New Efficient Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones Catalyzed by Benzotriazolium-Based Ionic Liquids under Solvent-Free Conditions
Abstract
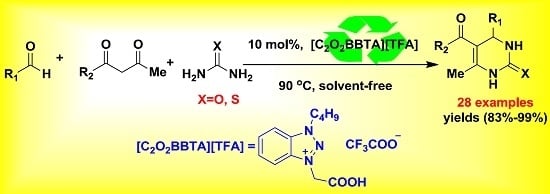
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Procedure for the Synthesis of 1-Butyl-3-carboxymethyl-benzotriazolium Trifluoroacetate
3.2. General Procedure for the Synthesis of 3,4-Dihydropyrimidin-2(1H)-(thio)ones
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tayebee, R.; Amini, M.M.; Ghadamgahi, M.; Armaghan, M. H5PW10V2O40/Pip-SBA-15: A novel reusable organic-inorganic hybrid material as potent Lewis acid catalyst for one-pot solvent-free synthesis of 3,4-dihydropyrimidinones. J. Mol. Catal. A Chem. 2013, 366, 266–274. [Google Scholar]
- Shaabani, A.; Seyyedhamzeh, M.; Maleki, A.; Hajishaabanha, F. Diketene as an alternative substrate for a new Biginelli-like multicomponent reaction: One-pot synthesis of 5-carboxamide substituted 3,4-dihydropyrimidine-2(1H)ones. Tetrahedron 2010, 66, 4040–4042. [Google Scholar] [CrossRef]
- Ismaili, L.; Nadaradjane, A.; Nicod, L.; Guyon, C.; Xicluna, A.; Robert, J.F.; Refouvelet, B. Synthesis and antioxidant activity evaluation of new hexahydropyrimido[5,4-c]quinoline-2,5-diones and 2-thioxohexahydropyrimido[5,4-c]quinoline-5-ones obtained by Biginelli reaction in two steps. Eur. J. Med. Chem. 2008, 43, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Litvic, M.; Vecenaj, I.; Ladisic, Z.M.; Lovric, M.; Vinkovic, V.; Filipan-Litvic, M. First application of hexaaquaaluminium(III) tetrafluoroborate as a mild, recyclable, non-hygroscopic acid catalyst in organic synthesis: A simple and efficient protocol for the multigram scale synthesis of 3,4-dihydropyrimidinones by Biginelli reaction. Tetrahedron 2010, 66, 3463–3471. [Google Scholar] [CrossRef]
- Mandhane, P.G.; Joshi, R.S.; Nagargoje, D.R.; Gill, C.H. An efficient synthesis of 3,4-dihydropyrimidin-2(1H)-ones catalyzed by thiamine hydrochloride in water under ultrasound irradiation. Tetrahedron Lett. 2010, 51, 3138–3140. [Google Scholar] [CrossRef]
- Gore, S.; Baskaran, S.; Koenig, B. Efficient synthesis of 3,4-dihydropyrimidin-2-ones in low melting tartaric acid-urea mixtures. Green Chem. 2011, 13, 1009–1013. [Google Scholar] [CrossRef]
- Kargar, M.; Hekmatshoar, R.; Mostashari, A.; Hashemi, Z. Efficient and green synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones using imidazol-1-yl-acetic acid as a novel, reusable and water-soluble organocatalyst. Catal. Commun. 2011, 15, 123–126. [Google Scholar] [CrossRef]
- Narahari, S.R.; Reguri, B.R.; Gudaparthi, O.; Mukkanti, K. Synthesis of dihydropyrimidinones via Biginelli multi-component reaction. Tetrahedron Lett. 2012, 53, 1543–1545. [Google Scholar] [CrossRef]
- Rahmatpour, A. Polyvinylsulfonic acid: An efficient, water-soluble and reusable bronsted acid catalyst for the three-component synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones in water and ethanol. Catal. Lett. 2012, 142, 1505–1511. [Google Scholar] [CrossRef]
