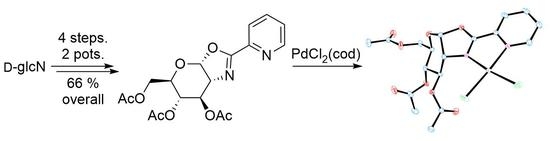
Sugar-Annulated Oxazoline Ligands: A Novel Pd(II) Complex and Its Application in Allylic Substitution
Abstract
:1. Introduction
2. Results and Discussion
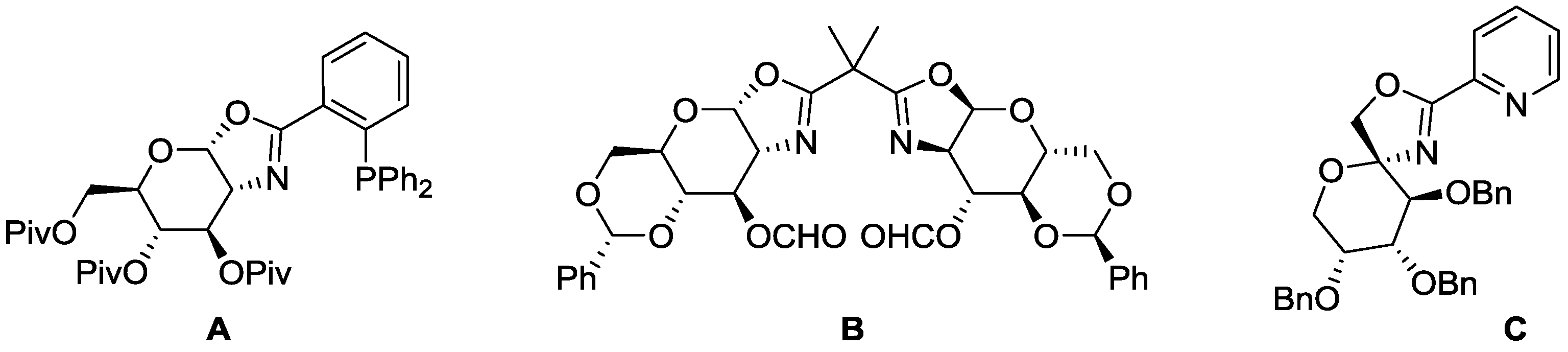
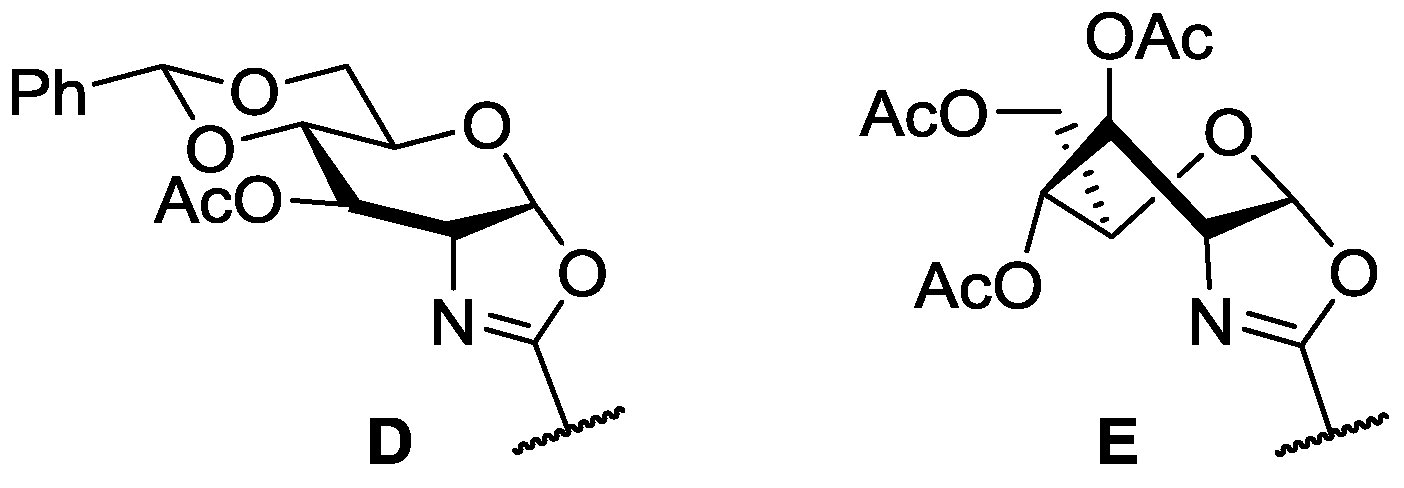
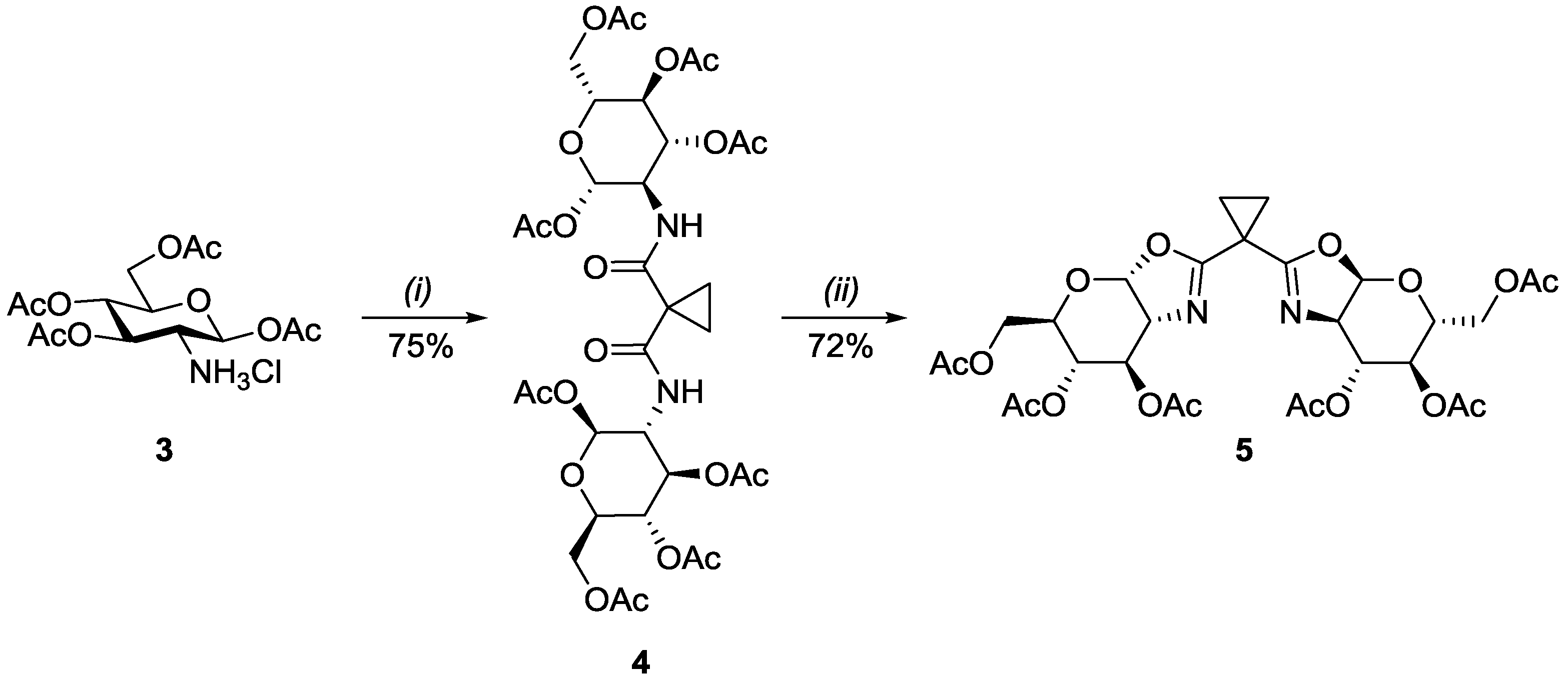
2.1. Synthesis of Oxazoline Ligands
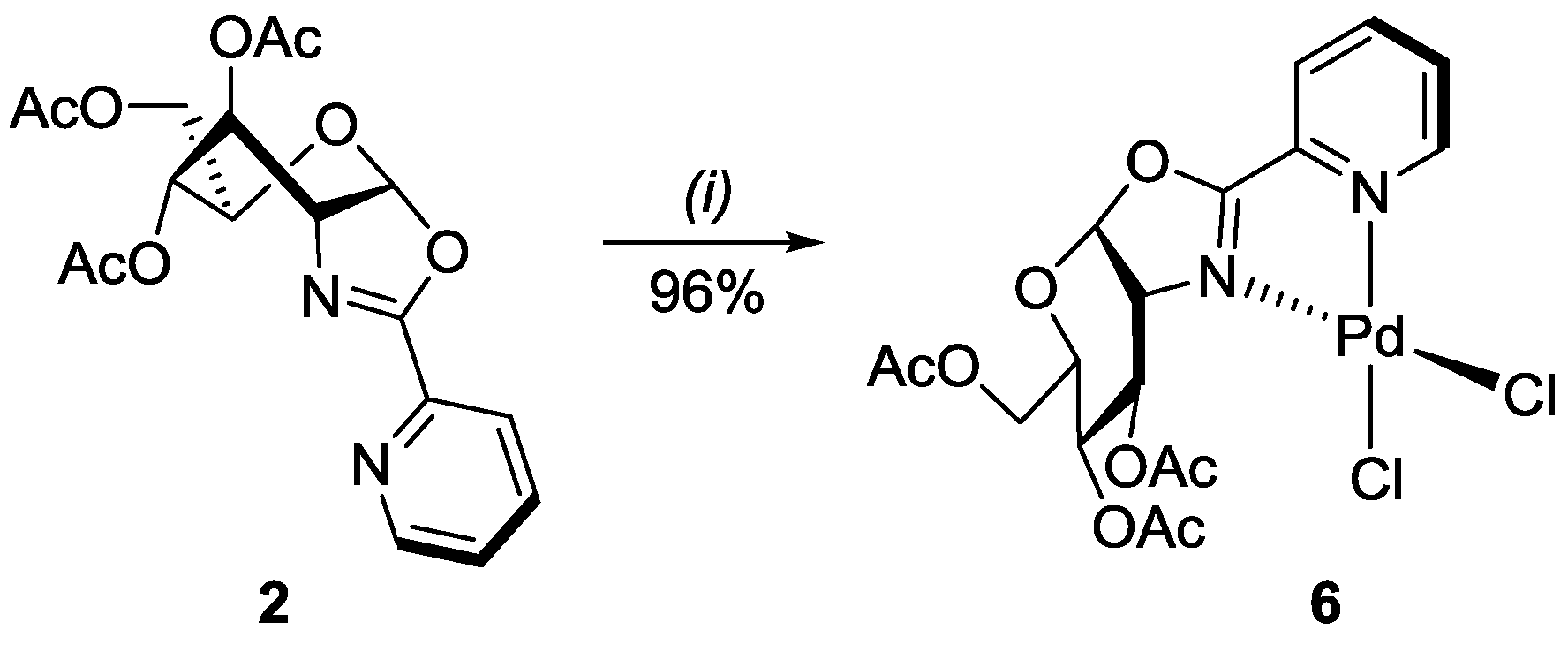
2.2. Palladium(II) Complex of Ligand 2
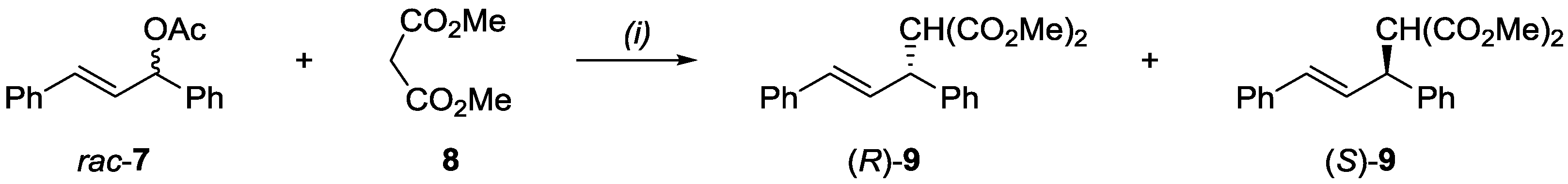
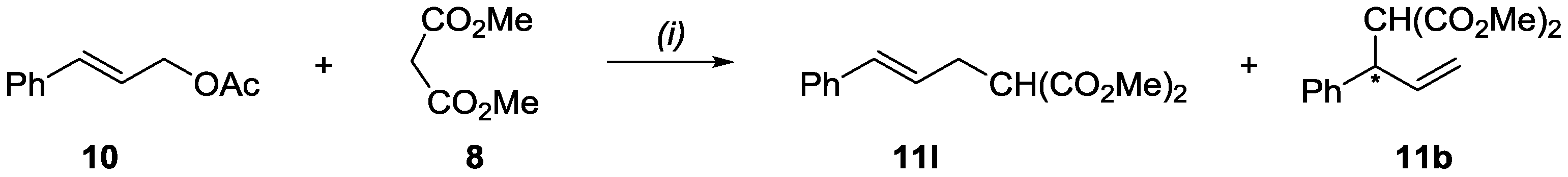
2.3. Application of Ligands in Asymmetric Allylic Substitution
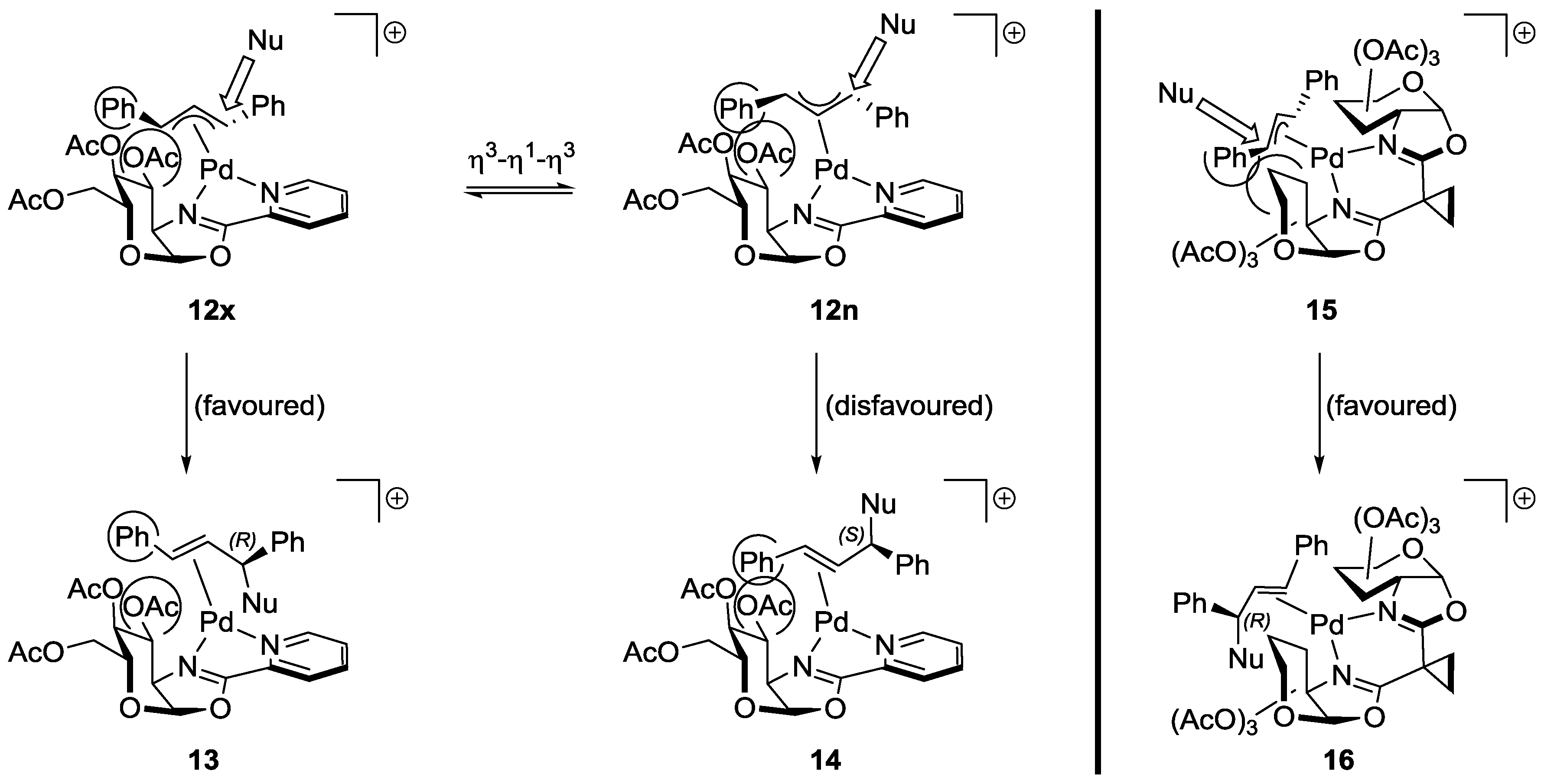
2.4. Proposed Mechanism
3. Materials and Methods
3.1. General Remarks
3.2. Synthesis of Compounds
3.3. Crystallography
3.4. Asymmetric Allylic Alkylation (Tsuji-Trost Reaction)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Boysen, M.M.K. Carbohydrates—Tools for Stereoselective Synthesis; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Lehnert, T.; Özüduru, G.; Gruel, H.; Albrecht, F.; Telligmann, S.M.; Boysen, M.M.K. More than just sweet-sugar-derived stereo differentiating agents for asymmetric synthesis. Synthesis 2011, 17, 2685–2708. [Google Scholar]
- Benessere, V.; Del Litto, R.; De Roma, A.; Ruffo, F. Carbohydrates as building blocks of privileged ligands. Coord. Chem. Rev. 2010, 254, 390–401. [Google Scholar] [CrossRef]
