1. Introduction
Wheat germ is a by-product of the wheat milling industry. It contains about 8%–14% oil and plays a crucial role in the food industry [
1]. WGO is the most important source of tocopherol of all vegetable oils, up to about 2500 mg/kg [
2], especially the content of α-tocopherol, which represents around 60% of the total tocopherol. WGO is also a rich source of unsaponifiable phytosterols, in particular sitosterol (60%–70%) and campesterol (20%–30%) [
3], polycosanols (POC), thiamine, riboflavin and niacin. In addition, tetracosanol, hexa-cosanol, and octacosanol are the major POC components in all varieties of WGO [
4,
5,
6]. Furthermore, WGO is high in human essential unsaturated fatty acids, including about 80% of linoleic (18:2) and linolenic (18:3) acids [
4]. In recent years, WGO has been used as a functional additive in natural foods or cosmetic products. However, the presence of polyunsaturated fatty acids and bioactive compounds affects its shelf life because they are prone to oxidation and degradation [
7].
The trends of natural and safe products have driven supercritical fluid technology to be a primary alternative to traditional solvent extraction (SE) for active ingredients of the food industry [
8]. Although supercritical carbon dioxide extraction (SCE) has been widely used in the food industry [
9], the higher processing pressure and operation cost have limited its application. Subcritical fluid extraction is performed at a lower temperature and pressure than those employed in SCE, which endow the subcritical fluid extraction with a higher application potential. Besides the mild temperature and pressure, the use of short-chain hydrocarbons, including propane or
n-butane, allows the reduction of the extraction time because of their high density, diffusivity and low viscosity [
10], while improving the quality of the oil including no use of toxic residual solvents, higher oxidation stability and reducing the degradation of the bioactive components [
11,
12]. Some results have been reported and have proved profitable and effective with the use of compressed n-butane [
13] and some results have been reported with the use of compressed propane [
10,
14]. There are several works which compared the different extraction methods of SCE and SE and the extract composition. Fiori et al. investigated the mass transfer kinetics of SCE grape oil and compared the lipid profiles and tocol with that of SE [
15]. However, there are few reports regarding the differences in the different extraction methods of SCE, subcritical fluid extraction and SE in aspects related to the yield, physicochemical properties, and fatty acid composition of the WGO.
Crude vegetable oils need to be refined to produce high quality and highly stable oils through elimination of undesirable compounds. A significant portion of the nutritional oil components is lost during conventional refining processes. Ghazani et al. investigated the minor constituents in canola oil processed by traditional and minimal refining methods and found that traditional neutralization removed 19.6% of the total tocopherol and 23.6% of the total free sterols [
16]. Wang and Johnson examined the effect of conventional oil refining processes on WGO quality and found that deodorization conditions reduced the tocopherol content of WGO significantly [
4].
Molecular distillation, a special high-vacuum distillation technology, is widely used in the oil industry because of its shorter residence time and good thermal stability, etc. It has been reported that molecular distillation exhibits outstanding performance for separation or purification of high-boiling-point mixtures such as octacosanol from transesterified rice bran wax [
17], triacylglycerol from free fatty acids mixtures [
18], carotenoids from palm oil [
19] and the production of palm olein–enriched diacylglycerol [
20]. Martinello et al. demonstrated that this method can obtain refined grape seed oil with a lower free fatty acid content and higher tocopherol recovery, using molecular distillation at the feed flow of 1.5 mL/min and the temperature of 220 °C [
21]. Wu et al. optimized the molecular distillation conditions of crude low-calorie cocoa butter [
22]. Solaesa et al. produced monoacylglycerols (MAGs)-enriched ω-3 polyunsaturated fatty acids using molecular distillation [
23]. Plenty of studies pointed out that molecular distillation was an appropriate method to separate heat-sensitive and low-volatile compounds [
24], and it provides a new and efficient approach to solving the rancidity of oils and fats in food. However, there are few works to be reported in the field of the molecular distillation–based deacidification of WGO.
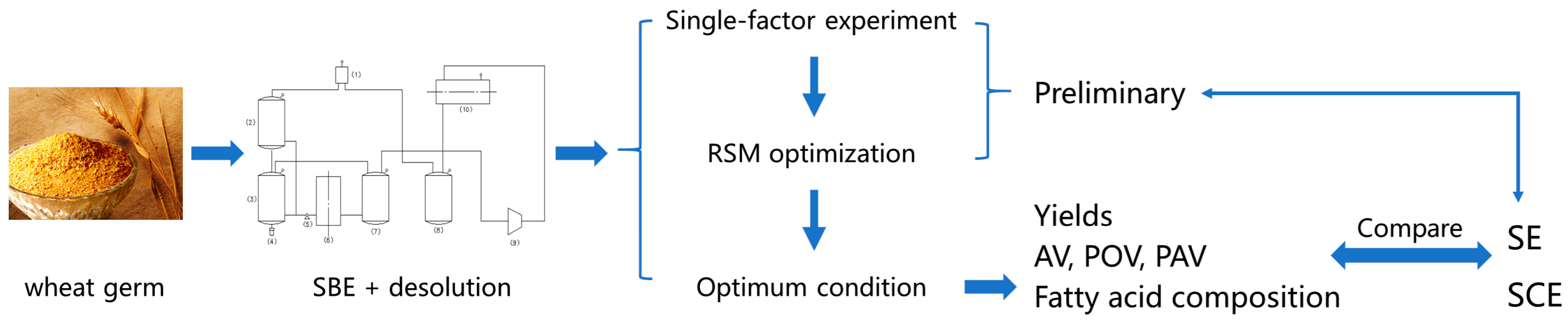
The purpose of this research was to assess the feasibility of subcritical butane extraction (SBE) in WGO extraction and compare it to SCE and SE on the basis of the yield, quality and fatty acid composition of the crude WGO. Furthermore, the effects of the molecular distillation temperature on the acid value, peroxide value, anisidine value, tocopherol content, polyphenols content and phytosterols content of WGO were also investigated.