- Ashok, M.; Holla, B.S.; Kumari, N.S. Convenient one pot synthesis of some novel derivatives of thiazolo[2,3-b]dihydropyrimidinone possessing 4-methylthiophenyl moiety and evaluation of their antibacterial and antifungal activities. Eur. J. Med. Chem. 2007, 42, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Kolosov, M.A.; Orlov, V.D.; Beloborodov, D.A.; Dotsenko, V.V. A chemical placebo: NaCl as an effective, cheapest, non-acidic and greener catalyst for Biginelli-type 3,4-dihydropyrimidin-2(1H)-ones (-thiones) synthesis. Mol. Div. 2009, 13, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Chitra, S.; Devanathan, D.; Pandiarajan, K. Synthesis and in vitro microbiological evaluation of novel 4-aryl-5-isopropoxycarbonyl-6-methyl-3,4-dihydropyrimidinones. Eur. J. Med. Chem. 2010, 45, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Ishitani, H.; Iwamoto, M. Highly ordered aluminium-planted mesoporous silica as active catalyst for Biginelli reaction and formyl C-H insertion reaction with diazoester. Phys. Chem. Chem. Phys. 2010, 12, 14452–14455. [Google Scholar] [CrossRef] [PubMed]
- Tamaddon, F.; Rami, Z.; Jafari, A.A. Synthesis of 3,4-dihydropyrimidin-2(1H)-ones and 1,4-dihydropyridines using ammonium carbonate in water. Tetrahedron Lett. 2010, 51, 1187–1189. [Google Scholar] [CrossRef]
- Zych, A.J.; Wang, H.-J.; Sakwa, S.A. Synthesis and Suzuki-Miyaura reactions of 5-halo-3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron Lett. 2010, 51, 5103–5105. [Google Scholar] [CrossRef]
- Akhaja, T.N.; Raval, J.P. 1,3-Dihydro-2H-indol-2-ones derivatives: Design, synthesis, in vitro antibacterial, antifungal and antitubercular study. Eur. J. Med. Chem. 2011, 46, 5573–5579. [Google Scholar] [CrossRef] [PubMed]
- Karade, H.N.; Acharya, J.; Kaushik, M.P. An efficient and rapid dehydrogenation of 4-aryl-3,4-dihydropyrimidin-2(1H)-ones (DHPMs) using CAN/HCl. Tetrahedron Lett. 2012, 53, 5541–5543. [Google Scholar] [CrossRef]
- Starcevich, J.T.; Laughlin, T.J.; Mohan, R.S. Iron(III) tosylate catalyzed synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones via the Biginelli reaction. Tetrahedron Lett. 2013, 54, 983–985. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Welton, T. Room-temperature ionic liquids. Solvents for Synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.M.; Ponce de Leon y Tobio, A.Y.; dosSantos, M.R.; de Oliveira, H.C.B.; Gomes, A.F.; Gozzo, F.C.; de Oliveira, A.L.; Neto, B.A.D. Mechanistic studies on lewis acid catalyzed biginelli reactions in ionic liquids: Evidence for the reactive intermediates and the role of the reagents. J. Org. Chem. 2012, 77, 10184–10193. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sharma, U.K.; Kumar, R.; Richa; Sinha, A.K. Green and recyclable glycine nitrate (GlyNO3) ionic liquid triggered multicomponent Biginelli reaction for the efficient synthesis of dihydropyrimidinones. RSC Adv. 2012, 2, 10648–10651. [Google Scholar] [CrossRef]
- Nagarajan, S.; Shaikh, T.M.; Kandasamy, E. Synthesis of 1-alkyl triazolium triflate room temperature ionic liquids and their catalytic studies in multi-component Biginelli reaction. J. Chem. Sci. 2015, 127, 1539–1545. [Google Scholar] [CrossRef]