- Diéguez, M.; Claver, C.; Pàmies, O. Recent progress in Asymmetric catalysis using chiral carbohydrate-based ligands. Eur. J. Org. Chem. 2007, 28, 4621–4634. [Google Scholar] [CrossRef]
- Castillón, S.; Claver, C.; Díaz, Y. C1 and C2-symmetric carbohydrate phosphorus ligands in asymmetric catalysis. Chem. Soc. Rev. 2005, 34, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Diéguez, M.; Pàmies, O.; Claver, C. Ligands derived from carbohydrates for asymmetric catalysis. Chem. Rev. 2004, 104, 3189–3215. [Google Scholar] [CrossRef] [PubMed]
- Diéguez, M.; Pàmies, O.; Ruiz, A.; Díaz, Y.; Castillón, S.; Claver, C. Carbohydrate derivative ligands in asymmetric catalysis. Coord. Chem. Rev. 2004, 248, 2165–2192. [Google Scholar] [CrossRef]
- Trost, B.M.; Crawley, M.L. Asymmetric transition-metal-catalyzed allylic alkylations: Applications in total synthesis. Chem. Rev. 2003, 103, 2921–2943. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Crawley, M.L. Asymmetric transition metal-catalyzed allylic alkylations. Chem. Rev. 1996, 96, 395–422. [Google Scholar] [CrossRef] [PubMed]
- Gläser, B.; Kunz, H. Enantioselective allylic substitution using a novel (Phosphino-α-d-glucopyrano-oxazoline)palladium catalyst. Synlett 1998, 53–54. [Google Scholar] [CrossRef]
