Design, Synthesis and Structure-Activity Relationships of Novel Chalcone-1,2,3-triazole-azole Derivates as Antiproliferative Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
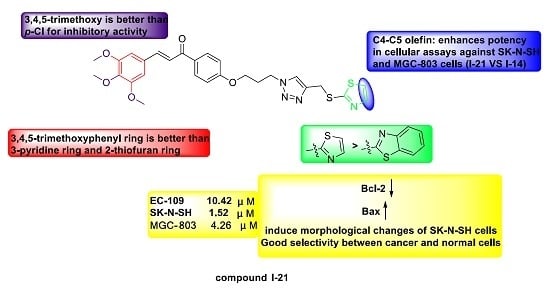
2.2. Antiproliferative Activity and Structure Activity Relationship Analysis
2.3. Apoptosis Analysis with Hoechst-33258 Staning
2.4. Western Blots Analysis
3. Materials and Methods
3.1. General Experimental Procedures
3.1.1. General Procedure of Compounds 5a–5d
3.1.2. General Procedure of Compounds 7a–7d
3.1.3. General Procedure of Compounds I-13–I-27
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ma, L.Y.; Wang, B.; Pang, L.P.; Zhang, M.; Wang, S.Q.; Zheng, Y.C.; Shao, K.P.; Xue, D.Q.; Liu, H.M. Design and synthesis of novel 1,2,3-triazole–pyrimidine–urea hybrids. Bioorg. Med. Chem. Lett. 2015, 25, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.-C.; Zheng, Y.-C.; Li, X.-C.; Wang, M.-M.; Ye, X.-W.; Guan, Y.-Y.; Liu, G.-Z.; Zheng, J.-X.; Liu, H.-M. Design, synthesis and antiproliferative activity studies of novel 1,2,3-triazole–dithiocarbamate–urea hybrids. Eur. J. Med. Chem. 2013, 64, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Wang, K.; Edler, M.C.; Hamel, E.; Mooberry, S.L.; Paige, M.A.; Brown, M.L. A boronic acid chalcone analog of combretastatin A-4 as a potent anti-proliferation agent. Bioorg. Med. Chem. 2010, 18, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Alcaráz, L.; Blanco, S.; Puig, O.; Tomás, F.; Ferretti, F. Antibacterial activity of flavonoids against methicillin-resistant Staphylococcus aureus strains. J. Theor. Biol. 2000, 205, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Mallavadhani, U.; Sahoo, L.; Kumar, K.; Murty, U. Synthesis and antimicrobial screening of some novel chalcones and flavanones substituted with higher alkyl chains. Med. Chem. Res. 2014, 23, 2900–2908. [Google Scholar] [CrossRef]
- Awoussong, P.; Zaharia, V.; Ngameni, B.; Kuete, V.; Ntede, H.; Fokunang, C.; Abegaz, B.; Ngadjui, B. Heterocycles 26: Synthesis, characterisation, and anticancer activity of some thiazolic chalcones. Med. Chem. Res. 2015, 24, 131–141. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, S.; Ali, F.; Anthal, S.; Gupta, V.; Khan, I.; Singh, S.; Sangwan, P.; Suri, K.; Gupta, B.; et al. Synthesis and biologic activities of some novel heterocyclic chalcone derivatives. Med. Chem. Res. 2013, 22, 3969–3983. [Google Scholar] [CrossRef]
- Nowakowska, Z. A review of anti-infective and anti-inflammatory chalcones. Eur. J. Med. Chem. 2007, 42, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-K.; Lee, T.-H.; Wang, J.-P.; Wang, J.-J.; Lin, C.-N. Synthesis and Anti-inflammatory Effect of Chalcones and Related Compounds. Pharm. Res. 1998, 15, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Z.; Cheng, C.-C.; Shen, L.-L.; Wang, Z.-K.; Wu, S.-B.; Li, W.-L.; Chen, S.-H.; Zhou, R.-P.; Qiu, P.-H. Synthetic Chalcones with Potent Antioxidant Ability on H2O2-Induced Apoptosis in PC12 Cells. Int. J. Mol. Sci. 2014, 15, 18525–18539. [Google Scholar] [CrossRef] [PubMed]
- Vasil’ev, R.F.; Kancheva, V.D.; Fedorova, G.F.; Batovska, D.I.; Trofimov, A.V. Antioxidant activity of chalcones: The chemiluminescence determination of the reactivity and the quantum chemical calculation of the energies and structures of reagents and intermediates. Kinet. Catal. 2010, 51, 507–515. [Google Scholar] [CrossRef]
- Syam, S.; Abdelwahab, S.I.; Al-Mamary, M.A.; Mohan, S. Synthesis of chalcones with anticancer activities. Molecules 2012, 17, 6179–6195. [Google Scholar] [CrossRef] [PubMed]
