A Novel Synthesis of Fused Uracils: Indenopyrimidopyridazines, Pyrimidopyridazines, and Pyrazolopyrimidines for Antimicrobial and Antitumor Evalution
Abstract
:1. Introduction
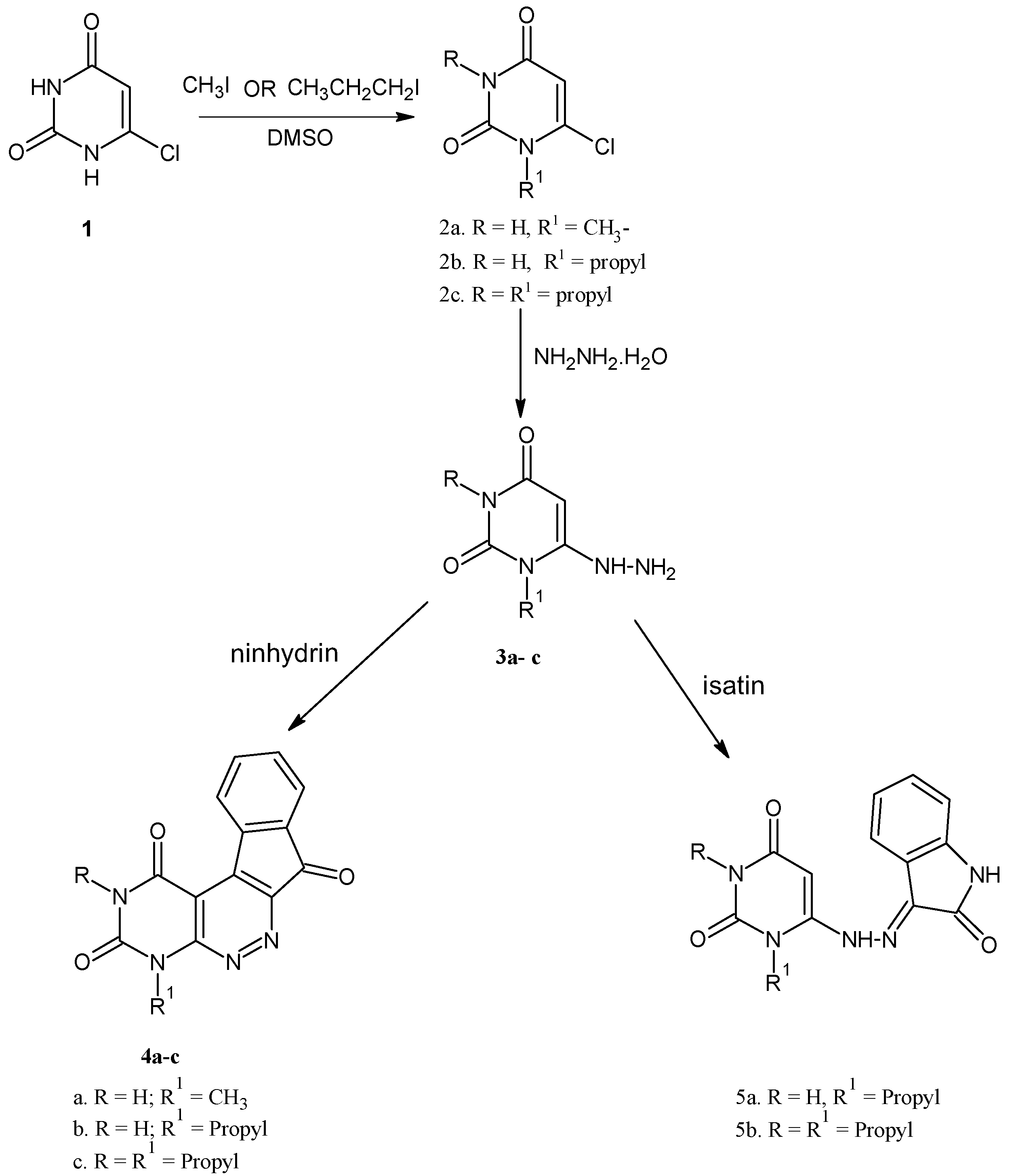
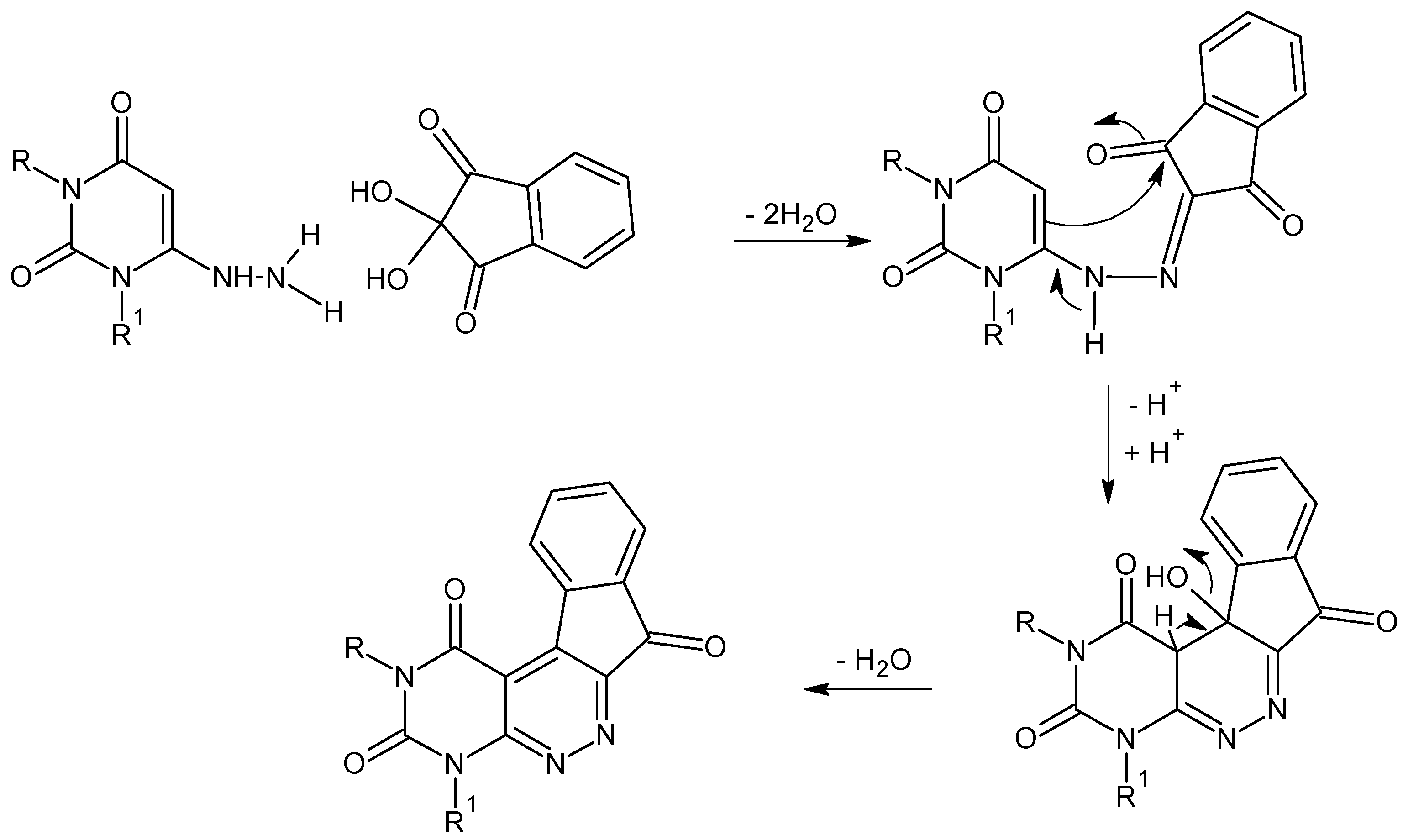
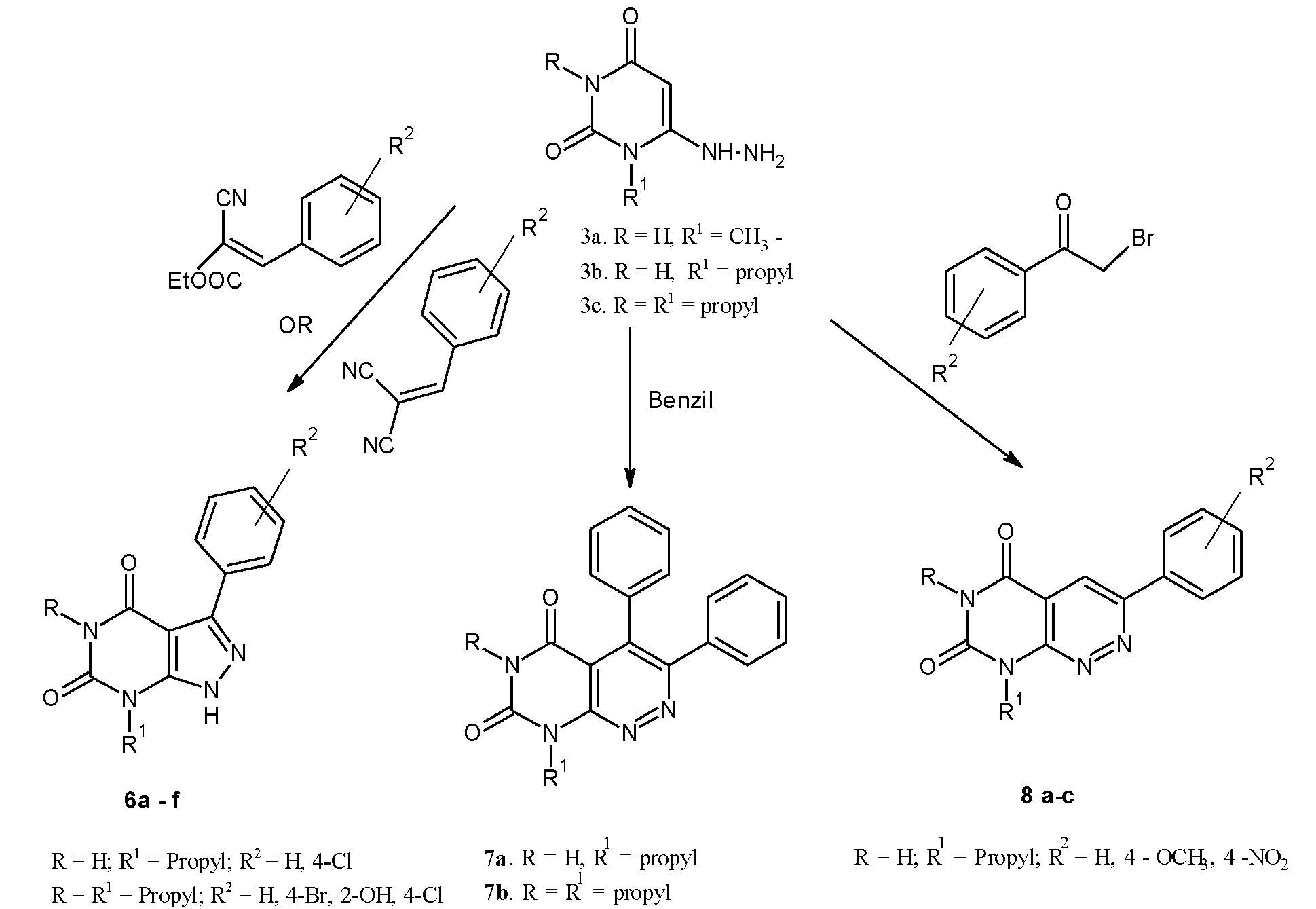
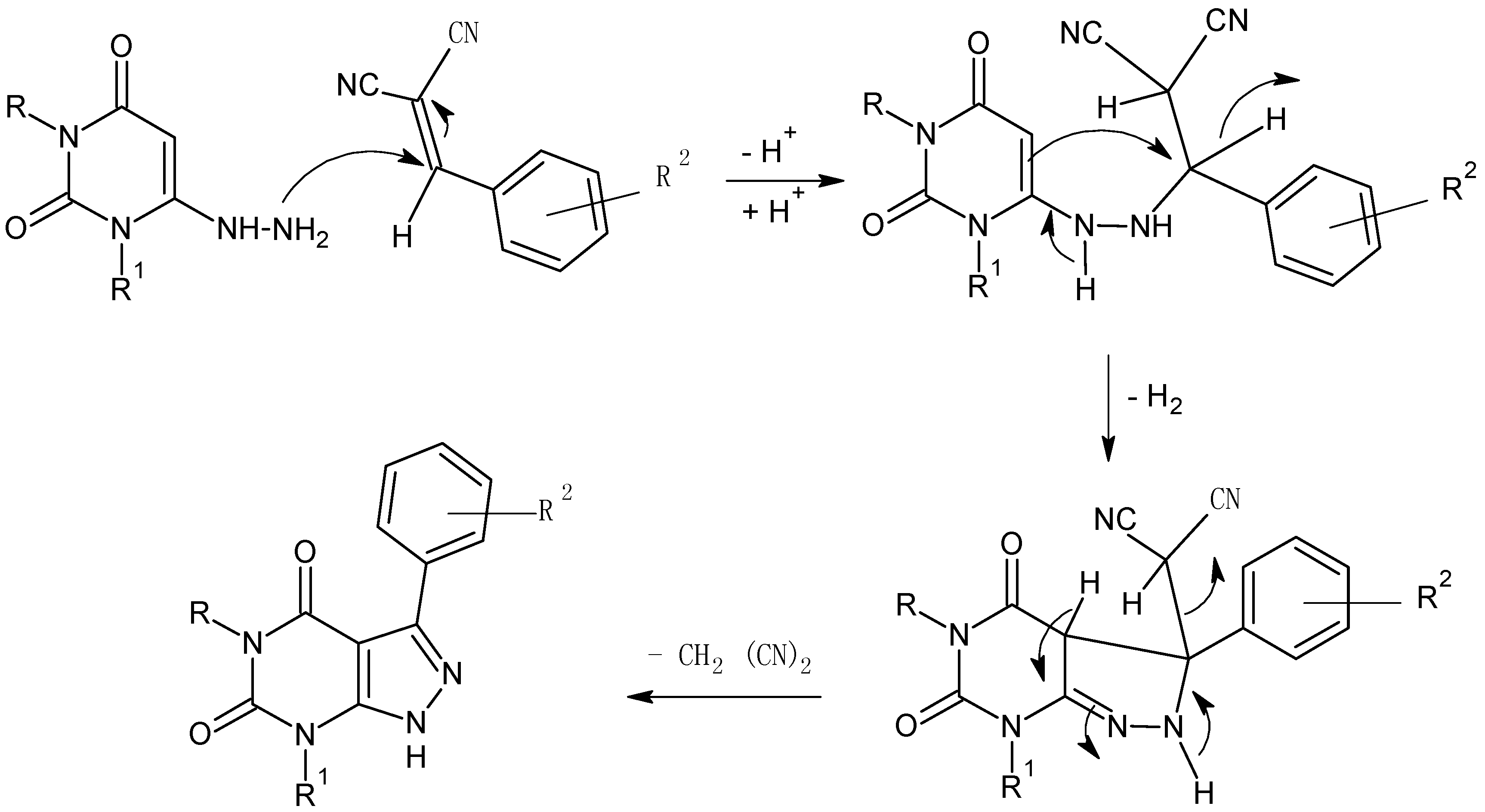
2. Results and Discussion
2.1. Chemistry
2.2. Antimicrobial Screening
Anticancer Activity
3. Experimental Section
3.1. General
3.2. Biological Evaluation
3.2.1. Antimicrobial Bioassay by Using the Agar Diffusion Cylinder Method [36]
3.2.2. Determination of the Minimum Inhibitory Concentration (MIC)
3.2.3. Evaluation of the Antitumor Activity Using Viability Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rashad, A.E.; Shamroukh, A.H.; Abdel-Megeid, R.E.; Ali, S.H. Synthesis and isomerization of some novel pyrazolopyrimidine and pyrazolotriazolopyrimidine derivatives. Molecules 2014, 19, 5459–5469. [Google Scholar] [CrossRef] [PubMed]
- Shamroukh, A.H.; Rashad, A.E.; Ali, H.S.; Abdel-Megeid, F.M.E. Synthesis, reactions and antimicrobial evaluation of β-enaminonitriles of pyrazole. J. Heterocycl. Chem. 2013, 50, 758–765. [Google Scholar] [CrossRef]
- Chauhan, M.; Kumar, R. Medicinal attributes of pyrazolo[3,4-d]pyrimidines: A review. Bioorg. Med. Chem. 2013, 21, 5657–5668. [Google Scholar] [CrossRef] [PubMed]