- Irmak, M.; Groschner, A.; Boysen, M.M.K. glucoBox—A new carbohydrate-based bis(oxazoline) ligand. Synthesis and first application. Chem. Commun. 2007, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Minuth, T.; Irmak, M.; Groschner, A.; Lehnert, T.; Boysen, M.M.K. Sweets for Catalysis-Facile Optimisation of Carbohydrate-Based Bis(oxazoline) Ligands. Eur. J. Org. Chem. 2009, 7, 997–1008. [Google Scholar] [CrossRef]
- Minuth, T.; Boysen, M.M.K. Bis(oxazolines) based on glycopyranosides-steric, configurational and conformational influences on stereoselectivity. Beilstein J. Org. Chem. 2010, 6. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.; Ziegler, T. Synthesis of spirofused carbohydrate-oxazoline based palladium(II) complexes. Carbohydr. Res. 2015, 411, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.; Golkowski, M.; Ziegler, T. Spiro-fused carbohydrate oxazoline ligands: Synthesis and application as enantio-discrimination agents in asymmetric allylic alkylation. Beilstein J. Org. Chem. 2016, 12, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, R.U.; Hendriks, K.B.; Stick, R.V.; James, K. Halide Ion Catalyzed Glycosidation Reactions Syntheses of α-Linked Disaccharides. J. Am. Chem. Soc. 1975, 97, 4056–4062. [Google Scholar] [CrossRef]
- Hermange, P.; Lindhardt, A.T.; Taaning, R.H.; Bjerglund, K.; Lupp, D.; Skyrdstrup, T. Ex Situ Generation of Stoichiometric and Substoichiometric 12CO and 13CO and Its Efficient Incorporation in Palladium Catalyzed Aminocarbonylations. J. Am. Chem. Soc. 2011, 133, 6061–6071. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.C.; Chen, W.; Lu, H.Z.; Yang, Q.; Dong, Y.H.; Wang, D.Q.; Zhang, J.J. Synthesis of NAG-thiazoline-derived inhibitors for β-N-acetyl-d-hexosaminidases. Carbohydr. Res. 2015, 413, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Holder, J.C.; Zou, L.F.; Marziale, A.N.; Liu, P.; Lan, Y.; Gatti, M.; Kikushima, K.; Houk, K.N.; Stoltz, B.M. Mechanism and Enantioselectivity in Palladium-Catalyzed Conjugate Addition of Arylboronic Acids to β-Substituted Cyclic Enones: Insights from Computation and Experiment. J. Am. Chem. Soc. 2013, 135, 14996–15007. [Google Scholar] [CrossRef] [PubMed]
- Dodd, D.W.; Toews, H.E.; Carneiro, M.C.; Jennings, M.C.; Jones, N.D. Model intermolecular asymmetric Heck reactions catalyzed by chiral pyridyloxazoline palladium(II) complexes. Inorg. Chim. Acta 2006, 359, 2850–2858. [Google Scholar] [CrossRef]
- Svensson, M.; Bremberg, U.; Hallman, K.; Csoregh, I.; Moberg, C. (Hydroxyalkyl)pyridinooxazolines in palladium-catalyzed allylic substitutions. Conformational preferences of the ligand. Organometallics 1999, 18, 4900–4907. [Google Scholar] [CrossRef]
- Nashed, M.A.; Slife, C.W.; Kiso, M.; Anderson, L. O-Benzylated Oxazoline Derivatives of 2-Acetamido-2-Deoxy-d-Glucopyranose from 1-Propenyl Glycosides-Synthesis of the Propenyl Glycosides and Their Direct Cyclization. Carbohydr. Res. 1980, 82, 237–252. [Google Scholar] [CrossRef]