- Liu, C.J.; Wang, J.D. Ultrasound-assisted synthesis of novel 4-(2-pheny-1,2,3-triazol-4-yl)-3,4-dihydropyrimidin-2(1H)-(thio)ones catalyzed by Sm(ClO4)3. Molecules 2010, 15, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Wang, J.D. Copper(II) sulfamate: an efficient catalyst for the one-pot synthesis of 3,4-dihydropyrimidine-2(1H)-ones and thiones. Molecules 2009, 14, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Wang, B.; Zhang, X.M.; Huang, J.B.; Liu, C.J. An efficient synthesis of 3, 4-dihydropyrimidin-2(1H)-ones and thiones catalyzed by a novel Brønsted acidic ionic liquid under solvent-free conditions. Molecules 2015, 20, 3811–3820. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.H.; Macquarrie, D.J.; Sherwood, J. The combined role of catalysis and solvent effects on the Biginelli reaction: Improving efficiency and sustainability. Chem. Eur. J. 2013, 19, 5174–5182. [Google Scholar] [CrossRef] [PubMed]
- Folkers, K.; Harwd, H.J.; Johnson, T.B. Researches on pyrimidines. Cxxx. synthesis of 2-keto-l,2,3,4-tetrahydropymidin. J. Am. Chem. Soc. 1932, 54, 3751–3758. [Google Scholar] [CrossRef]
- Rao, G.B.D.; Acharya, B.N.; Verma, S.K.; Kaushik, M.P. N,N'-Dichlorobis(2,4,6-trichlorophenyl) urea (CC-2) as a new reagent for the synthesis of pyrimidone and pyrimidine derivatives via Biginelli reaction. Tetrahedron Lett. 2011, 52, 809–812. [Google Scholar] [CrossRef]
- Gholap, A.R.; Venkatesan, K.; Daniel, T.; Lahoti, R.J.; Srinivasan, K.V. Ionic liquid promoted novel and efficient one pot synthesis of 3,4-dihydropyrimidin-2-(1H)-ones at ambient temperature under ultrasound irradiation. Green Chem. 2004, 6, 147–150. [Google Scholar] [CrossRef]
- Boumoud, T.; Boumoud, B.; Mosset, P.; Debache, A. Gypsum-catalyzed one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones under solvent-free conditions. Eur. J. Chem. 2011, 8, 312–318. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Sridhar, P.; Reddy, J.S.S.; Nagaiah, K.; Lingaiah, N.; Saiprasad, P.S. Green protocol for the Biginelli three-component reaction: Ag3PW12O40 as a novel, water-tolerant heteropolyacid for the synthesis of 3,4-dihydropyrimidinones. Eur. J. Org. Chem. 2004, 3, 552–557. [Google Scholar] [CrossRef]
- Li, W.M.; Zhou, G.B.; Zhang, P.F.; Lai, Y.F.; Xu, S.F. One-pot synthesis of dihydropyrimidiones via environmentally friendly enzyme-catalyzed Biginelli reaction. Heterocycles 2011, 83, 2067–2077. [Google Scholar] [CrossRef]
- Boumoud, T.; Boumoud, B.; Rhouati, S.; Belfaitah, A.; Debache, A.; Mosset, P. An efficient and recycling catalyst for the one-pot three-component synthesis of substituted 3,4-dihydropyrimidin-2(1H)-ones. Eur. J. Chem. 2008, 5, 688–695. [Google Scholar] [CrossRef]
- Murata, H.; Ishitani, H.; Iwamoto, M. Synthesis of Biginelli dihydropyrimidinone derivatives with various substituents on aluminium-planted mesoporous silica catalyst. Org. Biomol. Chem. 2010, 8, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Pasunooti, K.K.; Chai, H.; Jensen, C.N.; Gorityala, B.K.; Wang, S.; Liu, X.W. A microwave-assisted, copper-catalyzed three-component synthesis of dihydropyrimidinones under mild conditions. Tetrahedron Lett. 2011, 52, 80–84. [Google Scholar] [CrossRef]
