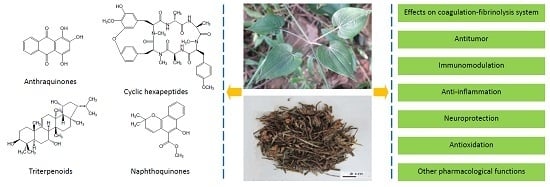
A Review of the Botany, Phytochemistry, Pharmacology and Toxicology of Rubiae Radix et Rhizoma
Abstract
:1. Introduction
2. Botany
3. Phytochemistry
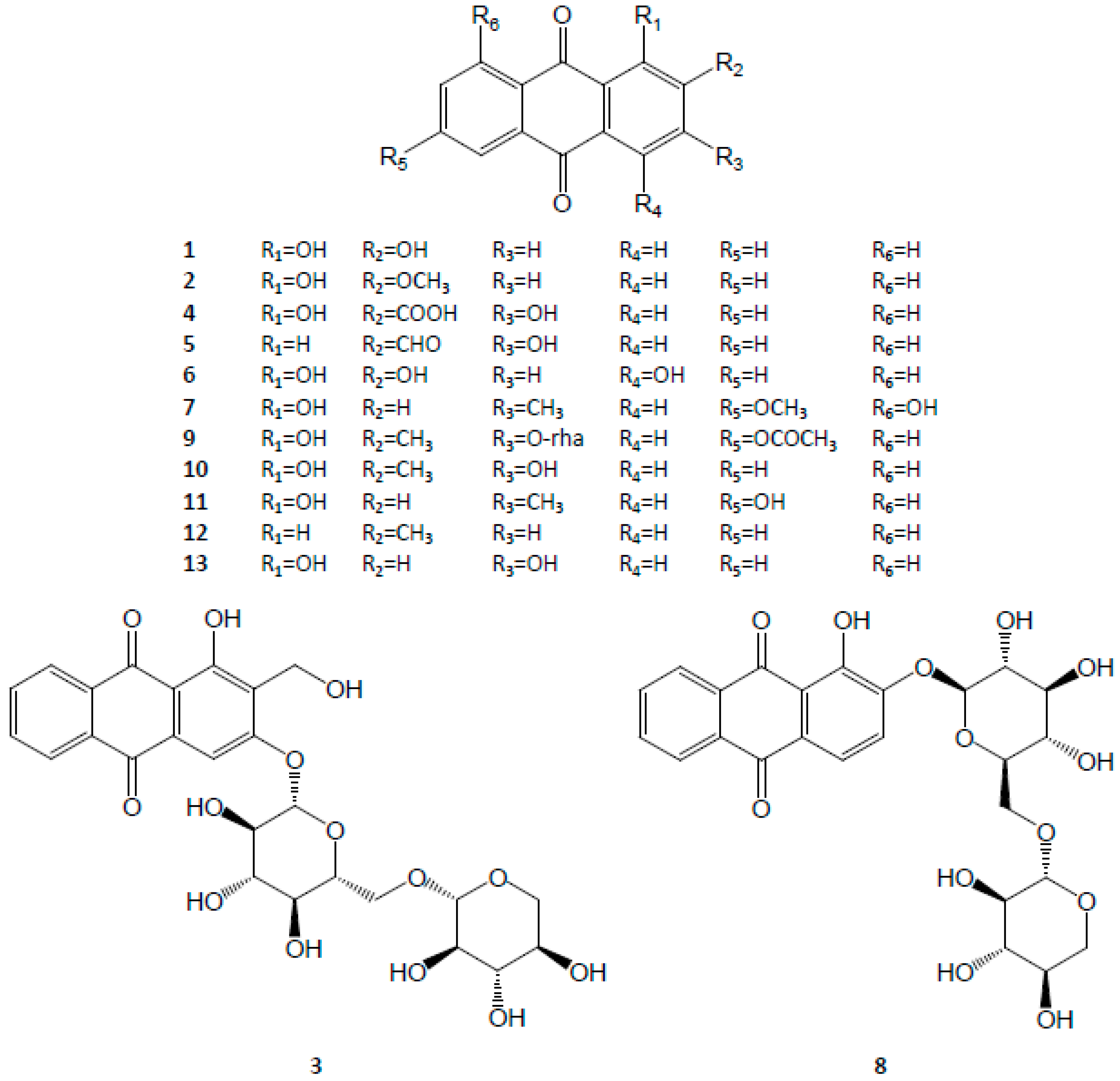
3.1. Anthraquinones
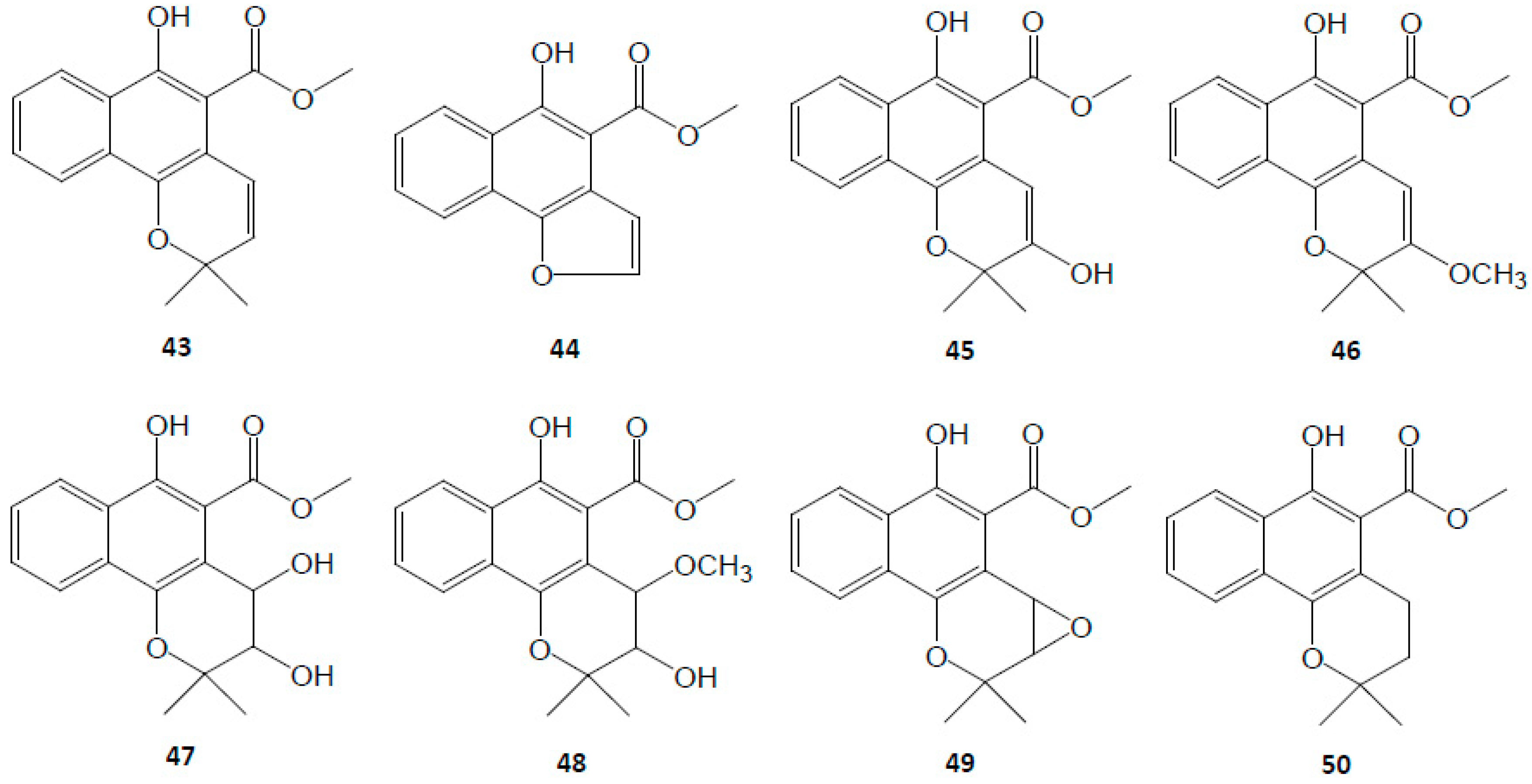
3.2. Naphthoquinones
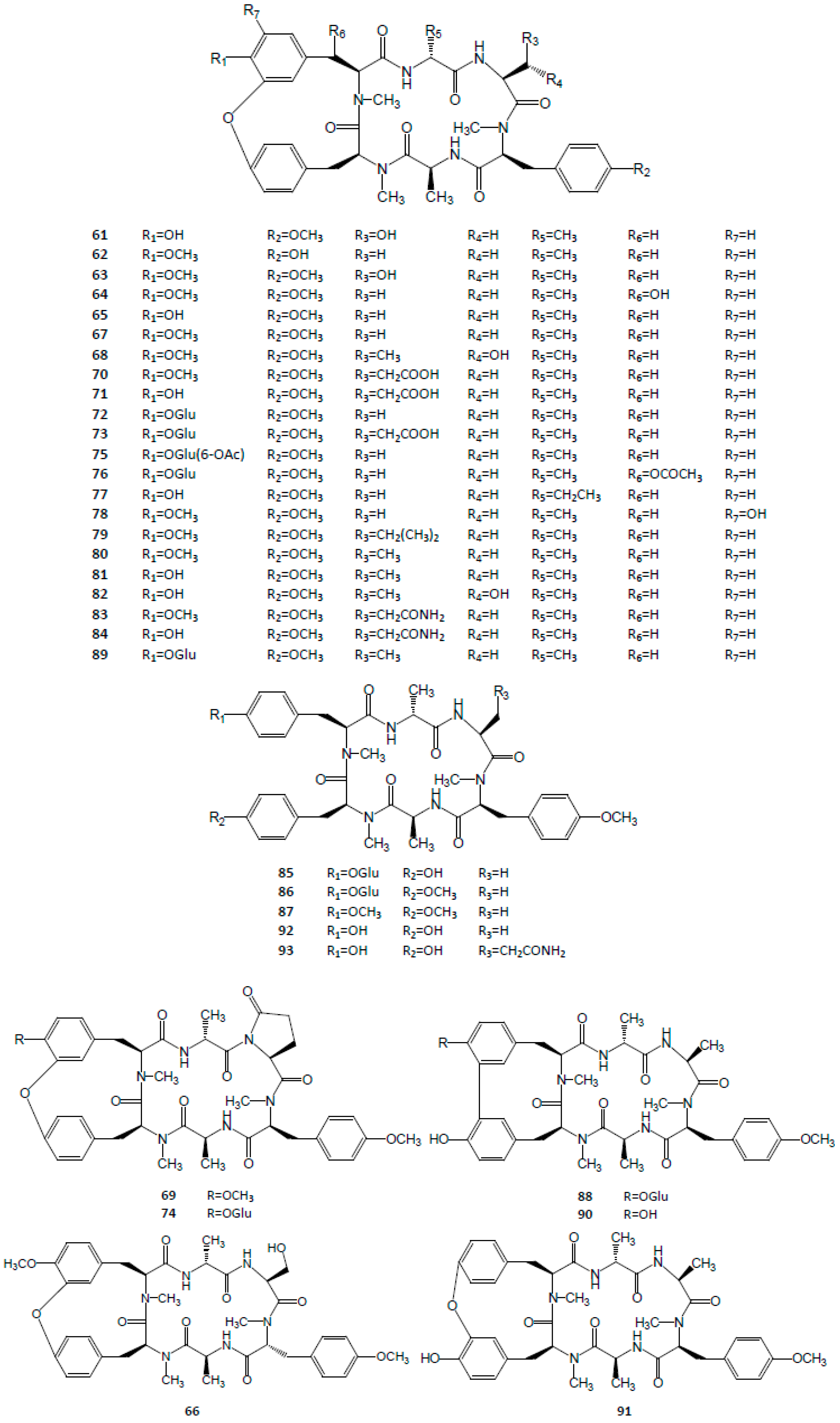
3.3. Cyclic Hexapeptides
3.4. Triterpenoids
3.5. Other Compounds
4. Pharmacology
4.1. Effects on the Coagulation-Fibrinolysis System
4.2. Antitumor
4.3. Immunomodulation
4.4. Anti-Inflammation
4.5. Neuroprotection
4.6. Antioxidation
4.7. Other Pharmacological Functions
5. Toxicology
6. Conclusions and Remarks on Future Work
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chinese Pharmacopeia Commission. Pharmacopoeia of the People’s Republic of China; Chinese Medical Science Press: Beijing, China, 2015; Volume 1, pp. 234–235. [Google Scholar]
- Akhtar, M.S.; Ali, M.; Madhurima; Mir, S.R.; Singh, O. New anthraquinones from Rubia cordifolia roots. Indian J. Chem. B 2006, 45, 1945–1950. [Google Scholar] [CrossRef]
- Chen, X.Q.; Zhao, S.M.; Wang, Z.; Zeng, G.Z.; Huang, M.B.; Tan, N.H. Rubicordins A-C, new cyclopeptides from Rubia cordifolia with cytotoxicity and inhibiting NF-κB signaling pathway. Tetrahedron 2015, 71, 9673–9678. [Google Scholar] [CrossRef]
- Hitotsuyanagi, Y.; Kusano, J.; Kim, I.H.; Hasuda, T.; Fukaya, H.; Takeya, K. O-Seco-RA-XXIV, a possible precursor of an antitumor peptide RA-XXIV, from Rubia cordifolia L. Phytochem. Lett. 2012, 5, 335–339. [Google Scholar] [CrossRef]
- Hitotsuyanagi, Y.; Odagiri, M.; Kato, S.; Kusano, J.; Hasuda, T.; Fukaya, H.; Takeya, K. Isolation, structure determination, and synthesis of allo-RA-V and neo-RA-V, RA-series bicyclic peptides from Rubia cordifolia L. Chem. Eur. J. 2012, 18, 2839–2846. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, H.; Mihara, K.; Takeya, K. Studies on a novel anthraquinone and its glycosides isolated from Rubia cordifolia and R. akane. Chem. Pharm. Bull. 1983, 31, 2353–2358. [Google Scholar] [CrossRef]
- Itokawa, H.; Qiao, Y.F.; Takeya, K. Anthraquinones and naphthohydroquinones from Rubia cordifolia. Phytochemistry 1989, 28, 3465–3468. [Google Scholar] [CrossRef]
- Itokawa, H.; Ibraheim, Z.Z.; Qiao, Y.F.; Takeya, K. Anthraquinones, naphthohydroquinones and naphthohydroquinone dimers from Rubia cordifolia and their cytotoxic activity. Chem. Pharm. Bull. 1993, 41, 1869–1872. [Google Scholar] [CrossRef] [PubMed]
- Singh, R. Isolation and synthesis of anthraquinones and related compounds of Rubia cordifolia. J. Serbian Chem. Soc. 2005, 70, 937–942. [Google Scholar] [CrossRef]
- Talapatra, S.K.; Sarkar, A.C.; Talapatra, B. Two pentacyclic triterpenes from Rubia cordifolia. Phytochemistry 1981, 20, 1923–1927. [Google Scholar] [CrossRef]
- Chakrabortee, S.; Liu, Y.; Zhang, L.; Matthews, H.R.; Zhang, H.R.; Pan, N.; Cheng, C.R.; Guan, S.H.; Guo, D.A.; Huang, Z.B. Macromolecular and small-molecule modulation of intracellular A beta(42)aggregationand associated toxicity. Biochem. J. 2012, 442, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Deoda, R.S.; Kumar, D.; Bhujbal, S.S. Gastroprotective effect of Rubia cordifolia Linn on aspirin plus pylorus-ligated ulcer. Evid. Based Complement. Altern. Med. 2011. [Google Scholar] [CrossRef] [PubMed]
- Lodi, S.; Sharma, V.; Kansal, L. The protective effect of Rubia cordifolia against lead nitrate-induced immune response impairment and kidney oxidative damage. Indian J. Pharmacol. 2011, 43, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Exposito, I.; Castillo, A.; Yang, N.; Liang, B.H.; Li, X.M. Chinese herbal extracts of Rubia cordifolia and Dianthus superbus suppress IgE production and prevent peanut-induced anaphylaxis. Chin. Med. 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shilpa, P.N.; Venkatabalasubramanian, S.; Devaraj, S.N. Induction of apoptosis by methanolic extract of Rubia Cordifolia Linn in HEp-2 cell line is mediated by reactive oxygen species. Asian Pac. J. Cancer Prev. 2012, 13, 2753–2758. [Google Scholar] [CrossRef] [PubMed]
