Catalytic Performance of a New 1D Cu(II) Coordination Polymer {Cu(NO3)(H2O)}(HTae)(4,4′-Bpy) for Knoevenagel Condensation
Abstract
:1. Introduction
2. Results and Discussion
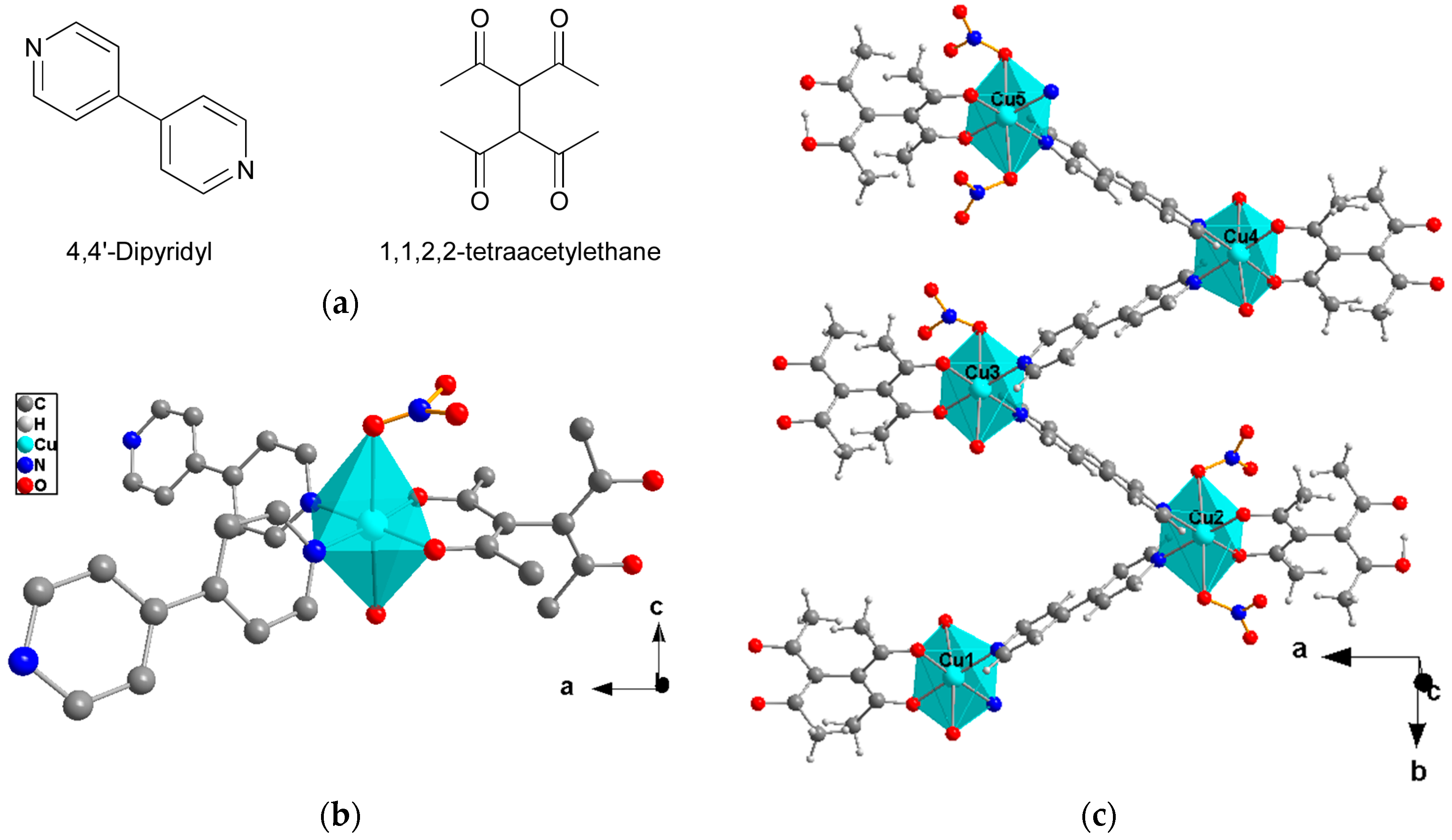
2.1. Crystal Structure Description
- Cu(II) atoms bonded to two coordinated water molecules.
- Cu(II) atoms bonded to one water molecule and one nitrate group.
- Cu(II) atoms connected to two nitrate anions.