- Ducki, S.; Rennison, D.; Woo, M.; Kendall, A.; Chabert, J.F.; McGown, A.T.; Lawrence, N.J. Combretastatin-like chalcones as inhibitors of microtubule polymerization. Part 1: Synthesis and biological evaluation of antivascular activity. Bioorg. Med. Chem. 2009, 17, 7698–7710. [Google Scholar] [CrossRef] [PubMed]
- Aher, N.G.; Pore, V.S.; Mishra, N.N.; Kumar, A.; Shukla, P.K.; Sharma, A.; Bhat, M.K. Synthesis and antifungal activity of 1,2,3-triazole containing fluconazole analogues. Bioorg. Med. Chem. Lett. 2009, 19, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Demaray, J.A.; Thuener, J.E.; Dawson, M.N.; Sucheck, S.J. Synthesis of triazole-oxazolidinones via a one-pot reaction and evaluation of their antimicrobial activity. Bioorg. Med. Chem. Lett. 2008, 18, 4868–4871. [Google Scholar] [CrossRef] [PubMed]
- Buckle, D.R.; Outred, D.J.; Rockell, C.J.M.; Smith, H.; Spicer, B.A. Studies on v-triazoles. 7. Antiallergic 9-oxo-1H,9H-benzopyrano[2,3-d]-v-triazoles. J. Med. Chem. 1983, 26, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Giffin, M.J.; Heaslet, H.; Brik, A.; Lin, Y.C.; Cauvi, G.; Wong, C.H.; McRee, D.E.; Elder, J.H.; Stout, C.D.; Torbett, B.E. A Copper(I)-Catalyzed 1,2,3-Triazole Azide−Alkyne Click Compound Is a Potent Inhibitor of a Multidrug-Resistant HIV-1 Protease Variant. J. Med. Chem. 2008, 51, 6263–6270. [Google Scholar] [CrossRef] [PubMed]
- Patpi, S.R.; Pulipati, L.; Yogeeswari, P.; Sriram, D.; Jain, N.; Sridhar, B.; Murthy, R.; DeviT, A.; Kalivendi, S.V.; Kantevari, S. Design, Synthesis, and Structure-Activity Correlations of Novel Dibenzo[b,d]furan, Dibenzo[b,d]thiophene, and N-Methylcarbazole Clubbed 1,2,3-Triazoles as Potent Inhibitors of Mycobacterium tuberculosis. J. Med. Chem. 2012, 55, 3911–3922. [Google Scholar] [CrossRef] [PubMed]
- De Simone, R.; Chini, M.G.; Bruno, I.; Riccio, R.; Mueller, D.; Werz, O.; Bifulco, G. Structure-Based Discovery of Inhibitors of Microsomal Prostaglandin E2 Synthase−1,5-Lipoxygenase and 5-Lipoxygenase-Activating Protein: Promising Hits for the Development of New Anti-inflammatory Agents. J. Med. Chem. 2011, 54, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, Y.; Wang, Q.; Yuan, Y.; Zhang, H.; Qian, X. The novel anti-tumor agents of 4-triazol-1,8-naphthalimides: Synthesis, cytotoxicity, DNA intercalation and photocleavage. Eur. J. Med. Chem. 2011, 46, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Stefely, J.A.; Palchaudhuri, R.; Miller, P.A.; Peterson, R.J.; Moraski, G.C.; Hergenrother, P.J.; Miller, M.J. N-((1-Benzyl-1H-1,2,3-triazol-4-yl)methyl)arylamide as a New Scaffold that Provides Rapid Access to Antimicrotubule Agents: Synthesis and Evaluation of Antiproliferative Activity Against Select Cancer Cell Lines. J. Med. Chem. 2010, 53, 3389–3395. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-C.; Duan, Y.-C.; Ma, J.-L.; Xu, R.-M.; Zi, X.; Lv, W.-L.; Wang, M.-M.; Ye, X.-W.; Zhu, S.; Mobley, D.; et al. Triazole–Dithiocarbamate Based Selective Lysine Specific Demethylase 1 (LSD1) Inactivators Inhibit Gastric Cancer Cell Growth, Invasion, and Migration. J. Med. Chem. 2013, 56, 8543–8560. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-Y.; Pang, L.-P.; Wang, B.; Zhang, M.; Hu, B.; Xue, D.-Q.; Shao, K.-P.; Zhang, B.-L.; Liu, Y.; Zhang, E.; et al. Design and synthesis of novel 1,2,3-triazole-pyrimidine hybrids as potential anticancer agents. Eur. J. Med. Chem. 2014, 86, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-Y.; Zheng, Y.-C.; Wang, S.-Q.; Wang, B.; Wang, Z.-R.; Pang, L.-P.; Zhang, M.; Wang, J.-W.; Ding, L.N.; Wang, C.; et al. Design, Synthesis, and Structure–Activity Relationship of Novel LSD1 Inhibitors Based on Pyrimidine–Thiourea Hybrids As Potent, Orally Active Antitumor Agents. J. Med. Chem. 2015, 58, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.