- Eweas, A.F.; Swelam, S.A.; Fathalla, O.A.; Fawzy, N.M.; Abdel-Moez, S.I. Synthesis, anti-microbial evaluation, and molecular modeling of new pyrazolo[3,4-d]pyrimidine derivatives. Med. Chem. Res. 2012, 21, 3848–3857. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sumran, G.; Garg, N.; Aggarwal, A.A. Regioselective synthesis of some new pyrazol-1′-ylpyrazolo[1,5-a]pyrimidines in aqueous medium and their evaluation as antimicrobial agents. Eur. J. Med. Chem. 2011, 46, 3038–3046. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, K.S.; Johns, B.A.; Weatherhead, J. Pyrazolopyrimidine and pyrazolo-triazines with potent activity against herpesviruses. Bioorg. Med. Chem. Lett. 2009, 19, 5689–5692. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, A.; Raghupathy, D.; Priyadarshini, M. Green Synthesis of Biologically Active Pyrazolopyrimidine Derivatives Using an Ionic liquid 2-Methyl-3-butylimidazolium chloride. Int. J. ChemTech Res. 2011, 3, 293–297. [Google Scholar]
- Ghorab, M.M.; Ragab, F.A.; Alqasoumi, S.I.; Alafeefy, A.M.; Aboulmagd, S.A. Synthesis of some new pyrazolo[3,4-d]pyrimidine derivatives of expected anticancer and radioprotective activity. Eur. J. Med. Chem. 2010, 45, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Abd El Razik, H.A.; Abdel Wahab, A.E. Synthesis and biological evaluation of some novel fused pyrazolopyrimidines as potential anticancer and antimicrobial agents. Arch. Pharm. 