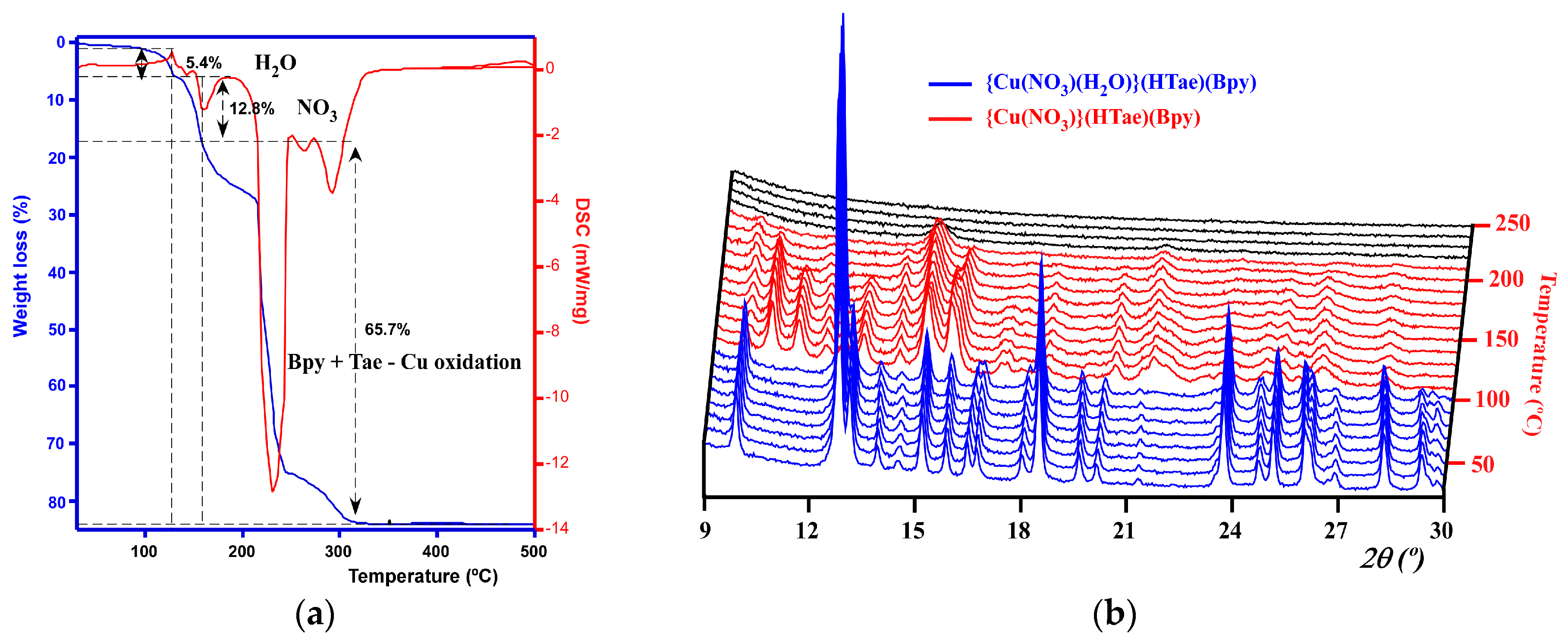
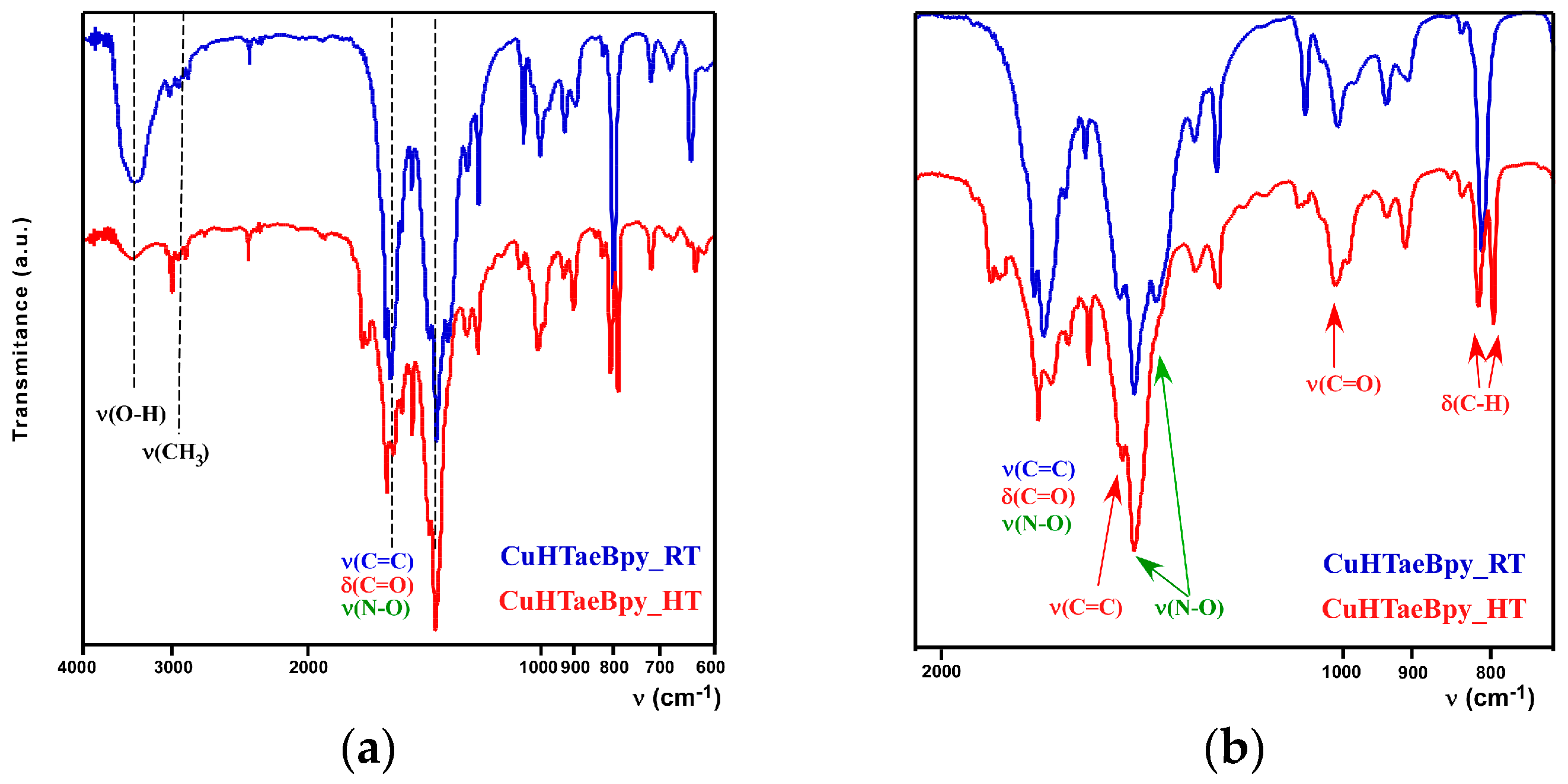
2.2. Thermal and Spectroscopic Characterization
2.3. Catalytic Activity Study
2.3.1. Knoevenagel Condensation
2.3.2. Recycling and Heterogeneity Tests
2.3.3. Catalyst Characterization
3. Materials and Methods
3.1. Synthesis
3.2. Characterization
3.3. Themal Studies
3.4. Catalytic Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Adams, J.; Pendlebury, D. Materials Science & Technology; Global Research Reports; Thomson Reuters: New York, NY, USA, 2011. [Google Scholar]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal–organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Abdel-Fattah, A.I.; Xu, H.; Zhao, Y.; Hickmott, D.D. Storage and Separation Applications of Nanoporous Metal–organic Frameworks. CrystEngComm 2010, 12, 1337–1353. [Google Scholar] [CrossRef]
- Verdegaal, W.M.; Wang, K.; Sculley, J.P.; Wriedt, M.; Zhou, H.-C. Evaluation of Metal–organic Frameworks and Porous Polymer Networks for CO2-Capture Applications. ChemSusChem 2016, 9, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kim, K.-H.; Kwon, E.E.; Szulejko, J.E. Metal–organic Frameworks for the Control and Management of Air Quality: Advances and Future Direction. J. Mater. Chem. A 2015, 4, 345–361. [Google Scholar] [CrossRef]
- Arriortua, M.I.; Barandika, G.; Bazan, B.; Calderon-Casado, A.; Urtiaga, M.K. Alcohol and Water Sensor Compounds, Detection Method and Device. WO2013057350 (A1), 25 April 2013. [Google Scholar]
- Jackson, S.L.; Rananaware, A.; Rix, C.; Bhosale, S.V.; Latham, K. Highly Fluorescent Metal–Organic Framework for the Sensing of Volatile Organic Compounds. Cryst. Growth Des. 2016. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Opanasenko, M.; Čejka, J.; Garcia, H. Metal Organic Frameworks as Solid Catalysts in Condensation Reactions of Carbonyl Groups. Adv. Synth. Catal. 2013, 355, 247–268. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Applications of Metal–Organic Frameworks in Heterogeneous Supramolecular Catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Metal–Organic Frameworks as Catalysts for Oxidation Reactions. Chem. Eur. J. 2016. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Metal–Organic Frameworks Catalyzed C–C and C–Heteroatom Coupling Reactions. Chem. Soc. Rev. 2015, 44, 1922–1947. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, B.; He, H.; Zhou, W.; Chen, B.; Qian, G. Metal–Organic Frameworks as Platforms for Functional Materials. Acc. Chem. Res. 2016, 49, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-C. “Joe”; Kitagawa, S. Metal–organic Frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Merkens, C.; Si, R.; Englert, U. Crosslinking of the Pd(acacCN)2 Building Unit with Ag(I) Salts: Dynamic 1D Polymers and an Extended 3D Network. CrystEngComm 2015, 17, 4383–4393. [Google Scholar] [CrossRef]
- Burrows, A.D.; Cassar, K.; Mahon, M.F.; Rigby, S.P.; Warren, J.E. Synthesis and Characterisation of Metal–Organic Frameworks Containing Bis(β-diketonate) Linkers. CrystEngComm 2008, 10, 1474–1479. [Google Scholar] [CrossRef]
- Yang, Q.; Tan, X.; Wang, S.; Zhang, J.; Chen, L.; Zhang, J.-P.; Su, C.-Y. Porous Organic–Inorganic Hybrid Aerogels Based on Bridging Acetylacetonate. Microporous Mesoporous Mater. 2014, 187, 108–113. [Google Scholar] [CrossRef]
- Luisi, B.S.; Kravtsov, V.C.; Moulton, B.D. An (8,3)-a 3D Coordination Network and Concomitant Three-Connected Supramolecular Isomers. Cryst. Growth Des. 2006, 6, 2207–2209. [Google Scholar] [CrossRef]
- Fernández de Luis, R.; Larrea, E.S.; Orive, J.; Lezama, L.; Arriortua, M.I. Commensurate Super-Structure of the {Cu(NO3)(H2O)}(HTae)(Bpy) Coordination Polymer. An Example of 2D Hydrogen Bonding Networks as Magnetic Exchange Pathway. Inorg. Chem. 2016. accepted. [Google Scholar]
- Xia, Q.-H.; Ge, H.-Q.; Ye, C.-P.; Liu, Z.-M.; Su, K.-X. Advances in Homogeneous and Heterogeneous Catalytic Asymmetric Epoxidation. Chem. Rev. 2005, 105, 1603–1662. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.A.; Faul, M.M.; Bilodeau, M.T. Copper-Catalyzed Aziridination of Olefins by (N-(p-toluenesulfonyl)imino)phenyliodinane. J. Org. Chem. 1991, 56, 6744–6746. [Google Scholar] [CrossRef]
- Müller, P.; Fruit, C. Enantioselective Catalytic Aziridinations and Asymmetric Nitrene Insertions into CH Bonds. Chem. Rev. 2003, 103, 2905–2920. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, A.; Leadbeater, N.E. Microencapsulated VO(acac)2: Preparation and Use in Allylic Alcohol Epoxidation. Org. Lett. 2002, 4, 1519–1521. [Google Scholar] [CrossRef] [PubMed]
- Valkenberg, M.H.; Hölderich, W.F. Preparation and Use of Hybrid Organic–Inorganic Catalysts. Catal. Rev. 2002, 44, 321–374. [Google Scholar] [CrossRef]
- De Vos, D.E.; Dams, M.; Sels, B.F.; Jacobs, P.A. Ordered Mesoporous and Microporous Molecular Sieves Functionalized with Transition Metal Complexes as Catalysts for Selective Organic Transformations. Chem. Rev. 2002, 102, 3615–3640. [Google Scholar] [CrossRef] [PubMed]
