahmad, H.; Anis, I.; Khan, A.; Choudhary, M.I.; Shah, M.R. Urease inhibitors from Hypericum oblongifolium WALL. J. Enzyme Inhib. Med. Chem. 2010, 25, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Kot, M.; Karcz, W.; Zaborska, W. 5-Hydroxy-1,4-naphthoquinone (juglone) and 2-hydroxy-1,4-naphthoquinone (lawsone) influence on jack bean urease activity: Elucidation of the difference in inhibition activity. Bioorg. Chem. 2010, 38, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Saeed, M.; Khan, A.; Adhikari, A.; Wadood, A.; Khan, K.; de Feo, V. A New Urease Inhibitor from Viola betonicifolia. Molecules 2014, 19, 16770–16778. [Google Scholar] [CrossRef] [PubMed]
- Kubo, J.; Lee, J.R.; Kubo, I. Anti-Helicobacter pylori agents from the cashew apple. J. Agric. Food Chem. 1999, 47, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Olech, Z.; Zaborska, W.; Kot, M. Jack bean urease inhibition by crude juices of Allium and Brassica plants. Determination of thiosulfinates. Food Chem. 2014, 145, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Veverka, K.A.; Johnson, K.L.; Mays, D.C.; Lipsky, J.J.; Naylor, S. Inhibition of aldehyde dehydrogenase by disulfiram and its metabolite methyl diethylthiocarbamoyl-sulfoxide. Biochem. Pharmacol. 1997, 53, 511–518. [Google Scholar] [CrossRef]
- Velasco-García, R.; Zaldívar-Machorro, V.J.; Mújica-Jiménez, C.; González-Segura, L.; Muñoz-Clares, R.A. Disulfiram irreversibly aggregates betaine aldehyde dehydrogenase—A potential target for antimicrobial agents against Pseudomonas aeruginosa. Biochem. Biophys. Res. Commun. 2006, 341, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Loi, C.M.; Day, J.D.; Jue, S.G.; Bush, E.D.; Costello, P.; Dewey, L.V.; Vestal, R.E. Dose-dependent inhibition of theophylline metabolism by disulfiram in recovering alcoholics. Clin. Pharmacol. Ther. 1989, 45, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Velasco-García, R.; Chacón-Aguilar, V.M.; Hervert-Hernández, D.; Muñoz-Clares, R.A. Inactivation of betaine aldehyde dehydrogenase from Pseudomonas aeruginosa and Amaranthus hypochondriacus L. leaves by disulfiram. Chem. Biol. Interact. 2003, 143–144, 149–158. [Google Scholar] [CrossRef]
- Dalecki, A.G.; Haeili, M.; Shah, S.; Speer, A.; Niederweis, M.; Kutsch, O.; Wolschendorf, F. Disulfiram and Copper Ions Kill Mycobacterium tuberculosis in a Synergistic Manner. Antimicrob. Agents Chemother. 2015, 59, 4835–4844. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-M.; Duh, Y.; Wang, S.-T.; Lai, M.M.C.; Yuan, H.S.; Lim, C. Using an old drug to target a new drug site: Application of disulfiram to target the Zn-site in HCV NS5A protein. J. Am. Chem. Soc. 2016, 138, 3856–3862. [Google Scholar] [CrossRef] [PubMed]
- Okyay, T.O.; Rodrigues, D.F. High throughput colorimetric assay for rapid urease activity quantification. J. Microbiol. Methods 2013, 95, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of all the compounds and enzyme preparation are available from the authors.