| Comp. | IC50 (μM) a | ||
|---|---|---|---|
| EC-109 | SK-N-SH | MGC-803 | |
| I-13 | >100 | 19.34 ± 0.47 | 37.19 ± 0.85 |
| I-14 | 3.57 ± 0.22 | 5.87 ± 1.05 | 8.52 ± 0.56 |
| I-15 | 42.36 ± 0.18 | 47.31 ± 1.57 | 63.84 ± 1.17 |
| I-16 | 19.28 ± 0.79 | 11.19 ± 0.26 | 8.69 ± 0.42 |
| I-17 | 9.54 ± 0.05 | >100 | 38.17 ± 0.58 |
| I-18 | 24.61 ± 3.80 | 14.81 ± 0.72 | 6.24 ± 0.05 |
| I-19 | 38.78 ± 1.05 | 37.83 ± 0.91 | 24.35 ± 0.71 |
| I-20 | 5.81 ± 0.21 | 3.75 ± 1.21 | 46.28 ± 1.64 |
| I-21 | 10.42 ± 0.24 | 1.52 ± 0.30 | 4.26 ± 0.32 |
| I-22 | 14.62 ± 1.21 | 12.43 ± 0.44 | 4.45 ± 0.82 |
| I-23 | 57.25 ± 1.63 | 20.96 ± 1.81 | 15.09 ± 0.85 |
| I-24 | 24.94 ± 1.09 | 28.37 ± 1.59 | 50.12 ± 0.97 |
| I-25 | 5.28 ± 0.56 | 6.98 ± 0.40 | 5.66 ± 0.55 |
| I-26 | 11.85 ± 0.74 | 4.49 ± 0.45 | 5.56 ± 0.45 |
| I-27 | >100 | 29.30 ± 0.85 | 38.12 ± 0.91 |
| 5-FU | 10.30 ± 0.32 | 9.85 ± 0.76 | 7.14 ± 0.28 |
| Comp. | R | IC50 (μM) a |
|---|---|---|
| I-14 |  | >64 |
| I-21 |  | 43.67 ± 1.35 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.-Y.; Fu, D.-J.; Yue, X.-X.; Liu, Y.-C.; Song, J.; Sun, H.-H.; Liu, H.-M.; Zhang, Y.-B. Design, Synthesis and Structure-Activity Relationships of Novel Chalcone-1,2,3-triazole-azole Derivates as Antiproliferative Agents. Molecules 2016, 21, 653. https://doi.org/10.3390/molecules21050653
Zhang S-Y, Fu D-J, Yue X-X, Liu Y-C, Song J, Sun H-H, Liu H-M, Zhang Y-B. Design, Synthesis and Structure-Activity Relationships of Novel Chalcone-1,2,3-triazole-azole Derivates as Antiproliferative Agents. Molecules. 2016; 21(5):653. https://doi.org/10.3390/molecules21050653
Chicago/Turabian StyleZhang, Sai-Yang, Dong-Jun Fu, Xiao-Xin Yue, Ying-Chao Liu, Jian Song, Hui-Hui Sun, Hong-Min Liu, and Yan-Bing Zhang. 2016. "Design, Synthesis and Structure-Activity Relationships of Novel Chalcone-1,2,3-triazole-azole Derivates as Antiproliferative Agents" Molecules 21, no. 5: 653. https://doi.org/10.3390/molecules21050653
APA StyleZhang, S.-Y., Fu, D.-J., Yue, X.-X., Liu, Y.-C., Song, J., Sun, H.-H., Liu, H.-M., & Zhang, Y.-B. (2016). Design, Synthesis and Structure-Activity Relationships of Novel Chalcone-1,2,3-triazole-azole Derivates as Antiproliferative Agents. Molecules, 21(5), 653. https://doi.org/10.3390/molecules21050653