- Dioos, B.M.L.; Vankelecom, I.F.J.; Jacobs, P.A. Aspects of Immobilisation of Catalysts on Polymeric Supports. Adv. Synth. Catal. 2006, 348, 1413–1446. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Silica-Bound Homogenous Catalysts as Recoverable and Reusable Catalysts in Organic Synthesis. Adv. Synth. Catal. 2006, 348, 1391–1412. [Google Scholar] [CrossRef]
- Pereira, C.; Silva, A.R.; Carvalho, A.P.; Pires, J.; Freire, C. Vanadyl Acetylacetonate Anchored onto Amine-Functionalised Clays and Catalytic Activity in the Epoxidation of Geraniol. J. Mol. Catal. Chem. 2008, 283, 5–14. [Google Scholar] [CrossRef]
- Jarrais, B.; Silva, A.R.; Freire, C. Anchoring of Vanadyl Acetylacetonate onto Amine-Functionalised Activated Carbons: Catalytic Activity in the Epoxidation of an Allylic Alcohol. Eur. J. Inorg. Chem. 2005, 2005, 4582–4589. [Google Scholar] [CrossRef]
- Silva, A.R.; Figueiredo, J.L.; Freire, C.; de Castro, B. Copper(II) Acetylacetonate Anchored onto an Activated Carbon as a Heterogeneous Catalyst for the Aziridination of Styrene. Catal. Today 2005, 102–103, 154–159. [Google Scholar] [CrossRef]
- Silva, A.R.; Wilson, K.; Whitwood, A.C.; Clark, J.H.; Freire, C. Amine-Functionalised Hexagonal Mesoporous Silica as Support for Copper(II) Acetylacetonate Catalyst. Eur. J. Inorg. Chem. 2006, 2006, 1275–1283. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Tayyari, S.F.; Milani-nejad, F. Vibrational Assignment of Acetylacetone. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 2679–2691. [Google Scholar] [CrossRef]
- Howard, D.L.; Kjaergaard, H.G.; Huang, J.; Meuwly, M. Infrared and Near-Infrared Spectroscopy of Acetylacetonate and Hexafluoroacetylacetonate. J. Phys. Chem. A 2015, 119, 7980–7990. [Google Scholar] [CrossRef] [PubMed]
- Tietze, L.F.; Beifuss, U. The Knoevenagel Reaction. In Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; Pergamon: Oxford, UK, 1991; pp. 341–394. [Google Scholar]
- Burgoyne, A.R.; Meijboom, R. Knoevenagel Condensation Reactions Catalysed by Metal–organic Frameworks. Catal. Lett. 2013, 143, 563–571. [Google Scholar] [CrossRef]
- Položij, M.; Rubeš, M.; Čejka, J.; Nachtigall, P. Catalysis by Dynamically Formed Defects in a Metal–Organic Framework Structure: Knoevenagel Reaction Catalyzed by Copper Benzene-1,3,5-tricarboxylate. ChemCatChem 2014, 6, 2821–2824. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds CuHTaeBpy_RT and CuHTaeBpy_HT are available from the authors.