- Shilpa, P.N.; Venkatabalasubramanian, S.; Devaraj, S.N. Ameliorative effect of methanol extract of Rubia cordifolia in N-nitrosodiethylamine-induced hepatocellular carcinoma. Pharm. Biol. 2012, 50, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.G.; Jin, H.; Yu, P.J.; Tian, Y.X.; Zhang, J.J.; Wu, S.G. Mollugin inhibits the inflammatory response in lipopolysaccharide-stimulated RAW264.7 macrophages by blocking the Janus kinase-signal transducers and activators of transcription signaling pathway. Biol. Pharm. Bull. 2013, 36, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Kumari, S.; Gulrajani, M. Dyeing studies with hydroxyanthraquinones extracted from Indian madder. Part 1: Dyeing of nylon with purpurin. Color. Technol. 2001, 117, 328–332. [Google Scholar] [CrossRef]
- Gupta, D.; Kumari, S.; Gulrajani, M. Dyeing studies with hydroxyanthraquinones extracted from Indian madder. Part 2: Dyeing of nylon and polyester with nordamncanthal. Color. Technol. 2001, 117, 333–336. [Google Scholar] [CrossRef]
- Vankar, P.S.; Shanker, R.; Mahanta, D.; Tiwari, S.C. Ecofriendly sonicator dyeing of cotton with Rubia cordifolia Linn using biomordant. Dyes Pigment 2008, 76, 207–212. [Google Scholar] [CrossRef]
- Yusuf, M.; Shahid, M.; Khan, S.A.; Khan, M.I.; Shahid-Ul-Islam; Mohammad, F.; Khan, M.A. Eco-dyeing of wool using aqueous extract of the roots of Indian Madder (Rubia cordifolia) as natural dye. J. Nat. Fibers 2013, 10, 14–28. [Google Scholar] [CrossRef]
- Namsa, N.D.; Mandal, M.; Tangjang, S.; Mandal, S.C. Ethnobotany of the Monpa ethnic group at Arunachal Pradesh, India. J. Ethnobiol. Ethnomed. 2011. [Google Scholar] [CrossRef] [PubMed]
- Xun, L. Quality Control Study of Rubia cordifolia L. and the Fakes. Master’s Thesis, Beijing University of Chinese Medicine, Beijing, China, June 2014. [Google Scholar]
- State Administration of Traditional Chinese Medicine. Chinese Materia Medica, 1st ed.Shanghai Scientific & Technical Publishers: Shanghai, China, 1998; Volume 18, pp. 470–475.
- Chen, S.B.; Feng, R.Z.; Chen, B.Z.; Jing, L.H.; Dong, X.; Gu, Z.P.; Wang, S.B. A study on the medicinal plants of genus Rubia. I. Botanical origins and resource of Chinese traditional drug “Qiancao” (Madder). Nat. Prod. Res. Dev. 1991, 3, 7–15. [Google Scholar]
- Arisawa, M.; Ueno, H.; Nimura, M.; Hayashi, T.; Morita, N. Rubiatriol, a new triterpenoid from the Chinese drug, Qian-Cao-Gen, Rubia cordifolia. J. Nat. Prod. 1986, 74, 2069–2080. [Google Scholar] [CrossRef]
- Gandhi, M.R.; Reegan, A.D.; Ganesan, P.; Sivasankaran, K.; Paulraj, M.G.; Balakrishna, K.; Ignacimuthu, S.; Al-Dhabi, N.A. Larvicidal and pupicidal activities of alizarin isolated from roots of Rubia cordifolia against Culex quinquefasciatus Say and Aedes aegypti (L.) (Diptera: Culicidae). Neotrop. Entomol. 2016, 45, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Murti, V.V.S.; Seshadri, T.R.; Sivakuma, S. Anthraquinones of Rubia cordifolia. Phytochemistry 1972, 11, 1524. [Google Scholar] [CrossRef]
- Son, J.K.; Jung, S.J.; Jung, J.H.; Fang, Z.; Lee, C.S.; Seo, C.S.; Moon, D.C.; Min, B.S.; Kim, M.R.; Woo, M.H. Anticancer Constituents from the Roots of Rubia cordifolia L. Chem. Pharm. Bull. 2008, 56, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.X.; Hua, H.M.; Wu, L.J.; Li, X.; Zhu, T.R. Study on anthraquinones from the roots of Rubia cordifolia L. Acta Pharm. Sin. 1992, 27, 743–747. [Google Scholar]
- Mischenko, N.P.; Fedoreyev, S.A.; Glazunov, V.P.; Chernoded, G.K.; Bulgakov, V.P.; Zhuravlev, Y.N. Anthraquinone production by callus cultures of Rubia cordifolia. Fitoterapia 1999, 70, 552–557. [Google Scholar] [CrossRef]
- Tessier, A.M.; Delaveau, P.; Champion, B. New anthraquinones in Rubia cordifolia roots. Planta Med. 1981, 41, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Han, D.Z.; Jin, G.Z. Study on the chemical constituents and anticancer activity from the roots of Rubia cordifolia L. Chin. Hosp. Pharm. J. 2012, 32, 1126–1128. [Google Scholar]
- Li, X.; Liu, Z.; Chen, Y.; Wang, L.J.; Zheng, Y.N.; Sun, G.Z.; Ruan, C.C. Rubiacordone A: A new anthraquinone glycoside from the roots of Rubia cordifolia. Molecules 2009, 14, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Dosseh, C.; Tessier, A.M.; Delaveau, P. New quinones in Rubia cordifolia L. Roots, III. Planta Med. 1981, 43, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Koyama, J.; Ogura, T.; Tagahara, K.; Konoshima, T.; Kozuka, M. Two naphthoquinones from Rubia cordifolia. Phytochemistry 1992, 31, 2907–2908. [Google Scholar] [CrossRef]