2011, 344, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Yewale, S.B.; Ganorkar, S.B.; Baheti, K.G.; Shelke, R.U. Novel 3-substituted-1-aryl-5-phenyl-6-anilinopyrazolo[3,4-d]pyrimidin-4-ones: Docking, synthesis and pharmacological evaluation as a potential anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2012, 22, 6616–6620. [Google Scholar] [CrossRef] [PubMed]
- El-Tombany, A.A. Synthesis, anti-Inflammatory, and ulcerogenicity studies of novel substituted and fused pyrazolo[3,4-d]pyrimidin-4-ones. Sci. Pharm. 2013, 81, 393–422. [Google Scholar] [CrossRef] [PubMed]
- Alcaro, S.; Artese, A.; Botta, M.; Zizzari, A.T.; Orallo, F.; Ortuso, F.; Schenone, S.; Brullo, C.; Yáñez, M. Hit identification and biological evaluation of anticancer pyrazolopyrimidines endowed with anti-inflammatory activity. ChemMedChem 2010, 5, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Rodrigues, L.M.; Esteves, A.P.; Oliveira-Campos, A.M.F.; Jose Nascimento, M.S.; Nazareth, N.; Cidade, H.; Neves, M.P.; Fernandes, E.; Pinto, M.; et al. Synthesis of N-aryl-5-amino-4-cyanopyrazole derivatives as potent xanthine oxidase inhibitors. Eur. J. Med. Chem. 2008, 43, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Ishibuchi, S.; Morimoto, H.; Oe, T.; Ikebe, T.; Inoue, H.; Fukunari, A.; Kamezawa, M.; Yamada, I.; Naka, Y. Synthesis and structure—Activity relationships of 1-phenylpyrazoles as xanthine oxidase inhibitors. Bioorg. Med. Chem. Lett. 2001, 11, 879–882. [Google Scholar] [CrossRef]