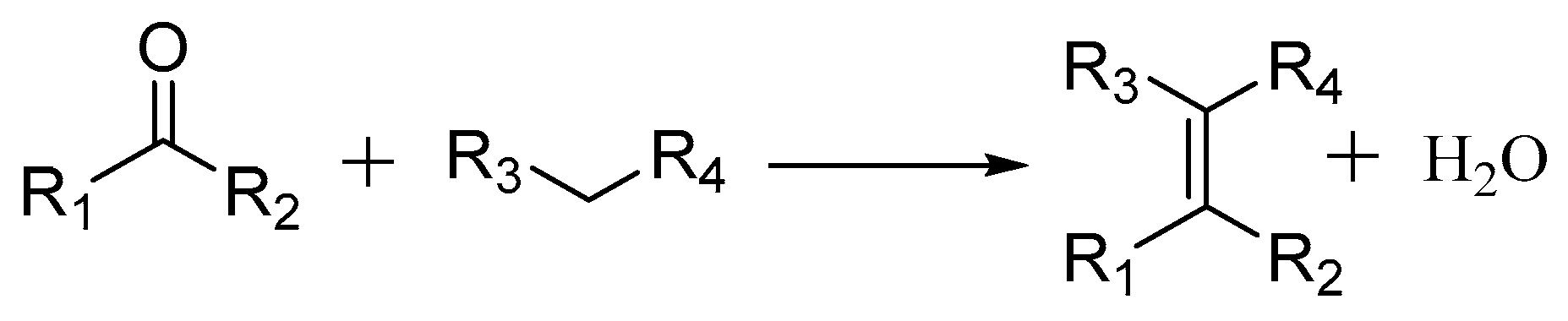
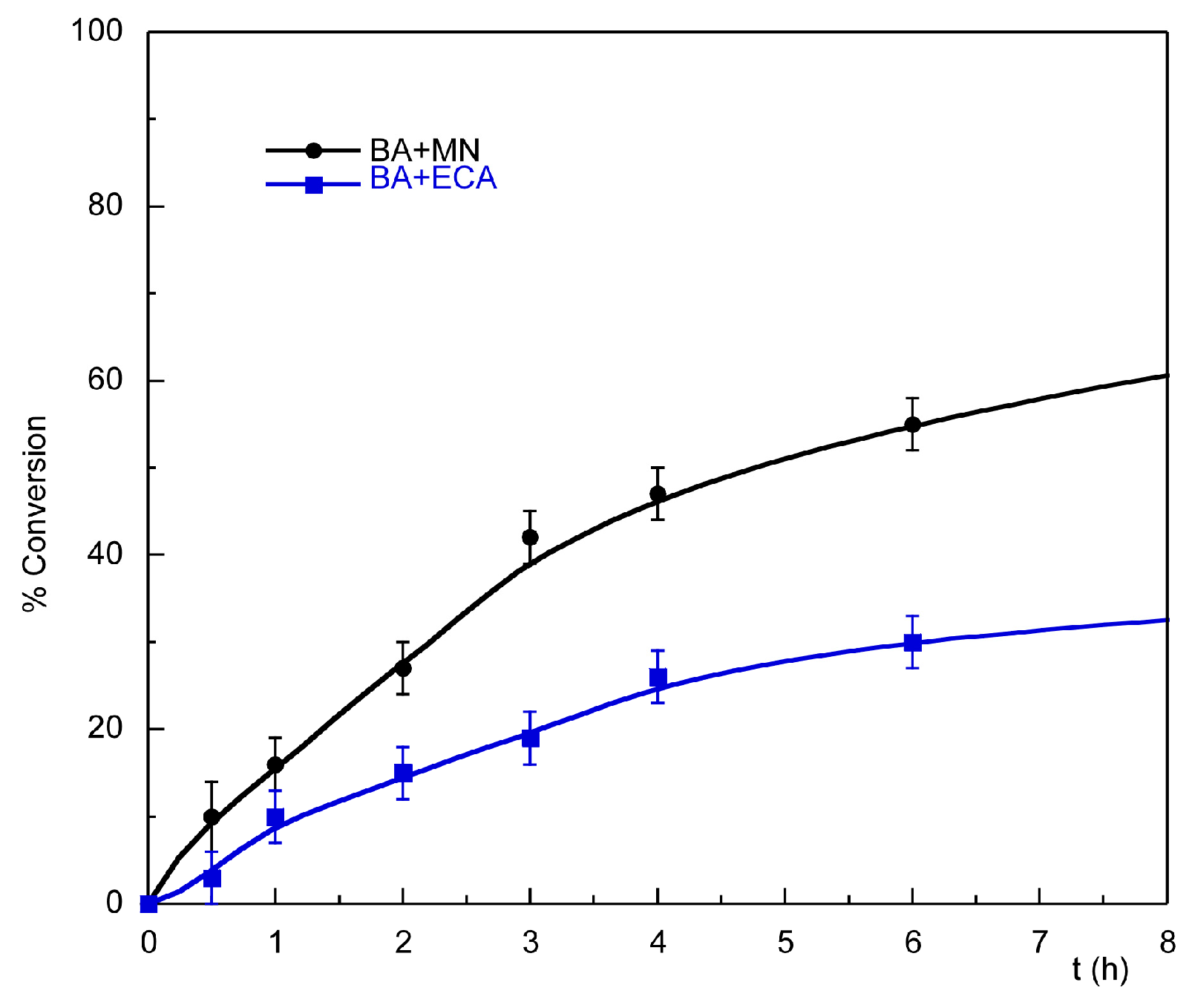
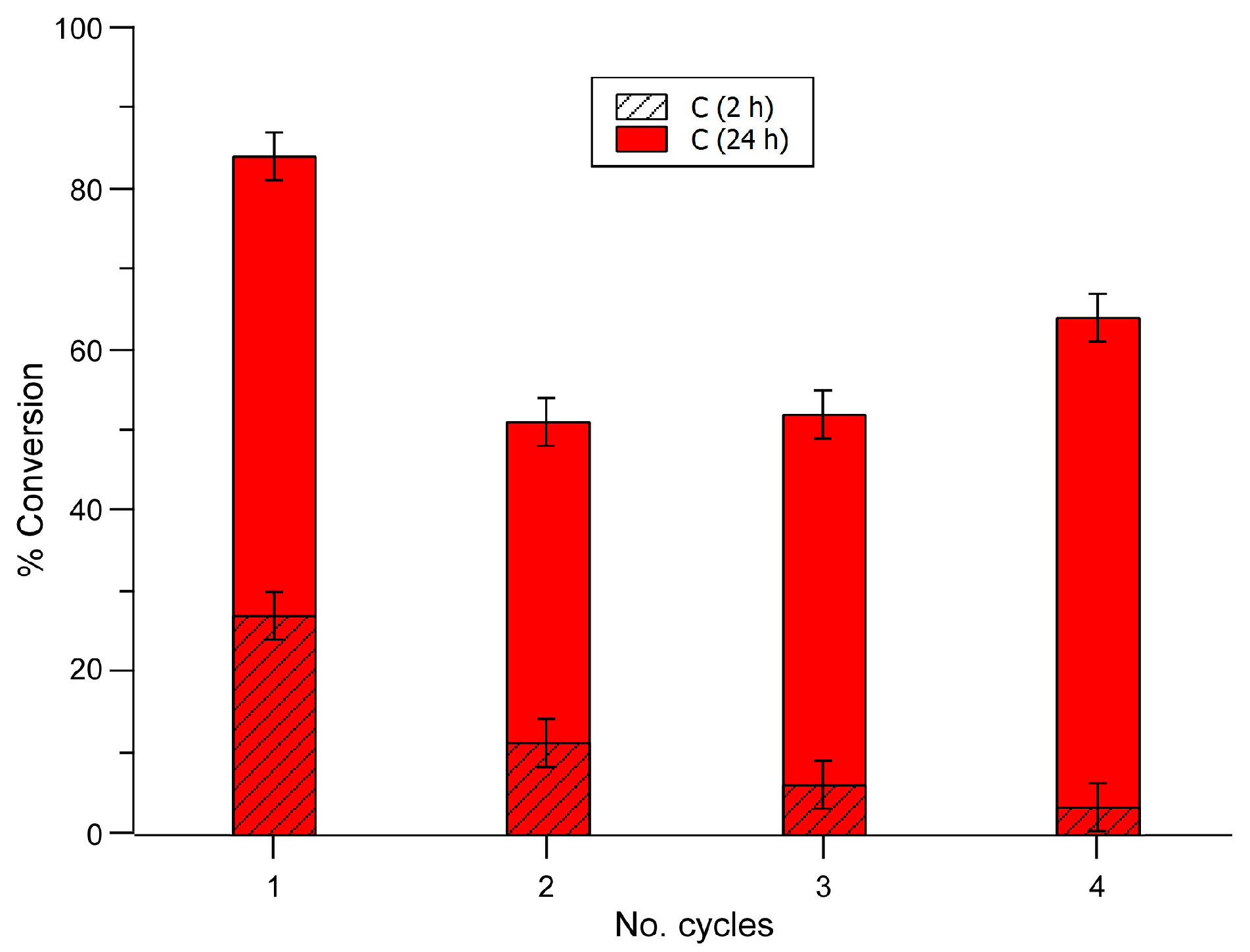
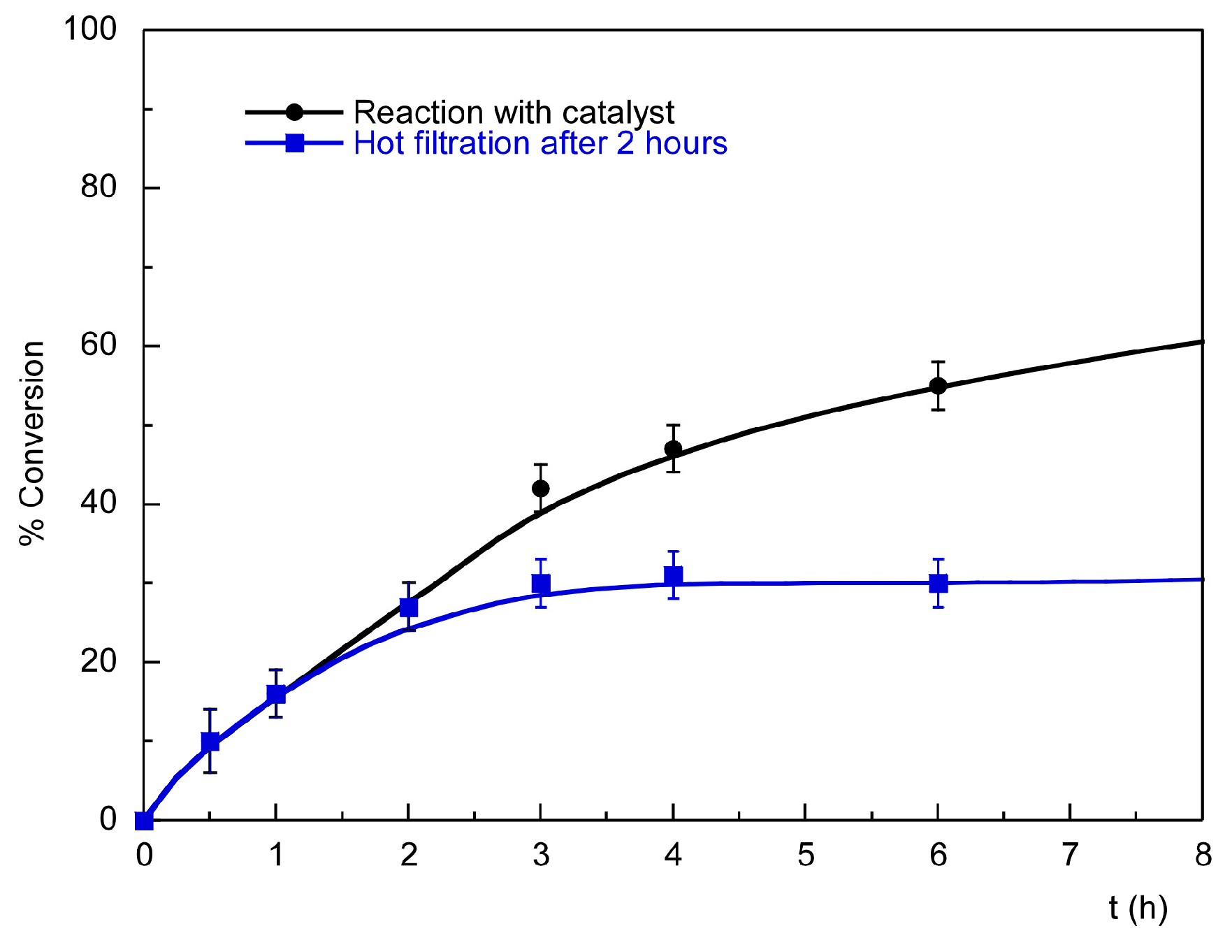
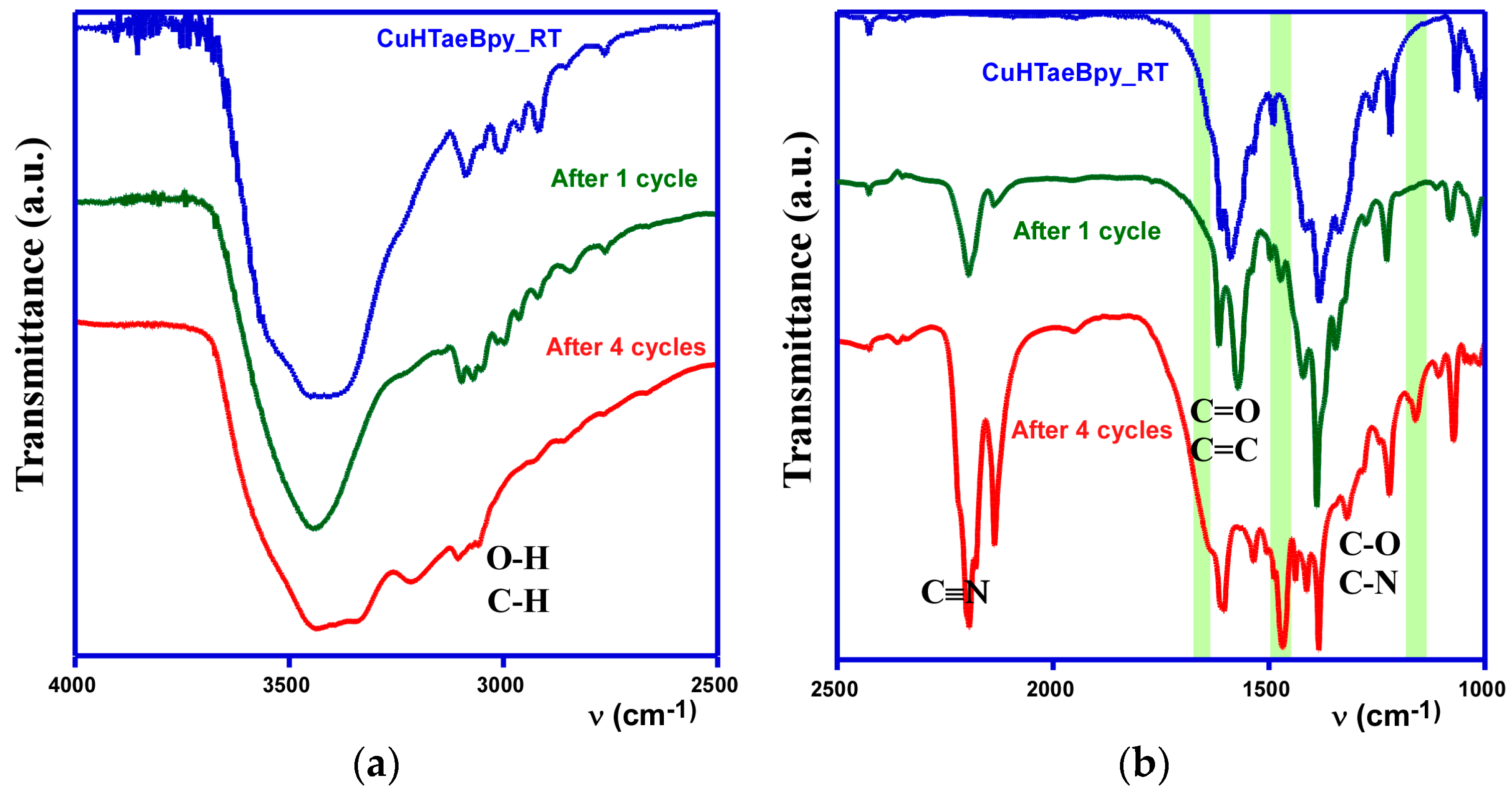
| Entry | Substrate | Donor | Conversion [%] (after 2 h) | Conversion [%] (after 24 h) |
|---|---|---|---|---|
| 1 | Benzaldehyde | Malononitrile | 27 | 84 |