- Liu, R.; Lu, Y.B.; Wu, T.X.; Pan, Y.J. Simultaneous isolation and purification of mollugin and two anthraquinones from Rubia cordifolia by HSCCC. Chromatographia 2008, 68, 95–99. [Google Scholar] [CrossRef]
- Lu, Y.B.; Hu, R.L.; Dai, Z.Y.; Pan, Y.J. Preparative separation of anti-oxidative constituents from Rubia cordifolia by column-switching counter-current chromatography. J. Sep. Sci. 2010, 33, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Q.; Gao, J.F.; Wu, L.; Yuan, X. Study of anthraquinone components from rhizome of Rubia cordifolia by preparative chromatogram of medium and low press. Guihaia 2011, 31, 857–860. [Google Scholar]
- Dosseh, C.; Tessier, A.M.; Delaveau, P. Rubia cordifolia roots. II: New quinones. Planta Med. 1981, 43, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J. Study on the Chemical and Biological Activity of Rubia cordifolia L. Collected from Henan Province. Master’s Thesis, Henan University, Kaifeng, China, June 2014. [Google Scholar]
- Abdullah, S.T.; Ali, A.; Hamid, H.; Ali, M.; Ansari, S.H.; Alam, M.S. Two new anthraquinones from the roots of Rubia cordifolia Linn. Pharmazie 2003, 58, 216–217. [Google Scholar] [PubMed]
- Son, J.K.; Jung, J.H.; Lee, C.S.; Moon, D.C.; Choi, S.W.; Min, B.S.; Woo, M.H. DNA Topoisomerases I and II inhibition and cytotoxicity of constituents from the roots of Rubia cordifolia. Bull. Korean Chem. Soc. 2006, 27, 1231–1234. [Google Scholar] [CrossRef]
- Gupta, P.P.; Srimal, R.C.; Verma, N.; Tandon, J.S. Biological activity of Rubia cordifolia and isolation of an active principle. Pharm. Biol. 1999, 37, 46–49. [Google Scholar] [CrossRef]
- Ho, L.K.; Don, M.J.; Chen, H.C.; Yeh, S.F.; Chen, J.M. Inhibition of hepatitis B surface antigen secretion on human hepatoma cells. Components from Rubia cordifolia. J. Nat. Prod. 1996, 59, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.J.; Wang, S.X.; Hua, H.M.; Li, X.; Zhu, T.R.; Miyase, T.; Ueno, A. 6-Methoxygeniposidic acid, an iridoid glycoside from Rubia cordifolia. Phytochemistry 1991, 30, 1710–1711. [Google Scholar] [CrossRef]
- Jun, D.Y.; Han, C.R.; Lee, J.Y.; Park, W.; Choi, M.S.; Woo, M.H.; Kim, Y.H. Anti-adipogenic activity of 2-carbomethoxy-2,3-epoxy-3-prenyl-1,4-naphthoquinone from Rubia cordifolia L. J. Med. Food 2011, 14, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, H.; Takeya, K.; Mori, N.; Sonobe, T.; Mihashi, S.; Hamanaka, T. Studies on antitumor cyctic hexapeptides RA obtained from Rubiae Radix, Rubiaceae.VI. Minor antitumor constituents. Chem. Pharm. Bull. 1986, 34, 3762–3768. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, H.; Morita, H.; Takeya, K.; Tomioka, N.; Itai, A.; Iitaka, Y. New antitumor bicyclic hexapeptides, RA-VI and RA-VIII from Rubia cordifolia-conformation-activity relationship II. Tetrahedron 1991, 47, 7007–7020. [Google Scholar] [CrossRef]
- Itokawa, H.; Yamamiya, T.; Morita, H.; Takeya, K. New antitumour bicyclic hexapeptides, RA-IX and -X from Rubia cordifolia. Part 3. Conformation-antitumour activity relationship. J. Chem. Soc. Perkin Trans. 1992, 14, 455–459. [Google Scholar] [CrossRef]
- Morita, H.; Yamamiya, T.; Takeya, K.; Itokawa, H. New antitumor bicyclic hexapeptides, RA-XI, RA-XII, RA-XIII and RA-XIV from Rubia cordifolia. Chem. Pharm. Bull. 1992, 40, 1352–1354. [Google Scholar] [CrossRef] [PubMed]
- Takeya, K.; Yamamiya, T.; Morita, H.; Itokawa, H. Two antitumour bicyclic hexapeptides from Rubia cordifolia. Phytochemistry 1993, 33, 613–615. [Google Scholar] [CrossRef]
- Hitotsuyanagi, Y.; Ishikawa, H.; Hasuda, T.; Takeya, K. Isolation, structural elucidation, and synthesis of RA-XVII, a novel bicyclic hexapeptide from Rubia cordifolia, and the effect of side chain at residue 1 upon the conformation and cytotoxic activity. Tetrahedron Lett. 2004, 45, 935–938. [Google Scholar] [CrossRef]
- Lee, J.E.; Hitotsuyanagi, Y.; Kim, I.H.; Hasuda, T.; Takeya, K. A novel bicyclic hexapeptide, RA-XVIII, from Rubia cordifolia: Structure, semi-synthesis, and cytotoxicity. Bioorg. Med. Chem. Lett. 2008, 18, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Hitotsuyanagi, Y.; Takeya, K. Structures of cytotoxic bicyclic hexapeptides, RA-XIX, -XX, -XXI, and -XXII, from Rubia cordifolia L. Tetrahedron 2008, 64, 4117–4125. [Google Scholar] [CrossRef]