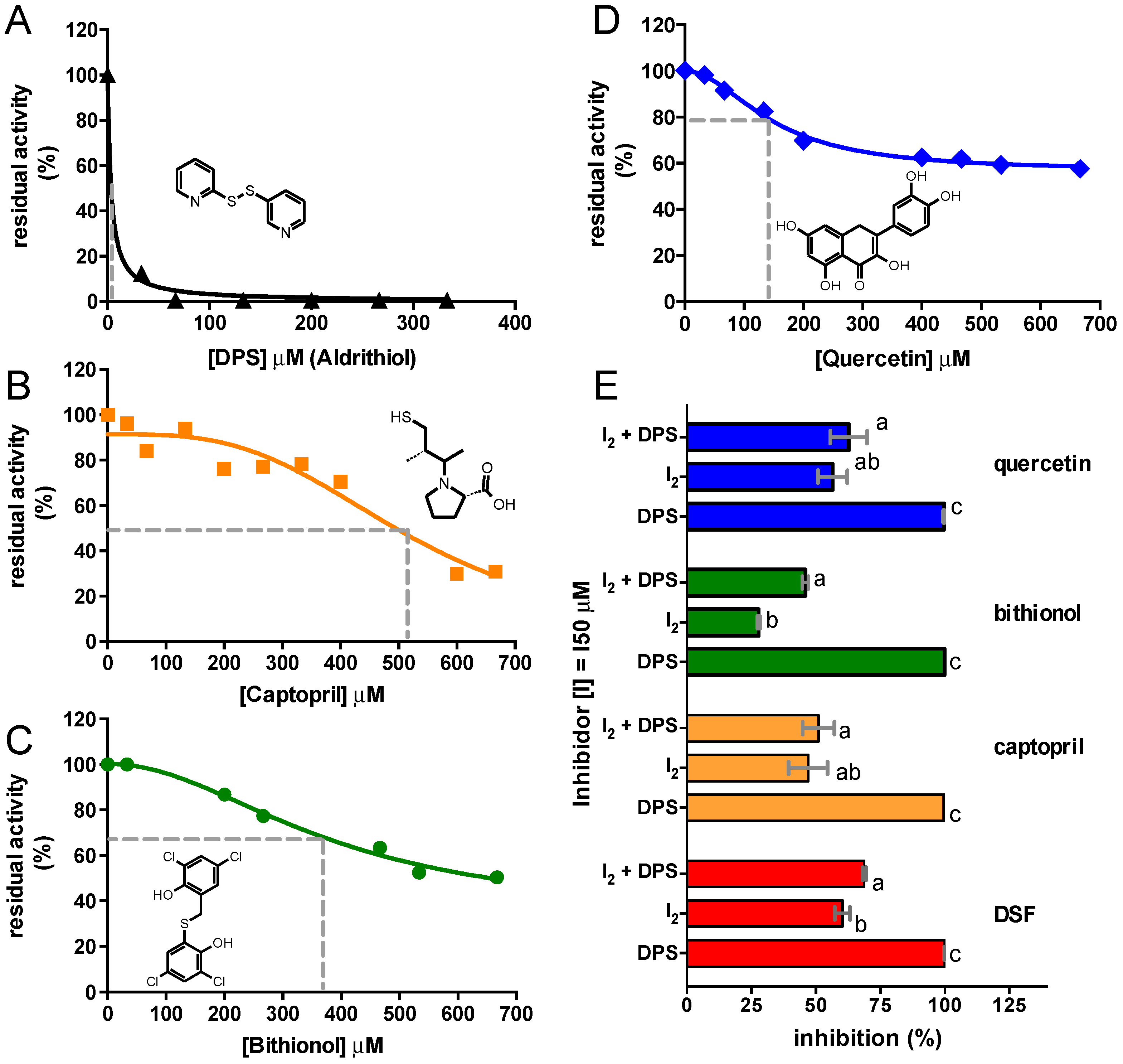
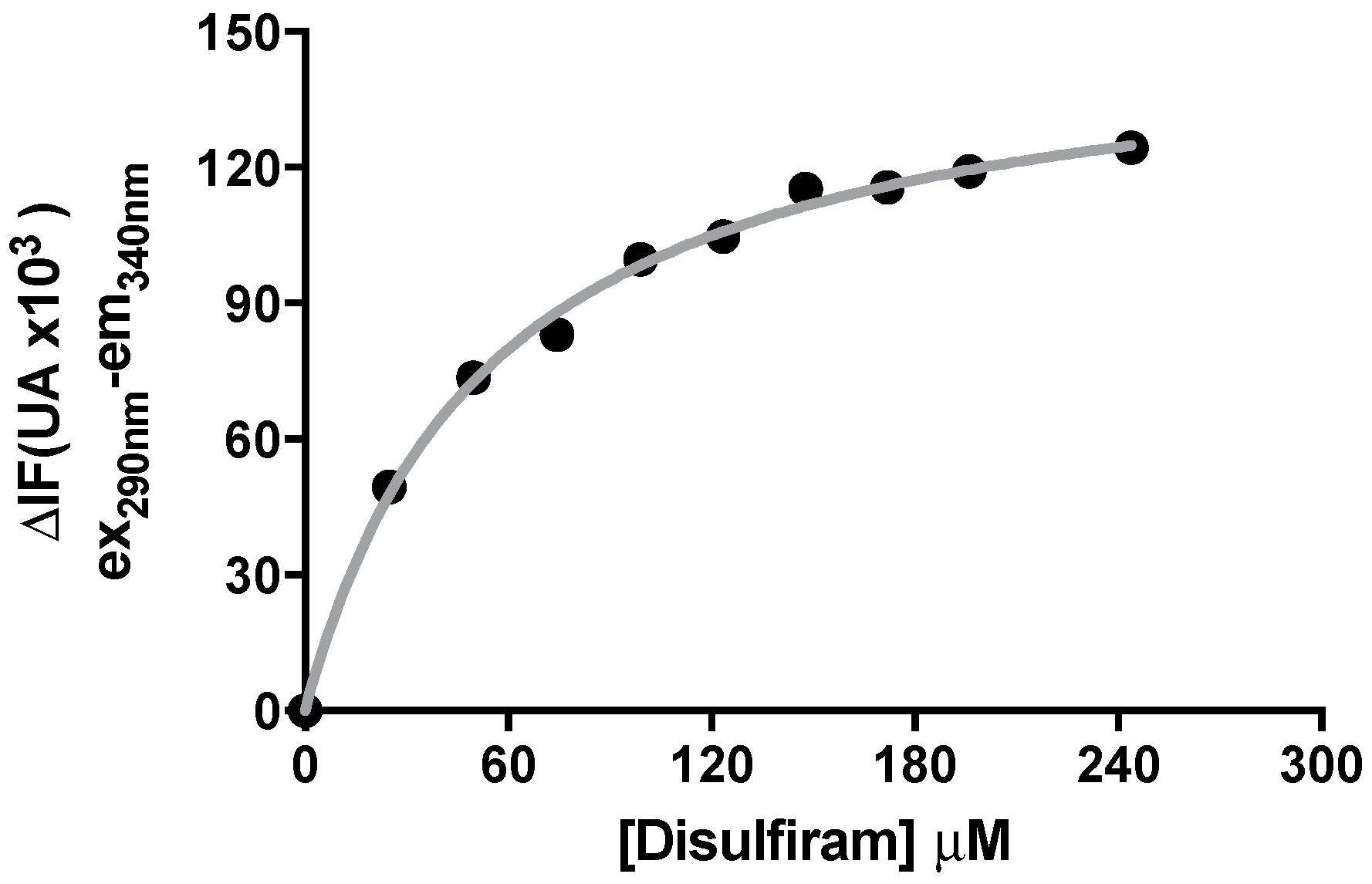

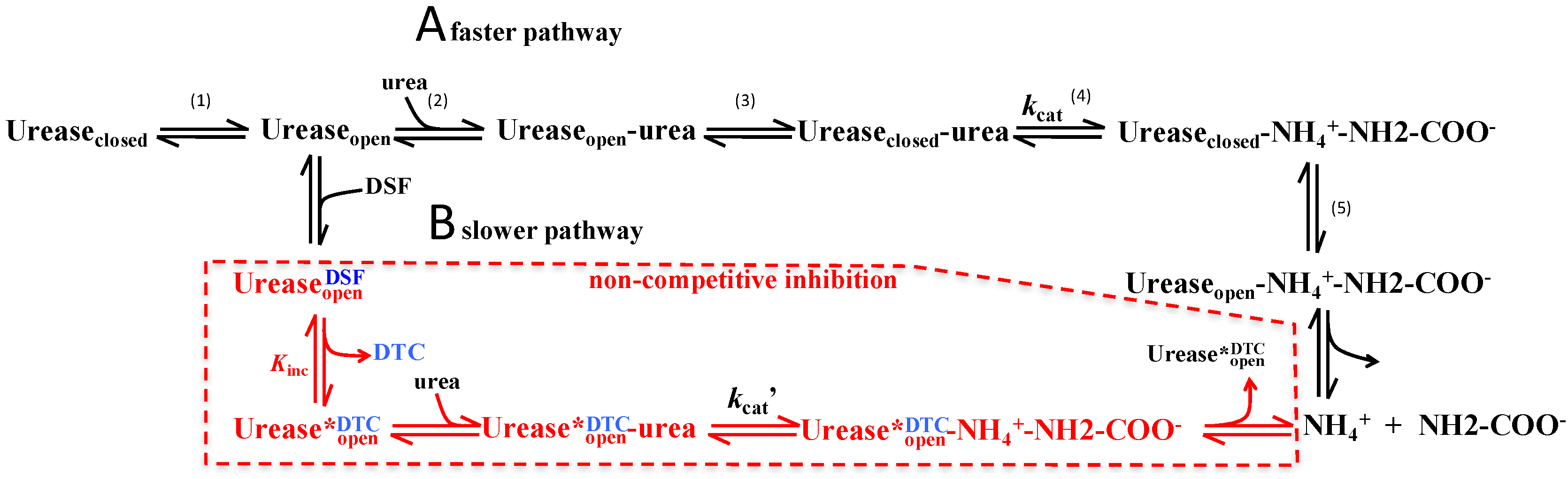
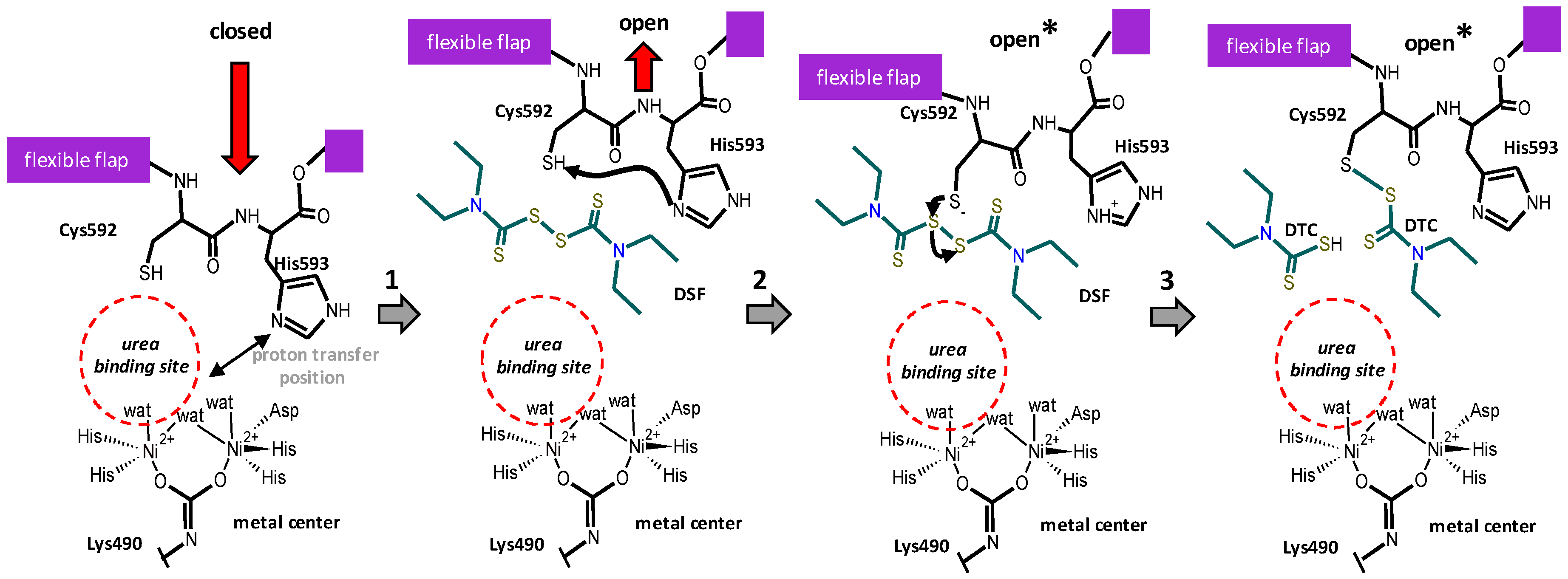
| Compound | IC50 (µM) | Plateau (% of Initial) | ½ of Span (% of Initial) |
|---|---|---|---|
| DSF | 80.02 ± 1.30 × 10−4 | 0.00 | 50.00 |
| Aldrithiol (DPS) | 3.35 ± 0.81 | 0.00 | 50.00 |
| Captopril | 523.90 ± 39.62 | 0.00 | 50.00 |
| Bithionol | 376.50 ± 120.40 | 34.53 ± 18.23 | 67.26 |