- Rimaz, M.; Pourhossein, P.; Khalili, B. Regiospecific one-pot, combinatorial synthesis of new substituted pyrimido[4,5-c]pyridazines as potential monoamine oxidase inhibitors. Turk. J. Chem. 2015, 39, 244–254. [Google Scholar]
- Khalafy, J.; Rimaz, M.; Panahi, L.; Rabiei, H. A Regiospecific One-Pot, Three Component Synthesis of 4-Aryl-6,8-dimethylpyrimido[4,5-c]pyridazine-5,7(6H,8H)-diones as New Potential Monoamine Oxidase Inhibitors. Bull. Korean Chem. Soc. 2011, 32, 2428–2432. [Google Scholar] [CrossRef]
- Hamilton, G.A. Progress in Bioorganic Chemistry; Kaiser, E.T., Kezdy, F.J., Eds.; Wiley: New York, NY, USA, 1971; Volume 1, p. 83. [Google Scholar]
- Altomare, C.; Cellamare, S.; Summo, L.; Catto, M.; Carotti, A.; Thull, U.; Carrupt, P.; Testa, B.; Stoeckli-Evans, H. Inhibition of monoamine oxidase-B by condensed pyridazines and pyrimidines: Effects of lipophilicity and structure-activity Relationships. J. Med. Chem. 1998, 41, 3812–3820. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.O.; Bari, S.B.; Firke, S.D.; Deshmukh, P.K.; Donda, S.T.; Patil, D.A. A comprehensive review on synthesis and designing aspects of coumarin derivatives as monoamine oxidase inhibitors for depression and Alzheimer’s disease. Bioorg. Med. Chem. 2013, 21, 2434–2450. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdullah, E.S. Synthesis and anticancer activity of some novel tetralin-6-yl-pyrazoline, 2-thioxopyrimidine, 2-Oxopyridine, 2-thioxo-pyridine and 2-iminopyridine derivatives. Molecules 2011, 16, 3410–3419. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, D.; Baruah, P.; Gogoi, B.J.; Sandhu, J.S. One-pot synthesis of novel 1H-pyrimido[4,5-c][1,2]diazepines and pyrazolo[3,4-d]pyrimidines. Beilstein J. Org. Chem. 2006, 2, 1–5. [Google Scholar]
- Ribone, S.R.; Schenfeld, E.M.; Madrid, M.; Pierini, A.B.; Quevedo, M.A. Evaluation and synthesis of AZT prodrugs with optimized chemical stabilities: Experimental and theoretical analyses. New J. Chem. 2016, 40, 2383–2392. [Google Scholar] [CrossRef]
- Huang, S.N.; Murai, J.; Rosa, I.D.; Dexheimer, T.S.; Naumova, A.; Gmeiner, W.H.; Pommier, Y. TDP1 repairs nuclear and mitochondrial DNA damage induced by chain-terminating anticancer and antiviral nucleoside analogs. Nucleic Acids Res. 2013, 41, 7793–7803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhao, L.; Ma, S.; Gu, M.; Zheng, X. Potent Anticancer Effects of Lentivirus Encoding a Drosophila Melanogaster Deoxyribonucleoside Kinase Mutant Combined with Brivudine. Asian Pac. J. Cancer Prev. 2012, 13, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Cui, H.; Wang, J.; Li, N.; Zhao, Q.; Zhou, Y.; Lv, Z.; Zhong, W. Enhancing