- Lee, J.E.; Hitotsuyanagi, Y.; Fukaya, H.; Kondo, K.; Takeya, K. New cytotoxic bicyclic hexapeptides, RA-XXIII and XXIV, from Rubia cordifolia L. Chem. Pharm. Bull. 2008, 56, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, H.; Morita, H.; Takeya, K.; Tomioka, N.; Itai, A. RAI-III and VI, Conformational isomers of antitumor cyclic hexapeptides, RA-III and VI from Rubia cordifolia. Chem. Lett. 1991, 12, 2217–2220. [Google Scholar] [CrossRef]
- Hitotsuyanagi, Y.; Aihara, T.; Takeya, K. RA-dimer A, a novel dimeric antitumor bicyclic hexapeptide from Rubia cordifolia L. Tetrahedron Lett. 2000, 41, 6127–6130. [Google Scholar] [CrossRef]
- Itokawa, H.; Qiao, Y.F.; Takeya, K. New arborane type triterpenoids from Rubia cordifolia var Pratensis and Rubia oncotricha. Chem. Pharm. Bull. 1990, 38, 1435–1437. [Google Scholar] [CrossRef]
- Itokawa, H.; Qiao, Y.F.; Takeya, K.; Iitaka, Y. New triterpenoids from Rubiacordifolia var Pratensis (Rubiaceae). Chem. Pharm. Bull. 1989, 37, 1670–1672. [Google Scholar] [CrossRef]
- Ibraheim, Z.Z.; Gouda, Y.G. Minor constituents from Rubia cordifolia L. root. Bull. Pharm. Sci. 2010, 33, 225–233. [Google Scholar]
- Wang, S.X.; Hua, H.M.; Wu, L.J.; Li, X.; Zhu, T.R.; Miyase, T.; Ueno, A. Structure identification of a new iridoid glycoside from Rubia cordifolia. J. Shenyang Coll. Pharm. 1991, 46, 58. [Google Scholar]
- Hassanean, H.A.; Ibraheim, Z.Z.; Takeya, K.; Itorawa, H. Further quinoidal derivatives from Rubia cordifolia L. Pharmazie 2000, 55, 317–319. [Google Scholar] [PubMed]
- Chang, L.C.; Chavez, D.; Gills, J.J.; Fong, H.H.S.; Pezzuto, J.M.; Kinghorn, A.D. Rubiasins A-C, new anthracene derivatives from the roots and stems of Rubia cordifolia. Tetrahedron Lett. 2000, 41, 7157–7162. [Google Scholar] [CrossRef]
- Huang, R.Q.; Wang, Z.H.; Wang, H.X. Studies on the constituents of polysaccharides RPS-1, RPS-2 and RPS-3 from R. cordifolia. Chin. Tradit. Patent Med. 1996, 19, 25–26. [Google Scholar]
- Wang, H.X.; Wang, B. Isolation, purification and structural analysis of polysaccharide QA2 from Indian Madder (Rubia cordifolia). Chin. Tradit Herb. Drugs 1998, 29, 219–221. [Google Scholar]
- Park, H.; Shim, J.S.; Kim, B.S.; Jung, H.J.; Huh, T.L.; Kwon, H.J. Purpurin inhibits adipocyte-derived leucineaminopeptidase and angiogenesis in a zebrafish model. Biochem. Biophys. Res. Commun. 2014, 450, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, S.R.; Sehovic, S.F.; Manojlovic, N.T.; Markovic, Z.S. Antioxidant and free radical scavenging activity of purpurin. Monatshefte Chem. 2012, 143, 427–435. [Google Scholar] [CrossRef]
- Zengin, G.; Degirmenci, N.S.; Alpsoy, L.; Aktumsek, A. Evaluation of antioxidant, enzyme inhibition, and cytotoxic activity of three anthraquinones (alizarin, purpurin, and quinizarin). Hum. Exp. Toxicol. 2016, 35, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Do, M.T.; Hwang, Y.P.; Kim, H.G.; Na, M.; Jeong, H.G. Mollugin inhibits proliferation and induces apoptosis by suppressing fatty acid synthase in HER2-overexpressing cancer cells. J. Cell. Physiol. 2013, 228, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.D.; Zhu, J.H.; Xu, J.G.; Ding, K. Mollugin induces tumor cell apoptosis and autophagy via the PI3K/AKT/mTOR/p70S6K and ERK signaling pathways. Biochem. Biophys. Res. Commun. 2014, 450, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.S.; Lee, D.S.; Kim, D.C.; Jahng, Y.; Son, J.K.; Lee, S.H.; Kim, Y.C. Neuroprotective and anti-inflammatory effects of mollugin via up-regulation of heme oxygenase-1 in mouse hippocampal and microglial cells. Eur. J. Pharmacol. 2011, 654, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Lee, J.S.; Kwak, M.K.; Choi, H.G.; Yong, C.S.; Kim, J.A.; Lee, Y.R.; Lyoo, W.S.; Park, Y.J. Anti-inflammatory action of mollugin and its synthetic derivatives in HT-29 human colonic epithelial cells is mediated through inhibition of NF-kappa B activation. Eur. J. Pharmacol. 2009, 622, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, Y.B.; Singh, A.V. Role of Rubia cordifolia Linn in radiation protection. Indian J. Exp. Biol. 2007, 45, 620–625. [Google Scholar] [PubMed]