- Da Silva, D.L.; Fernandes, S.A.; Sabino, A.A.; de Fatima, A. p-Sulfonic acid calixarenes as efficient and reusable organocatalysts for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/-thiones. Tetrahedron Lett. 2011, 52, 6328–6330. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Khazdooz, L.; Zarei, A. Brønsted acidic ionic liquid–catalyzed one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones and thiones under solvent-free conditions. Synth. Commun. 2011, 41, 2200–2208. [Google Scholar] [CrossRef]
- Nath, J.; Chaudhuri, M.K. Borax: An ecofriendly and efficient catalyst for one-pot synthesis of 3,4-dihydropyrimidine-2(1H)-ones under solvent-free conditions. Synth. Commun. 2010, 40, 2976–2982. [Google Scholar] [CrossRef]
- Chandak, H.S.; Lad, N.P.; Upare, P.P. Recyclable amberlyst-70 as a catalyst for Biginelli reaction: An efficient one-pot green protocol for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Catal Lett. 2009, 131, 469–473. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Yu, J.P.; Yang, H.H.; Miao, Z.W.; Chen, R.Y. Solvent-free Biginelli reaction: A green method for the synthesis of 3,4-dihydropyrimidin-2-ones catalyzed by protic acids in large-scale. Lett. Org. Chem. 2011, 8, 264–267. [Google Scholar] [CrossRef]
- Khabazzadeh, H.; Saidi, K.; Sheibani, H. Microwave-assisted synthesis of dihydropyrimidin-2(1H)-ones using graphite supported lanthanum chloride as a mild and efficient catalyst. Bioorg. Med. Chem. Lett. 2008, 18, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Dallinger, D.; Kappe, C.O. Automated generation of a dihydropyrimidine compound library using microwave-assisted processing. Nat. Protoc. 2007, 2, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Dilmaghani, K.A.; Zeynizadeh, B.; Yari, M. One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones and their sulfur derivatives with H2SO4 supported on silica gel or alumina. Phosphorus Sulfur Silicon Related Elem. 2009, 184, 1722–1728. [Google Scholar] [CrossRef]
- Jiang, C.; You, Q.D. An efficient and solvent-free one-pot synthesis of dihydropyrimidinones under microwave irradiation. Chin. Chem. Lett. 2007, 18, 647–650. [Google Scholar] [CrossRef]
- Zhang, S.; Hou, Y.; Huang, W.; Shan, Y. Preparation and characterization of novel ionic liquid based on benzotriazolium cation. Electrochim. Acta 2005, 50, 4097–4103. [Google Scholar] [CrossRef]
- Sample Availability: All samples are available from the authors.


| Entry | Solvent | IL (mol %) | Time (min) | Yield (%) b |
|---|---|---|---|---|
| 1 | H2O | 10 | 40 | 5 |
| 2 | MeOH | 10 | 40 | 3 |
| 3 | EtOH | 10 | 40 | 10 |
| 4 | CH2Cl2 | 10 | 40 | 16 |
| 5 | CH3CN | 10 | 40 | 19 |
| 6 | DMF | 10 | 40 | NR |
| 7 | Toluene | 10 | 40 | 5 |
| 8 c | solvent-free | 10 | 40 | 96, 95, 95, 94, 93, 92 |
| 9 | solvent-free | None | 40 | NR |
| 10 | solvent-free | 1 | 40 | 81 |
| 11 | solvent-free | 2.5 | 40 | 84 |
| 12 | solvent-free | 5 | 40 | 85 |