- Foces-Foces, C.; Cano, F.H.; Bernabe, M.; Penades, S.; Martin-Lomas, M. Conformation of 3,4,6-Tri-O-Acetyl-1,2-Dideoxy-α-d-Glucopyrano-[2,1-d]-2-Oxazolines. Carbohydr. Res. 1984, 135, 1–11. [Google Scholar] [CrossRef]
- Mata, Y.; Diéguez, M.; Pàmies, O.; Claver, C. New Carbohydrate-Based Phosphite-Oxazoline Ligands as Highly Versatile Ligands for Palladium-Catalyzed Allylic Substitution Reactions. Adv. Synth. Catal. 2005, 347, 1943–1947. [Google Scholar] [CrossRef]
- Khiar, N.; Navas, R.; Fernández, I. ‘ClickCarb’: Modular sugar based ligands via click chemistry. Tetrahedron Lett. 2012, 53, 395–398. [Google Scholar] [CrossRef]
- Helmchen, G. Enantioselective palladium-catalyzed allylic substitutions with asymmetric chiral ligands. J. Organomet. Chem. 1999, 576, 203–214. [Google Scholar] [CrossRef]
- Ramillien, M.; Vanthuyne, N.; Jean, M.; Gherase, D.; Giorgi, M.; Naubron, J.-V.; Piras, P.; Roussel, C. Enantiomers of dimethyl [(2E)-1,3-diphenylprop-2-en-1-yl]propanedioate resulting from allylic alkylation reaction: Elution order on major high-performance liquid chromatography chiral columns. J. Chromatogr. A 2012, 1269, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Von