- Baskar, R.; Bhakshu, L.M.; Bharathi, G.V.; Reddy, S.S.; Karuna, R.; Reddy, G.K.; Saralakumari, D. Antihyperglycemic activity of aqueous root extract of Rubia cordifolia in streptozotocin-induced diabetic rats. Pharm. Biol. 2006, 44, 475–479. [Google Scholar] [CrossRef]
- Rao, G.M.M.; Rao, C.V.; Pushpangadan, P.; Shirwaikar, A. Hepatoprotective effects of rubiadin, a major constituent of Rubia cordifolia Linn. J. Ethnopharmacol. 2006, 103, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, Y.B.; Shukla, S.D. Rubia cordifolia extract inhibits cell proliferation in A-431 cells. Phytother. Res. 1998, 12, 454–456. [Google Scholar] [CrossRef]
- Tripathi, Y.B.; Pandey, S.; Shukla, S.D. Anti-platelet activating factor property of Rubia cordifolia Linn. Indian J. Exp. Biol. 1993, 31, 533–535. [Google Scholar] [PubMed]
- Deshkar, N.; Tilloo, S.; Pande, V. A comeprehensive review of Rubia cordifolia Linn. Pharmacogn. Rev. 2008, 2, 124–135. [Google Scholar]
- Patil, R.; Mohan, M.; Kasture, V.; Kasture, S. Rubia cordifolia: A review. Orient. Pharm. Exp. Med. 2009, 9, 1–13. [Google Scholar] [CrossRef]
- Shan, M.Q.; Chen, X.; Li, J.; Yu, B.; Ding, A.W. Comparative study on effects of Rubiae Radix et Rhizoma and carbonized Rubiae Radix et Rhizoma on acute blood stasis rat model. China J. Chin. Mater. Med. 2014, 39, 493–497. [Google Scholar]
- Geng, Q.B.; Huang, H.L.; Weng, Z.F.; Chen, P.; Zhang, W.Z.; Cai, Y.C. Effects of Rubia cordifolia and charred R. Cordifolia on coagulation-fibrinolytic system of normal rats. Chin. J. Exp. Tradit. Med. Formulae 2013, 19, 279–282. [Google Scholar]
- Nishimura, N.; Takai, M.; Yamamoto, E.; Hasumi, K. Purpurin as a specific inhibitor of spermidine-induced autoactivation of the protease plasma hyaluronan-binding protein. Biol. Pharm. Bull. 2010, 33, 1430–1433. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Chandel, M.; Kumar, S.; Kumar, N.; Singh, B.; Kaur, S. Modulatory role of alizarin from Rubia cordifolia L. against genotoxicity of mutagens. Food Chem. Toxicol. 2010, 48, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Auh, Q.S.; Lee, D.W.; Kim, J.Y.; Jung, H.J.; Lee, S.H.; Kim, E.C. Involvement of Nrf2-mediated upregulation of hemeoxygenase-1 in mollugin-induced growth inhibition and apoptosis in human oral cancer cells. Biomed. Res. Int. 2013. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sarma, M.D.; Patra, A.; Hazra, B. Anti-inflammatory and anticancer compounds isolated from Ventilago madraspatana Gaertn, Rubia cordifolia Linn and Lantana camara Linn. J. Pharm. Pharmacol. 2010, 62, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.T.; Anap, R.M.; Ghodasara, J.V.; Kuchekar, B.S. Protective effect of hydroalcoholic root extract of Rubia cordifolia in indomethacin-induced enterocolitis in rats. Indian J. Pharm. Sci. 2011, 73, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.A.; Kasture, S.B. Protective effect of Rubia cordifolia on reserpine-induced orofacial dyskinesia. Nat. Prod. Res. 2012, 26, 2159–2161. [Google Scholar] [PubMed]
- Cai, Y.; Sun, M.; Xing, J.; Corke, H. Antioxidant phenolic constituents in roots of Rheum officinale and Rubia cordifolia: Structure-radical scavenging activity relationships. J. Agric. Food Chem. 2004, 52, 7884–7890. [Google Scholar] [CrossRef] [PubMed]
- Divakar, K.; Pawar, A.T.; Chandrasekhar, S.B.; Dighe, S.B.; Divakar, G. Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food Chem. Toxicol. 2010, 48, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.X.; Jiao, B.W.; Che, C.T.; Zuo, Z.; Mok, C.F.; Zhao, M.; Ho, W.K.K.; Tse, W.P.; Lam, K.Y.; Fan, R.Q.; et al. Ethyl acetate fraction of the root of Rubia cordifolia L. inhibits keratinocyte proliferation in vitro and promotes keratinocyte differentiation in vivo: Potential application for psoriasis treatment. Phytother. Res. 2010, 24, 1056–1064. [Google Scholar] [PubMed]
- Joy, J.; Nair, C.K. Amelioration of cisplatin induced nephrotoxicity in Swiss albino mice by Rubia cordifolia extract. J. Cancer Res. Ther. 2008, 4, 111–115. [Google Scholar] [PubMed]