| Quercetin | 154.70 ± 10.15 | 58.86 ± 1.52 | 79.43 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Sánchez, Á.G.; Alvarez-Parrilla, E.; Martínez-Martínez, A.; Aguirre-Reyes, L.; Orozpe-Olvera, J.A.; Ramos-Soto, M.A.; Núñez-Gastélum, J.A.; Alvarado-Tenorio, B.; De la Rosa, L.A. Inhibition of Urease by Disulfiram, an FDA-Approved Thiol Reagent Used in Humans. Molecules 2016, 21, 1628. https://doi.org/10.3390/molecules21121628
Díaz-Sánchez ÁG, Alvarez-Parrilla E, Martínez-Martínez A, Aguirre-Reyes L, Orozpe-Olvera JA, Ramos-Soto MA, Núñez-Gastélum JA, Alvarado-Tenorio B, De la Rosa LA. Inhibition of Urease by Disulfiram, an FDA-Approved Thiol Reagent Used in Humans. Molecules. 2016; 21(12):1628. https://doi.org/10.3390/molecules21121628
Chicago/Turabian StyleDíaz-Sánchez, Ángel Gabriel, Emilio Alvarez-Parrilla, Alejandro Martínez-Martínez, Luis Aguirre-Reyes, Jesica Aline Orozpe-Olvera, Miguel Armando Ramos-Soto, José Alberto Núñez-Gastélum, Bonifacio Alvarado-Tenorio, and Laura Alejandra De la Rosa. 2016. "Inhibition of Urease by Disulfiram, an FDA-Approved Thiol Reagent Used in Humans" Molecules 21, no. 12: 1628. https://doi.org/10.3390/molecules21121628
APA StyleDíaz-Sánchez, Á. G., Alvarez-Parrilla, E., Martínez-Martínez, A., Aguirre-Reyes, L., Orozpe-Olvera, J. A., Ramos-Soto, M. A., Núñez-Gastélum, J. A., Alvarado-Tenorio, B., & De la Rosa, L. A. (2016). Inhibition of Urease by Disulfiram, an FDA-Approved Thiol Reagent Used in Humans. Molecules, 21(12), 1628. https://doi.org/10.3390/molecules21121628