Matt, P.; Lloyd-Jones, G.C.; Minidis, A.B.E.; Pfaltz, A.; Macko, L.; Neuburger, M.; Zehnder, M.; Rüegger, H.; Pregosin, P.S. Enantioselective Allylic Substitution Catalyzed by Chiral [Bis(Dihydrooxazole)]Palladium Complexes-Catalyst Structure and Possible Mechanism of Enantioselection. Helv. Chim. Acta 1995, 78, 265–284. [Google Scholar] [CrossRef]
- Prétôt, R.; Pfaltz, A. New Ligands for Regio- and Enantiocontrol in Pd-Catalyzed Allylic Alkylations. Angew. Chem. Int. Ed. 1998, 37, 323–325. [Google Scholar] [CrossRef]
- Sjögren, M.P.T.; Hansson, S.; Åkermark, B.; Vitagliano, A. Stereo- and Regiocontrol in Palladium-Catalyzed Allylic Alkylation Using 1,10-Phenanthrolines as Ligands. Organometallics 1994, 13, 1963–1971. [Google Scholar] [CrossRef]
- Chelucci, G.; Medici, S.; Saba, A. Chiral oxazolinylpyridines as ligands for enantioselective palladium catalysed allylic substitution. Tetrahedron: Asymmetry 1997, 8, 3183–3184. [Google Scholar] [CrossRef]
- Nordström, K.; Macedo, E.; Moberg, C. Enantioselective allylic substitutions catalyzed by [(hydroxyalkyl)pyridinooxazoline]- and [(alkoxyalkyl)pyridinooxazoline]palladium complexes. J. Org. Chem. 1997, 62, 1604–1609. [Google Scholar] [CrossRef]
- Pfaltz, A. Chiral Semicorrins and Related Nitrogen-Heterocycles as Ligands in Asymmetric Catalysis. Acc. Chem. Res. 1993, 26, 339–345. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS; University of Goettingen: Göttingen, Germany, 2008. [Google Scholar]