the antitumor effect of methotrexate in vitro and in vivo by a novel targeted single-walled carbon nanohorn-based drug delivery system. RSC Adv. 2016, 6, 47272–47280. [Google Scholar] [CrossRef]
- Billings, B.K.; Wagner, J.A.; Cook, P.D.; Castle, R.N. The synthesis of pyrimido[4,5-c]pyridazines and pyrido[2,3-d]pyrimidines related to toxoflavine and fervenulin. J. Het. Chem. 1975, 12, 1221–1224. [Google Scholar] [CrossRef]
- El-kalyoubi, S.; Agili, F.; Youssif, S. Novel 2-thioxanthine and dipyrimidopyridine derivatives: Synthesis and antimicrobial activity. Molecules 2015, 20, 19263–19276. [Google Scholar] [CrossRef] [PubMed]
- Mousa, B.A.; Bayoumi, A.H.; Korraa, M.M.; Assy, M.G.; El-Kalyoubi, S.A. A Novel One-Pot and Efficient Procedure for Synthesis of New Fused Uracil Derivatives for DNA Binding. Int. J. Org. Chem. 2015, 5, 37–47. [Google Scholar] [CrossRef]
- Mousa, B.A.; Bayoumi, A.H.; Korraa, M.M.; Assy, M.G.; El-Kalyoubi, S.A. Synthesis, DNA Binding Studies Of New Pyrimidothiazepine And Pyrimidobenzothiazepine Derivatives. Afinidad 2012, 69, 144–149. [Google Scholar]
- Cresswell, R.M.; Wood, H.C.S. The biosynthesis of pteridines. Part I. The synthesis of riboflavin. J. Chem. Soc. 1960, 4768–4775. [Google Scholar] [CrossRef]
- Youssif, S.; El-Kafrawy, A.; Bayoumy, B.; El-Bahaie, S. A novel synthesis of 3,9-dialkyl and 8-aryl-3,9-dimethylxanthines. Bull. Korean Chem. Soc. 2002, 23, 374–380. [Google Scholar]