| 2 | Benzaldehyde | Ethyl cyanoacetate | 15 | 40 |
| 3 | p-Tolualdehyde | Malononitrile | 36 | 68 |
| 4 | p-Metoxybenzaldehyde | Malononitrile | 22 | 75 |
| 5 | p-Fluorobenzaldehyde | Malononitrile | 28 | 74 |
| 6 | Heptanal | Malononitrile | 51 | 89 |
| 7 | Cyclohexanone | Malononitrile | 2 | 11 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larrea, E.S.; Fernández de Luis, R.; Arriortua, M.I. Catalytic Performance of a New 1D Cu(II) Coordination Polymer {Cu(NO3)(H2O)}(HTae)(4,4′-Bpy) for Knoevenagel Condensation. Molecules 2016, 21, 1651. https://doi.org/10.3390/molecules21121651
Larrea ES, Fernández de Luis R, Arriortua MI. Catalytic Performance of a New 1D Cu(II) Coordination Polymer {Cu(NO3)(H2O)}(HTae)(4,4′-Bpy) for Knoevenagel Condensation. Molecules. 2016; 21(12):1651. https://doi.org/10.3390/molecules21121651
Chicago/Turabian StyleLarrea, Edurne S., Roberto Fernández de Luis, and María I. Arriortua. 2016. "Catalytic Performance of a New 1D Cu(II) Coordination Polymer {Cu(NO3)(H2O)}(HTae)(4,4′-Bpy) for Knoevenagel Condensation" Molecules 21, no. 12: 1651. https://doi.org/10.3390/molecules21121651