- Parial, S.D.; Sharma, D.K.; Mistry, S.; Middha, A.; Agarwal, K. Estrogenic and progestational activity of roots of Rubia cordifolia. Asian J. Chem. 2007, 19, 2861–2865. [Google Scholar]
- Baek, J.M.; Kim, J.Y.; Jung, Y.; Moon, S.H.; Choi, M.K.; Kim, S.H.; Lee, M.S.; Kim, I.; Oh, J. Mollugin from Rubia cordifolia suppresses receptor activator of nuclear factor-kappa B ligand-induced osteoclastogenesis and bone resorbing activity in vitro and prevents lipopolysaccharide-induced bone loss in vivo. Phytomedicine 2015, 22, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Sabde, S.; Bodiwala, H.S.; Karmase, A.; Deshpande, P.J.; Kaur, A.; Ahmed, N.; Chauthe, S.K.; Brahmbhatt, K.G.; Phadke, R.U.; Mitra, D.; et al. Anti-HIV activity of Indian medicinal plants. J. Nat. Med. 2011, 65, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Yoshida, M.; Takahashi, M.; Fujimoto, H.; Ohnishi, K.; Nakashima, K.; Shibutani, M.; Hirose, M.; Nishikawa, A. Possible contribution of rubiadin, a metabolite of madder color, to renal carcinogenesis in rats. Food Chem. Toxicol. 2009, 47, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Yoshida, M.; Takahashi, M.; Fujimoto, H.; Shibutani, M.; Hirose, M.; Nishikawa, A. Carcinogenic potential of alizarin and rubiadin, components of madder color, in a rat medium-term multi-organ bioassay. Cancer Sci. 2009, 100, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Li, J.S.; Huang, G.X.; Yan, L.J.; Wang, Q.Q. Study on potential toxicity of madder pigment. Guangdong Agric. Sci. 2013, 40, 35–36, 39. [Google Scholar]
- Wu, D.Z.; Liu, Q.D.; Zhang, X.G.; Liu, D.P.; Liu, L.L. The pharmacokinetics and leukocytotic effect of 1,4-naphthodiol-2,3-dicarboxylic acid diethyl ester (NDDE). Bull. Acad. Mil. Med. Sci. 1984, 32, 437–442. [Google Scholar]
- Zhang, Z.H. Clinical observation of diethylester Rubia cordifolia L. promoting the increase of leucocyte count in leucopenia. Chin. J. Integr. Tradit. West. Med. 1983, 3, 98–99. [Google Scholar]
- Xie, Y.Q. Rubidatum. Pharm. Ind. 1987, 18, 92–93. [Google Scholar]
- Haruna, K.; Kanezaki, H.; Tanabe, K.; Dai, W.M.; Nishimoto, S. Effects of structural modification on the DNA binding properties and photo-induced cleavagere activity of propargylic sulfones conjugated with an anthraquinone structure. Bioorg. Med. Chem. 2006, 14, 4427–4432. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, A.; Gandhidasan, R.; Murugesan, R. Photosensitisation and photoinduced DNA cleavage by four naturally occurring anthraquinones. J. Photochem. Photobiol. A 2004, 168, 67–73. [Google Scholar] [CrossRef]

| Category | No. | Compound | Molecular Formula | Reference |
|---|---|---|---|---|
| Anthraquinones | 1 | Alizarin | C14H8O4 | [6,9,26,27,28] |
| 2 | Alizarin 2-methyl ether | C15H10O4 | [29] | |
| 3 | Lucidinprimeveroside | C26H28O14 | [6,9,30] | |
| 4 | Munjistin | C15H8O6 | [7,31] | |
| 5 | Nordamnacanthal | C15H8O5 | [32] | |
| 6 | Pupurin | C14H8O5 | [9,28,31] | |
| 7 | Physcion | C16H12O5 | [32,33] | |
| 8 | Ruberythric acid | C25H26O13 | [6,9,28,30] | |
| 9 | Rubiacordone A | C23H22O10 | [34] | |
| 10 | Rubiadin | C15H10O4 | [7,9,35] | |
| 11 | Soranjidiol | C15H10O4 | [29] | |
| 12 | Tectoquinone | C15H10O2 | [9,36,37,38] | |
| 13 | Xanthopurpurin | C14H8O4 | [7,9,28,36] | |
| 14 | 1-Hydroxyanthraquinone | C14H8O3 | [30] | |
| 15 | 1-Hydroxy-2-methylanthraquinone | C15H10O3 | [6,7,9,32,36,37] | |
| 16 | 1,4-Dihydroxy-6-methylanthraquinone | C15H10O4 | [32] | |
| 17 | 1-Hydroxy-2-methoxyanthraquinone | C15H10O4 | [35] | |
| 18 | 1,3-Dimethoxy-2-carboxylanthraquinone | C17H12O6 | [35] | |
| 19 | 1,3,6-Trihydroxy-2-methylanthraquinone | C15H10O5 | [6,7,8,26,30,39] | |
| 20 | 1,4-dihydroxy-2-methylanthraquinone | C15H10O4 | [9,36,40] | |
| 21 | 1,4-Dihydroxy-2,3-dimethylanthraquinone | C16H12O4 | [41] | |
| 22 | 1,5-Dihydroxy-2-methylanthraquinone | C15H10O4 | [40] | |
| 23 | 1,3-Dihydroxy-2-ethoxymethylanthraquinone | C17H14O5 | [6,41] | |
| 24 | 1,4-Dihydroxy-2-methy-5-methoxylanthraquinone | C16H12O5 | [9] | |
| 25 | 1-Acetoxy-3-methoxyanthraquinone | C17H12O5 | [29] | |
| 26 | 1-Hydroxy-3-carbomethoxyanthraquinone | C16H10O5 | [36] | |
| 27 | 1-Hydroxy-2-hydroxymethylanthraquinone | C15H10O4 | [8,36] | |
| 28 | 1-Hydroxy-3-hydroxymethylanthraquinone | C15H10O4 | [41] | |
| 29 | 1-Hydroxy-3-ethylanthraquinone | C16H12O3 | [41,42] | |