- Sheldrick, G.M. SHELXS 97, Program for the Solution of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. Phase Annealing in SHELXL-90—Direct Methods for Larger Structures. Acta Crystallogr. Sect. A 1990, 46, 467–473. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL, Bruker Analytical X-ray Division; Bruker: Madison, WI, USA, 2001. [Google Scholar]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Leutenegger, U.; Umbricht, G.; Fahrni, C.; von Matt, P.; Pfaltz, A. 5-Aza-Semicorrins—A New Class of Bidentate Nitrogen Ligands for Enantioselective Catalysis. Tetrahedron 1992, 48, 2143–2156. [Google Scholar] [CrossRef]
- Co, T.T.; Paek, S.W.; Shim, S.C.; Cho, C.S.; Kim, T.-J.; Choi, D.W.; Kang, S.O.; Jeong, J.H. 1,2-Ferrocenediylazaphosphinines: An unusual coordination behavior and application to allylic alkylation. Organometallics 2003, 22, 1475–1482. [Google Scholar] [CrossRef]
- Sample Availability: Samples of compounds are available from the authors.


| 2 a OS2 | 6 a 4H5 | |
|---|---|---|
| Proton chemical shifts (ppm) | ||
| H-1 | 6.23 | 6.71 |
| H-2 | 4.44 | 4.67 |
| H-3 | 5.29 | 6.09 |
| H-4 | 4.92 | 4.95 |
| H-5 | 3.65 | 3.92 |
| H-6a | 4.13–4.06 | 4.26–4.17 |
| H-6b | ||
| Vicinal coupling constants (Hz) | ||
| J1,2 | 7.4 | 7.6 |
| J2,3 | 2.7 | 3.3 |
| J2,4 | 1.2 | 1.3 |
| J3,4 | 2.3 | 3.0 |
| J4,5 | 9.1 | 7.1 |