- Youssef, S.; Pfleiderer, W. Purines XIV. Reactivity of 8-bromo-3,9-dimethylxanthine towards some nucleophilic reagent. J. Heterocycl. Chem. 1998, 35, 949–954. [Google Scholar] [CrossRef]
- Dudkin, S.; Iaroshenko, V.O.; Sosnovskikh, V.Y.; Tolmachev, A.A.; Villingera, A.; Langer, P. Synthesis and reactivity of 5-polyfluoroalkyl-5-deazaalloxazines. Org. Biomol. Chem. 2013, 11, 5351–5361. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Maki, Y. 3-Aminopyrazolo[3,4-d]pyrimidine Derivatives and Production Thereof. U.S. Patent Number 4,603,203, 29 July 1986. [Google Scholar]
- Youssif, S.; Assy, M. Fervenulin, 4-deazafervenulin and 5-deazaalloxazine analogues. J. Chem. Res. 1996, 442, 2546. [Google Scholar]
- Ibrahim, H.S.; Eldehna, W.M.; Abdel-Aziz, H.A.; Elaasser, M.M.; Abdel-Aziz, M.M. Improvement of antibacterial activity of some sulfa drugs through linkage to certain phthalazin-1(2H)-one scaffolds. Eur. J. Med. Chem. 2014, 85, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Riyadh, S.M.; Mahmmoud, E.A.; Elaasser, M.M. Synthesis and anticancer activities of thiazoles, 1,3-thiazines, and thiazolidine using chitosan-grafted-poly(vinylpyridine) as basic catalyst. Heterocycles 2015, 91, 1227–1243. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available upon request.