3. Materials and Methods
3.1. Chemicals
Wheat germ was obtained from WUDELI flour group co., LTD (Handan, Hebei, China). Mixed tocopherol standard (including α-, β-, γ-, δ-, 95%) (Roche, Basel, Switzerland), Gallic acid, isopropanol and dichloromethane were purchased from Sinopharm Chemical Reagent Co. (Shanghai, China).
3.2. Preparation of WGO
Untreated wheat germ was obtained from WUDELI flour group co., Ltd. (Handan, Hebei, China). After microwave enzyme inactivation at power of 600 W and time of 3 min, WG was extracted by SBE, then decolorized using activated clay of 3% at the temperature of 90 °C for 30 min. The decolorized WGO was disacidified by molecular distillation under the different temperature. The flow path of Preparation of WGO was shown as following:
Wheat germ → enzyme inactivation using microwave → extraction by SBE → decolorization → molecular distillation.
3.3. Extraction of WGO
WG after microwave enzyme inactivation was processed by subcritical butane extraction (SBE), and compared with the supercritical carbon dioxide extraction (SCE) and solvent extraction (SE), respectively according to the following methods.
3.3.1. Extraction of WGO by SBE
SBE was performed using the apparatus (CBE-5L, Henan Yalinjie Biological Technology Co., Ltd., Anyang, China).The wheat germ (400 g) was loaded into the extractor, and the butane was compressed through a syringe-type pump, transferred to the extractor. The SBE was carried out at the pressure of 0.35 MPa and temperature of 35 °C for 1 h on the basis of the preliminary experiments. Butane was then exhausted at a temperature of 50 °C and the pressure of 0.1 MPa for 45 min. The obtained oil in the separators was collected, weighed and stored in sealed containers at 4 °C for further analysis. The flow diagram was shown in
Figure 2.
3.3.2. Extraction of WGO by SCE
All SCE trials were carried out in a HA121-50-01 SFE device (Hua’an Supercritical Fluid Extraction corp., Nantong, China) to get the WGO. The operating methodology was as follows: Approximately 400 g of wheat germ were loaded into the extraction vessel, carbon dioxide was pumped into the extractor until the desired extraction pressure values was reached. The wheat germ was extracted at pressure of 35 MPa and temperature of 45 °C for 2 h, and the liquid CO2 flow rate was set at 26 L/h. The operating conditions of the separators were set at 8 MPa and 30 °C. When the predetermined time was achieved, the obtained oil in the separators were collected and weighted and stored in sealed containers at 4 °C for further analysis.
3.3.3. Extraction of WGO by SE
SE of wheat germ was performed with n-hexane as the extracting solvent. Then 400 g of wheat germ powders with 2 L hexane was placed into the beaker and stirred 5 h by digital-heating mantle at 50 °C and 400 rpm. After extraction, the hexane solution was filtered, and subsequently evaporated from the extracted oil in a rotary evaporator. The obtained oil was collected, weighed and stored in sealed containers at 4 °C for further analysis.