| 30 | 1-Hydroxy-2,7-dimethylanthraquinone | C16H12O3 | [2] | |
| 31 | 1,2,4,6-Tetrahydroxyanthraquinone | C14H8O6 | [43] | |
| 32 | 1,2,4-Trihydroxylanthraquinone | C14H8O5 | [30] | |
| 33 | 2-Hydroxy-6-methylanthraquinone | C15H10O3 | [2] | |
| 34 | 2,6-Dihydroxyanthraquinone | C14H8O4 | [2] | |
| 35 | 1,3,6-Trihydroxy-2-methyl-9,10-anthraquinone-3-O-β-glucoside | C21H20O10 | [7,30] | |
| 36 | 1,3,6-Trihydroxy-2-methylanthraquinone-3-O-α-rhamnosyl-(1→2)-β-glucoside | C27H30O14 | [6,7,39] | |
| 37 | 1,3,6-Trihydroxy-2-methylanthraquinone-3-O-(3′-O-acetyl)-α-rhamnosyl-(1→2)-β-glucoside | C29H32O15 | [7] | |
| 38 | 1,3,6-Trihydroxy-2-methylanthraquinone-3-O-(6′-O-acetyl)-α-rhamnosyl-(1→2)-β-glucoside | C29H32O15 | [6,7] | |
| 39 | 1,3,6-Trihydroxy-2-methylanthraquinone-3-O-(4′,6′-O-diacetyl)-α-rhamnosyl-(1→2)-β-glucoside | C31H34O16 | [7] | |
| 40 | 1,3,6-Trihydroxy-2-methylanthraquinone-3-O-(3′,6′-O-diacetyl)-α-rhamnosyl-(1→2)-β-glucoside | C31H34O16 | [7,39] | |
| 41 | 1,3,6-Trihydroxy-2-methylanthraquinone-3-O-(6′-O-acetyl)-α-xylopyranosyl-(1→2)-β-glucoside | C28H30O15 | [30] | |
| 42 | 1,8-Dihydroxy-11,20(15-pentylnaphthaquinonyl) phenanthrene | C26H20O4 | [42] | |
| Naphthoquinones | 43 | Mollugin | C17H16O4 | [6,7,9,36,44,45] |
| 44 | Furomollugin | C14H10O4 | [29,44,45,46] | |
| 45 | 2′-Hydroxymollugin | C17H16O5 | [8,38] | |
| 46 | 2′-Methoxymollugin | C18H18O5 | [8] | |
| 47 | 1′,2′-Dihydroxydihydromollugin | C17H18O6 | [8] | |
| 48 | 1′-Methoxy-2′-hydroxydihydromollugin | C18H20O6 | [8] | |
| 49 | Epoxymollugin | C17H16O5 | [29] | |
| 50 | Dihydromollugin | C17H18O4 | [7,33] | |
| 51 | 3-Prenyl-5-methoxynaphthoquinone | C16H16O3 | [40] | |
| 52 | 3-Prenyl-8-methoxynaphthoquinone | C16H16O3 | [40] | |
| 53 | 2-Carbamoyl-3-hydroxynaphthoquinone | C11H7NO4 | [36] | |
| 54 | 2-Carbnmoyl-3-methoxynaphthoquinone | C12H9NO4 | [36] | |
| 55 | Dehydro-α-lapachone | C15H12O3 | [36] | |
| 56 | 2-Carboxymethyl-3-prenyl-2,3-epoxynaphthoquinone | C17H16O5 | [8,29,33,47] | |
| 57 | 2-Carbomethoxy-3-(3′-hydroxy)isopentyl-1,4-naphthohydroquinone 4-O-β-glucoside | C23H30O10 | [7] | |
| 58 | 2-Carbomethoxy-3-prenyl-1,4-naphthohydroquinone 1,4-di-O-β-glucoside | C29H38O14 | [7] | |
| 59 | 5-Hydroxy-2-[7-hydroxy-4-(1-hydroxy-1-methylethyl)-2-methyl-6-oxo-2,3,3a,6-tetrahydro-4H-1,5-dioxabenzo-[de]anthracen-2-yl]-naphtho[1,2-b]furan-4-carboxylic acid methyl ester | C33H28O9 | [8] | |
| 60 | 6-Hydroxy-2-(5-hydroxy-4-methoxycarbonyl-naphtho-[1,2-b]furan-2-yl)-2-methyl-3,4-dihydro-2H-benzo[h]-chromene-5-carboxylic acid methyl ester | C30H24O8 | [8] | |
| Cyclic hexapeptides | 61 | RA-I | C40H48N6O10 | [3,48] |
| 62 | RA-II | C40H48N6O9 | [48] | |
| 63 | RA-III | C41H50N6O10 | [3,48] | |
| 64 | RA-IV | C41H50N6O10 | [48] | |
| 65 | RA-V | C40H48N6O9 | [3,48] | |
| 66 | RA-VI | C41H50N6O10 | [49] | |
| 67 | RA-VII | C41H50N6O9 | [3,48] | |
| 68 | RA-VIII | C41H50N6O10 | [49] | |
| 69 | RA-IX | C43H51N6O10 | [50] | |
| 70 | RA-X | C43H52N6O11 | [50] | |
| 71 | RA-XI | C42H50N6O11 | [51] | |
| 72 | RA-XII | C46H58N6O14 | [51] | |
| 73 | RA-XIII | C48H60N6O16 | [51] | |
| 74 | RA-XIV | C48H58N6O15 | [51] | |
| 75 | RA-XV | C48H60N6O15 | [52] | |
| 76 | RA-XVI | C48H60N6O16 | [52] | |
| 77 | RA-XVII | C41H50N6O9 | [53] | |
| 78 | RA-XVIII | C41H50N6O10 | [54] | |
| 79 | RA-XIX | C44H57N6O9 | [55] | |
| 80 | RA-XX | C42H52N6O9 | [55] | |
| 81 | RA-XXI | C41H50N6O9 | [55] | |
| 82 | RA-XXII | C41H50N6O10 | [55] | |
| 83 | RA-XXIII | C43H53N7O10 | [56] | |
| 84 | RA-XXIV | C42H51N7O10 | [56] | |
| 85 | Rubicordin A | C46H60N6O14 | [3] | |
| 86 | Rubicordin B | C47H62N6O14 | [3] | |
| 87 | Rubicordin C | C42H54N6O9 | [3] | |
| 88 | Rubiyunnanin B | C46H58N6O14 | [3] | |
| 89 | RY-II | C47H60N6O14 | [3] | |
| 90 | neo-RA-V | C40H48N6O9 | [5] | |
| 91 | allo-RA-V | C40H48N6O9 | [5] | |
| 92 | O-seco-RA-V | C40H50N6O9 | [5] | |
| 93 | O-seco-RA-XXIV | C42H53N7O10 | [4] | |
| 94 | RAI-III | C41H50N6O10 | [57] | |
| 95 | RAI-VI | C41H50N6O10 | [57] | |
| 96 | RA-dimer A | C80H94N12O18 | [58] | |
| Triterpenoids | 97 | Oleanolic acid | C30H48O3 | [29,33] |
| 98 | Oleanolic aldehyde acetate | C32H50O3 | [46] | |
| 99 | Rubiarbonol A | C30H50O4 | [43,59] | |