| Tested Compounds | Gram-Positive Bacteria | Gram-Negative Bacteria | Fungi | |||
|---|---|---|---|---|---|---|
| Bacillus subtilis | Streptococcus pneumoniae | Escherichia coli | Pseudomonas aeruginosa | Candida albicans | Aspergillus fumigatus | |
| 4a | 20.6 ± 0.63 | 18.3 ± 0.72 | 20.6 ± 1.2 | 15.2 ± 0.58 | NA | 18.3 ± 1.5 |
| 4b | 25.3 ± 1.2 | 22.6 ± 0.72 | 28.3 ± 0.72 | 24.2 ± 0.58 | NA | 23.4 ± 1.5 |
| 4c | 23.6 ± 0.63 | 21.1 ± 1.5 | 23.4 ± 1.2 | 19.2 ± 1.5 | NA | 21.5 ± 1.2 |
| 5a | 21.3 ± 0.72 | 22.4 ± 0.63 | 21.3 ± 0.37 | 20.1 ± 0.63 | NA | 19.3 ± 0.63 |
| 5b | 18.1 ± 0.63 | 17.3 ± 0.63 | NA | 17.3 ± 0.63 | NA | NA |
| 6b | 22.3 ± 1.5 | 20.1 ± 0.58 | 22.4 ± 0.58 | 18.6 ± 1.2 | NA | 21.3 ± 1.2 |
| 6c | 17.3 ± 0.63 | 19.2 ± 0.72 | 16.3 ± 0.46 | 17.3 ± 0.63 | NA | 17.3 ± 0.63 |
| 6d | 20.9 ± 1.5 | 19.2 ± 1.2 | 21.3 ± 0.37 | 17.3 ± 0.63 | NA | 18.9 ± 1.2 |
| 6f | 15.2 ± 0.63 | NA | NA | NA | NA | 13.6 ± 0.63 |
| 7a | NA | NA | NA | NA | NA | NA |
| 7b | NA | NA | NA | NA | NA | NA |
| Tetracycline | 28.7 ± 0.5 | 26.4 ± 0.7 | 30.2 ± 0.6 | 27.4 ± 0.8 | ||
| Amphotericin B | - | - | - | - | 25.4 ± 0.63 | 23.7 ± 0.72 |
| Sample Tested Microorganisms | 4a | 4b | 4c | 5a | 6d | Standard |
|---|---|---|---|---|---|---|
| Minimum Inhibitory Concentration (µg/mL) | ||||||
| Fungi | Amphotericin B | |||||
| Aspergillus fumigatus (RCMB 02568) | 7.81 | 1.95 | 3.9 | 3.9 | 3.9 | 1.95 |
| Candida albicans (RCMB 05036) | NA | NA | NA | NA | NA | 0.98 |
| Gram Positive Bacteria: | Ampicillin | |||||
| Streptococcuspneumonia (RCMB 010010) | 7.81 | 1.95 | 3.9 | 3.9 | 3.9 | 1.95 |
| Bacillis subtilis (RCMB 010067) | 3.9 | 0.98 | 0.98 | 1.95 | 3.9 | 0.49 |
| Gram negative Bacteria: | Gentamicin | |||||
| Pseudomonas aeruginosa (RCMB 010043-5) | 62.5 | 0.98 | 3.9 | 3.9 | 15.63 | 0.98 |
| Escherichia coli (RCMB 010052-6) | 3.9 | 0.49 | 1.95 | 1.95 | 3.9 | 0.49 |
| Tested Compounds | IC50 Values (μg/mL) | ±Standard Deviation |
|---|---|---|
| 4a | 3.6 | 0.4 |
| 4b | 47.6 | 2.8 |
| 4c | 4.6 | 0.3 |
| 5a | >200 | >8 |
| 5b | 95.1 | 2.6 |
| 6a | 106.7 | 2.5 |
| 6b | 42.2 | 1.9 |
| 6c | 160.4 | 5.8 |
| 6d | 189.9 | 7.6 |
| 6e | 49.8 | 2.4 |
| 6f | 34.8 | 3.2 |
| 7a | 104.5 | 4.9 |
| 7b | 68.1 | 1.7 |
| 8a | 20.4 | 0.8 |
| 8c | 86.1 | 1.7 |
| 5-Flurouracil | 4.1 | 0.6 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Kalyoubi, S.; Agili, F. A Novel Synthesis of Fused Uracils: Indenopyrimidopyridazines, Pyrimidopyridazines, and Pyrazolopyrimidines for Antimicrobial and Antitumor Evalution. Molecules 2016, 21, 1714. https://doi.org/10.3390/molecules21121714
El-Kalyoubi S, Agili F. A Novel Synthesis of Fused Uracils: Indenopyrimidopyridazines, Pyrimidopyridazines, and Pyrazolopyrimidines for Antimicrobial and Antitumor Evalution. Molecules. 2016; 21(12):1714. https://doi.org/10.3390/molecules21121714
Chicago/Turabian StyleEl-Kalyoubi, Samar, and Fatimah Agili. 2016. "A Novel Synthesis of Fused Uracils: Indenopyrimidopyridazines, Pyrimidopyridazines, and Pyrazolopyrimidines for Antimicrobial and Antitumor Evalution" Molecules 21, no. 12: 1714. https://doi.org/10.3390/molecules21121714