3.4. Molecular Distillation of WGO
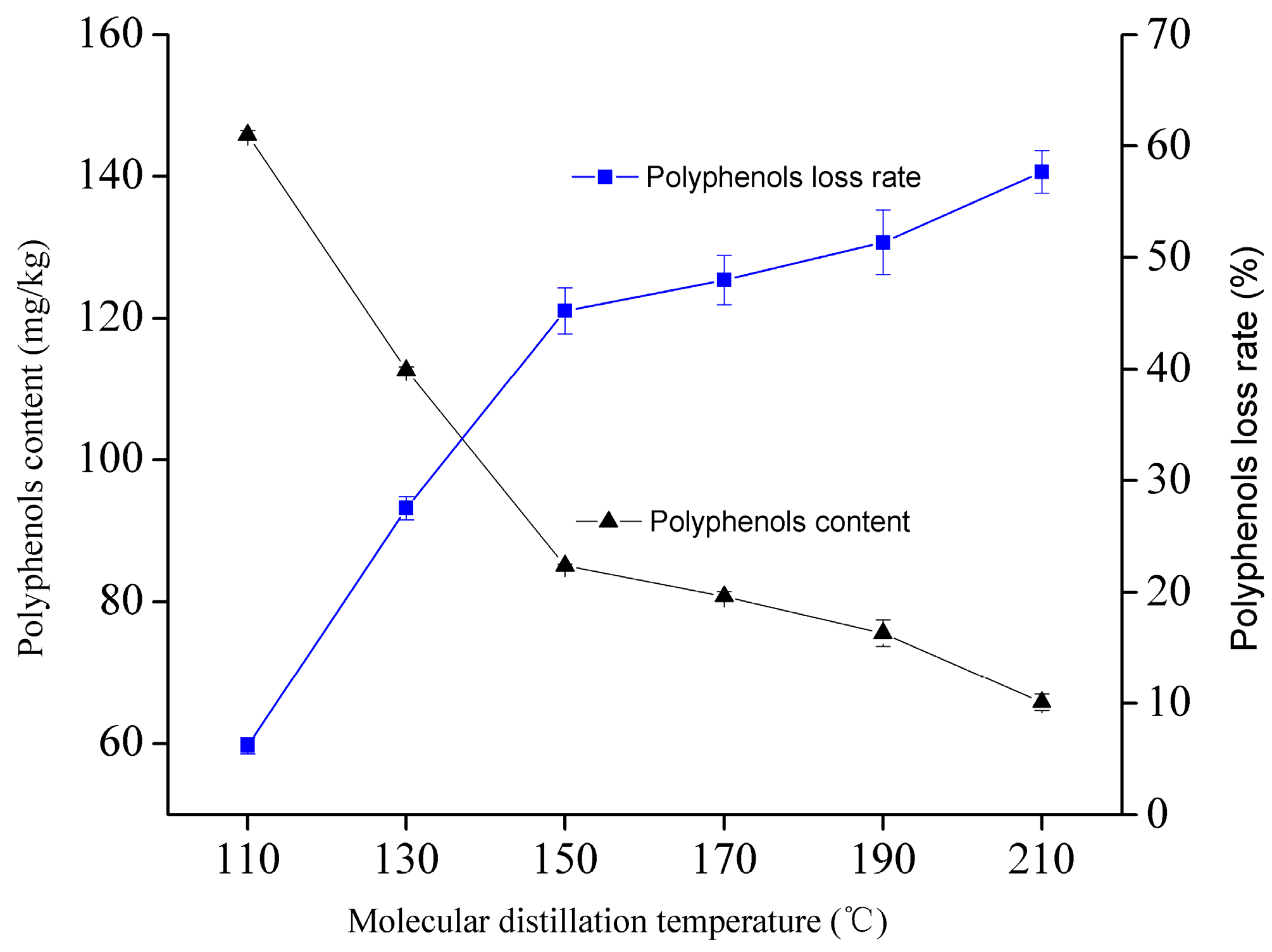
The WGO were added to short-range molecular distillation equipment (KDL2UIC GmbH, Alzenau, Germany). The preheating and condensing temperature were set at 45 °C and 25 °C, respectively. The vacuum degree was adjusted to 0.01 mbar, and scraping film speed was set to 120 rpm. The feed rate was adjusted to 1 mL/min and distillation temperature was set at 110 °C, 130 °C, 150 °C, 170 °C, 190 °C, 210 °C, respectively.
3.5. Analytical Methods
The fatty acid composition of WGO was analyzed according to the IUPAC method 2.302 [
30]. The tocopherol (α-, β-, γ-, and δ-isomers) contents of WGO were determined according to the method described by Liang, Yang and Ma [
31]. Acid value (AV), peroxide value (POV), and
p-anisidine value (PAV) were analyzed according to the method described by Li et al. [
32]. The total phenolic content was determined by the Folin-Ciocaulteu method based on the method described by Li et al. [
33]. Phytosterols were analyzed using a GC-MS (Thermo Electron, Waltham, MA, USA) according to the method described by Mitei et al. [
34].
3.6. Statistical Analysis
Statistical analysis was carried out with SPSS 16.0 for Windows software (SPSS China, Shanghai, China). Graphics rendering was performed using Origin 8.6 software (OriginLab, Northampton, MA, USA). Differences were considered significant at p < 0.05.
4. Conclusions
SBE is an appropriate method for crude WGO extraction. When extracted by SBE at the pressure of 0.35 MPa and temperature of 35 °C for 1 h, the yield of WGO was 9.10%, which was higher than that of SCE and similar to that of SE. The AV, POV and PAV of WGO were 8.97 mg KOH/g, 4.69 meq/kg and 1.25, respectively. At the same time, the fatty acid composition of WGO extracted by SBE has no significant difference compared with that of SE and SCE.
Molecular distillation at 150 °C is an appropriate condition for WGO deacidification. At this condition, the deacidification efficiency of crude WGO was 77.78% and the contents of tocopherol, polyphenols and phytosterols were 3285.2 mg/kg, 85.14 mg/kg and 16.9 mg/g, respectively.