| 100 | Rubiarbonol B | C30H50O3 | [43] | |
| 101 | Rubiatriol | C30H50O3 | [26] | |
| 102 | Rubicoumaric acid | C39H54O6 | [10] | |
| 1037 | Rubifolic acid | C30H48O4 | [10] | |
| 104 | Rubiprasin A | C32H52O5 | [60] | |
| 105 | Rubiprasin B | C32H52O4 | [60] | |
| 106 | Rubiprasin C | C32H50O5 | [60] | |
| 107 | Ursolic acid | C30H48O3 | [41] | |
| 108 | 3-β-Friedelinol | C30H52O6 | [61] | |
| Other compounds | 109 | β-Sitostenone | C29H48O | [43] |
| 110 | β-Sitosterol | C29H50O | [33,41] | |
| 111 | 5-Methoxygeniposidic acid | C17H24O11 | [62] | |
| 112 | 6-Methoxygeniposidic acid | C17H24O11 | [46] | |
| 113 | 3,5-di-(p-hydroxybenzyl)phenol | C20H18O3 | [2] | |
| 114 | n-Heptadecane | C17H36 | [2] | |
| 115 | n-Nonadecane | C19H40 | [2] | |
| 116 | (+)-Lariciresinol | C20H24O6 | [38] | |
| Other compounds | 117 | 3,3′-bis(3,4-Dihydro-4-hydroxy-6-methoxy-2H-1-benzopyran) | C20H22O6 | [29] |
| 118 | 8-Hydroxy n-pentadecanyl decan-4-en-1-oate | C25H48O3 | [2] | |
| 119 | n-Octacosanyl octa-1-oate | C36H72O2 | [2] | |
| 120 | Rubilactone | C15H10O5 | [41,45,63] | |
| 121 | Rubioncolin B | C31H24O10 | [8] | |
| 122 | 2,3-Dihydro-2-(4-hydroxy-3-methoxyphenyl)-3-hydroxymethyl-5-ω-hydroxypropyl-7-methoxybenzofuran | C20H24O6 | [43] | |
| 123 | Palmitic acid | C16H32O2 | [41] | |
| 124 | Tricosanoic acid | C23H46O2 | [41] | |
| 125 | Rubiasin A | C15H16O2 | [38,64] | |
| 126 | Rubiasin B | C15H16O2 | [64] | |
| 127 | Rubiasin C | C15H16O2 | [64] | |
| 128 | Atraric acid | C10H12O4 | [61] | |
| 129 | Vanillic acid | C8H8O4 | [61] | |
| 130 | d-3-O-Methoxy-chiroinositol | C7H14O6 | [61] | |
| 131 | Polysaccharide RPS-1 | Not mentioned | [65] | |
| 132 | Polysaccharide RPS-2 | Not mentioned | [65] | |
| 133 | Polysaccharide RPS-3 | Not mentioned | [65] | |
| 134 | Polysaccharide QA2 | Not mentioned | [66] |
| Function | Inducer | Test Drug | Model | Efficacy Evaluation | Reference |
|---|---|---|---|---|---|
| Anti-adipogenic activity | 2-Carboxymethyl-3-prenyl-2,3-epoxynaphthoquinone | 3T3-L1 preadipocytes | Induced MMP loss, caspase-3 activation | [54] | |
| Reduced differentiation-associated accumulation of intracellular lipid | |||||
| Downregulatedexpressions of CCAAT/enhancer binding protein-α, PPAR γ1, PPAR γ2, adiponectin | |||||
| Anti-urolithiasis | Ethylene glycol | Ethanol extract | Male Wistar albino rats | Decreased calcium, oxalate levels and number of calcium oxalate crystals deposits in kidney tissue | [90] |
| Anti-psoriasis | Ethyl acetate fraction of ethanol extract | HaCaT cells | Decreased MMP | [91] | |
| Induced apoptosis | |||||
| Male BALB/c mice | Increased NGL, TGL and VET | ||||
| Anti-nephrotoxicity | Cisplatin | Ethanol extract | Swiss albino mice | Decreased values of serum urea and creatinine | [92] |
| Increased GPx, SOD and CAT | |||||
| Inhibited LPO in kidney and liver | |||||
| Estrogenic and progestational activity | Ethyl acetate precipitate of methanol extract | Old female albino rats | Increased the regularity of the estrous cycle | [93] | |
| Increased uterine weight and foetal survival | |||||
| Anti-osteoclastogenesis | NF-κB ligand | Mollugin | Mice BMMs | Inhibited osteoclast differentiation | [94] |
| Reduced the phosphorylation ofMAP kinase, Akt, and GSK3β | |||||
| Inhibited expression of c-Fos, NFATc1, | |||||
| OSCAR, TRAP, DC-STAMP, OC-STAMP, integrin αν, integrin β3, cathepsin K, and ICAM-1 | |||||
| Anti-HIV | HIV-1NL4.3 | Ethyl acetate extract | CEM-GFP cells | Reduced viral production | [95] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, M.; Yu, S.; Yan, H.; Chen, P.; Zhang, L.; Ding, A. A Review of the Botany, Phytochemistry, Pharmacology and Toxicology of Rubiae Radix et Rhizoma. Molecules 2016, 21, 1747. https://doi.org/10.3390/molecules21121747
Shan M, Yu S, Yan H, Chen P, Zhang L, Ding A. A Review of the Botany, Phytochemistry, Pharmacology and Toxicology of Rubiae Radix et Rhizoma. Molecules. 2016; 21(12):1747. https://doi.org/10.3390/molecules21121747
Chicago/Turabian StyleShan, Mingqiu, Sheng Yu, Hui Yan, Peidong Chen, Li Zhang, and Anwei Ding. 2016. "A Review of the Botany, Phytochemistry, Pharmacology and Toxicology of Rubiae Radix et Rhizoma" Molecules 21, no. 12: 1747. https://doi.org/10.3390/molecules21121747