| 13 | solvent-free | 15 | 40 | 95 |
| 14 | solvent-free | 20 | 40 | 93 |
| 15 | solvent-free | 10 | 10 | 75 |
| 16 | solvent-free | 10 | 20 | 91 |
| 17 | solvent-free | 10 | 30 | 94 |
| 18 | solvent-free | 10 | 50 | 91 |
| 19 | solvent-free | 10 | 60 | 93 |
| Entry | R1 | R2 | X | Yields b (%) | Mp (°C) c | |
|---|---|---|---|---|---|---|
| Found | Reported (lit.) | |||||
| 3a | C6H5 | EtO | O | 96 | 201–202 | 200–202 [29] |
| 3b | 2-F-C6H4 | EtO | O | 96 | 236–237 | 235–237 [30] |
| 3c | 3-F-C6H4 | EtO | O | 97 | 209–211 | 209–211 [31] |
| 3d | 4-F-C6H4 | EtO | O | 98 | 175–176 | 175–177 [32] |
| 3e | 2-Cl-C6H4 | EtO | O | 93 | 211–213 | 211–213 [33] |
| 3f | 2-Br-C6H4 | EtO | O | 93 | 204–205 | 205–207 [30] |
| 3g | 3-Br-C6H4 | EtO | O | 94 | 190–191 | 190–192 [26] |
| 3h | 3-Me-C6H4 | EtO | O | 92 | 228–230 | 228–230 [34] |
| 3i | 4-Me-C6H4 | EtO | O | 97 | 209–211 | 209–212 [35] |
| 3j | 3,4-(MeO)2-C6H3 | EtO | O | 98 | 171–172 | 172–174 [36] |
| 3k | 3-MeO-C6H4 | EtO | O | 93 | 219–221 | 219–220 [37] |
| 3l | 2-Cl-4-F-C6H3 | EtO | O | 88 | 195–197 | |
| 3m | 3-Br-4-F-C6H3 | EtO | O | 85 | 193–195 | |
| 3n | 3,4-(HO)2-C6H3 | EtO | O | 89 | 232–234 | 233–235 [37] |
| 3o | 4-N(CH3)2-C6H4 | EtO | O | 89 | 249–251 | 249–250 [38] |
| 3p | C6H5 | EtO | S | 83 | 202–204 | 202–204 [39] |
| 3q | 4-F-C6H4 | EtO | S | 86 | 192–193 | 191–192 [40] |
| 3r | 3-Me-C6H4 | EtO | S | 86 | 193–195 | 194–195 [41] |
| 3s | 4-Me-C6H4 | EtO | S | 90 | 184–186 | 185–186 [42] |
| 3t | 3-MeO-C6H4 | EtO | S | 93 | 140–142 | 141–143 [37] |
| 3u | 4-F-C6H4 | MeO | O | 98 | 188–189 | 188–190 [43] |
| 3v | 4-Me-C6H4 | MeO | O | 96 | 202–203 | 202–204 [44] |
| 3w | 3-MeO-C6H4 | MeO | O | 92 | 206–208 | 204–206 [29] |
| 3x | 4-OH-C6H4 | MeO | O | 99 | 231–233 | 231–233 [45] |
| 3y | 3-MeO-C6H4 | i-PrO | O | 94 | 196–198 | |
| 3z | 4-OH-C6H4 | i-PrO | O | 98 | 192–194 | |
| 3aa | 4-F-C6H4 | t-BuO | O | 99 | 147–149 | |
| 3ab | 3-MeO-C6H4 | t-BuO | O | 95 | 212–214 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Ma, R.; Cao, D.; Liu, C. New Efficient Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones Catalyzed by Benzotriazolium-Based Ionic Liquids under Solvent-Free Conditions. Molecules 2016, 21, 462. https://doi.org/10.3390/molecules21040462
Liu Z, Ma R, Cao D, Liu C. New Efficient Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones Catalyzed by Benzotriazolium-Based Ionic Liquids under Solvent-Free Conditions. Molecules. 2016; 21(4):462. https://doi.org/10.3390/molecules21040462
Chicago/Turabian StyleLiu, Zhiqing, Rong Ma, Dawei Cao, and Chenjiang Liu. 2016. "New Efficient Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones Catalyzed by Benzotriazolium-Based Ionic Liquids under Solvent-Free Conditions" Molecules 21, no. 4: 462. https://doi.org/10.3390/molecules21040462
APA StyleLiu, Z., Ma, R., Cao, D., & Liu, C. (2016). New Efficient Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones Catalyzed by Benzotriazolium-Based Ionic Liquids under Solvent-Free Conditions. Molecules, 21(4), 462. https://doi.org/10.3390/molecules21040462