| Entry | Ligand | Solvent | Yield b | ee c |
|---|---|---|---|---|
| 1 a | A | CH2Cl2 | 94% | 98% (R) |
| 2 | 2 | CH2Cl2 | 92% | 47% (R) |
| 3 | 2 | THF | 53% | 56% (R) |
| 4 | 2 | MeCN | 71% | 56% (R) |
| 5 | 2 | PhCH3 | traces | n.d. |
| 6 d | 2 | MeCN | 9% | 66% (R) |
| 7 e,f | 2 | MeCN | 38% | 47% (R) |
| 8 | 5 | CH2Cl2 | 48% | 98% (R) |
| 9 | 5 | THF | traces | n.d. |
| 10 | 5 | PhCH3 | traces | n.d. |
| 11 | 5 | MeCN | 35% | 97% (R) |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraft, J.; Mill, K.; Ziegler, T. Sugar-Annulated Oxazoline Ligands: A Novel Pd(II) Complex and Its Application in Allylic Substitution. Molecules 2016, 21, 1704. https://doi.org/10.3390/molecules21121704
Kraft J, Mill K, Ziegler T. Sugar-Annulated Oxazoline Ligands: A Novel Pd(II) Complex and Its Application in Allylic Substitution. Molecules. 2016; 21(12):1704. https://doi.org/10.3390/molecules21121704
Chicago/Turabian StyleKraft, Jochen, Katharina Mill, and Thomas Ziegler. 2016. "Sugar-Annulated Oxazoline Ligands: A Novel Pd(II) Complex and Its Application in Allylic Substitution" Molecules 21, no. 12: 1704. https://doi.org/10.3390/molecules21121704
APA StyleKraft, J., Mill, K., & Ziegler, T. (2016). Sugar-Annulated Oxazoline Ligands: A Novel Pd(II) Complex and Its Application in Allylic Substitution. Molecules, 21(12), 1704. https://doi.org/10.3390/molecules21121704