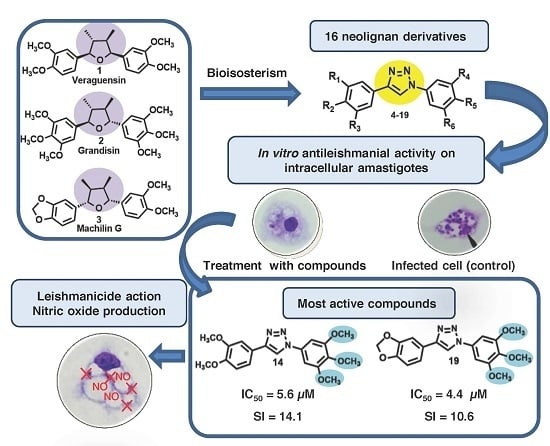
Antileishmanial Activity and Structure-Activity Relationship of Triazolic Compounds Derived from the Neolignans Grandisin, Veraguensin, and Machilin G
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
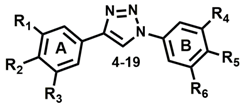
3.1. Triazole Derivatives of Neolignans
3.2. General Procedure for the Synthesis of Triazoles 4–19
3.3. ClogP
3.4. Antileishmanial Activity
3.4.1. Parasites and Peritoneal Macrophages
3.4.2. In Vitro Antileishmanial Activity on Intracellular Amastigotes
3.5. Nitric Oxide Production
3.6. Cytotoxicity Assay
3.7. Ethical Aspects
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| C.L. | Cutaneous Leishmaniasis |
| FCS | Fetal Calf Serum |
| RPMI | Roswell Park Memorial Institute |
| IC50 | Half maximal inhibitory concentration |
| DMSO | Dimethyl sulfoxide |
| S.I. | Selectivity Index |
References
- Brasil. Ministério da Saúde, Secretaria de Vigilância em Saúde. In Manual de Vigilância da Leishmaniose Tegumentar Americana/Ministério da Saúde/ Secretaria de Vigilância em Saúde., 2nd ed.; Editora do Ministério da Saúde: Brasília, Brazil, 2010; p. 180.
- WHO (World Health Organization). Leishmaniasis-Fact Sheet. Available online: http://www.who.int/mediacentre/factsheets/fs375/en/ (accessed on 11 March 2016).
- Herwaldt, B.L. Leishmaniasis. Lancet 1999, 354, 1191–1199. [Google Scholar] [CrossRef]
- Reithinger, R.; Dujardin, J.-C.; Louzir, H.; Pirmez, C.; Alexander, B.; Brooker, S. Cutaneous leishmaniasis. Lancet Infect. Dis. 2007, 7, 581–596. [Google Scholar] [CrossRef]
- Rath, S.; Trivelin, L.A.; Imbrunito, T.R.; Tomazela, D.M.; Jesús, M.N.; Marzal, P.C. Antimoniais empregados no tratamento da leishmaniose: Estado da arte. Quim. Nova 2003, 26, 550–555. [Google Scholar] [CrossRef]
- Alviano, D.S.; Barreto, A.L.S.; Dias, F.A.; Rodrigues, I.A.; Rosa, M.S.S.; Alviano, C.S.; Soares, R.M.A. Conventional therapy and promising plant-derived compounds against trypanosomatid parasites. Front. Microbiol. 2012, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Croft, S.L.; Sundar, S.; Fairlamb, A.H. Drug resistance in leishmaniases. Clin. Microbiol. Rev. 2006, 19, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.H.; Vederas, J.C. Drug discovery and natural products: end of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Lindoso, J.A.L.; Costa, J.M.L.; Queiroz, I.T.; Goto, H. Review of the current treatments for leishmaniases. Res. Rep. Trop. Med. 2012, 3, 69–77. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Rishton, G.M. Natural products as a robust source of new drugs and drug leads: Past successes and present day issues. Am. J. Cardiol. 2008, 101, 43D–49D. [Google Scholar] [CrossRef] [PubMed]
- Cassamale, T.B.; Costa, E.C.; Carvalho, D.B.; Cassemiro, N.S.; Tomazela, C.C.; Marques, M.C.S.; Ojeda, M.; Matos, M.F.C.; Albuquerque, S.; Arruda, C.C.P.; et al. Synthesis and Antitrypanosomastid Activity of 1,4-Diaryl-1,2,3-triazole Analogues of Neolignans Veraguensin, Grandisin and Machilin G. J. Braz. Chem. Soc. 2016. [Google Scholar] [CrossRef]
- Lopes, N.P.; Chicaro, R.; Kato, M.J.; Albuquerque, S.; Yoshida, M. Flavonoids and lignans from Virola surinamensis twigs and their in vitro activity against Trypanosoma cruzi. Planta Med. 1998, 64, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.B.; Souza-Fagundes, E.M.; Siqueira, H.A.J.; Leite, S.L.; Donicci, C.L.; Zani, C.L. Synthesis and evaluation of cytotoxic activity of arylfurans. Eur. J. Med. Chem. 2006, 41, 756–760. [Google Scholar] [PubMed]
- Oliveira, R.B.; Vaz, A.B.M.; Alves, R.O.; Liarte, D.B.; Donicci, C.L.; Romanha, A.J.; Zani, C.L. Arylfurans as potential Trypanosoma cruzi trypanothione reductase inhibitors. Mem. Inst. Oswaldo Cruz 2006, 101, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Silva Filho, A.A.; Costa, E.S.; Cunha, W.R.; Silva, M.L.; Nanayakkara, D.; Bastos, J.K. In vitro antileishmanial and antimalarial activities of tetrahydrofuran lignans isolated from Nectandra megapotamica (Lauraceae). Phytother. Res. 2008, 22, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Upegui, Y.; Gil, J.F.; Quiñones, W.; Torres, F.; Escobar, G.; Robledo, S.M.; Echeverri, F. Preparation of rotenone derivatives and in vitro analysis of their antimalarial, antileishmanial and selective cytotoxic activities. Molecules 2014, 19, 18911–18922. [Google Scholar] [CrossRef] [PubMed]
- Kayser, O.; Kiderlen, A.F. In vitro leishmanicidal activity of naturally occurring chalcones. Phytother. Res. 2001, 15, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, J.P.; Santos, L.S.; Santos, R.H.A.; Tozzo, E.; Ferreira, J.G.; Carmo, M.C.L.; Brasil, D.S.B.; Alves, C.N. Synthesis, X-ray crystal structure and theoretical calculations of antileishmanial neolignan analogues. J. Braz. Chem. Soc. 2010, 21, 1825–1837. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharm. Toxicol. Meth. 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Daunes, S.; D’Silva, C. QSAR Study on the contribution of log P and Es to the in vitro antiprotozoal activity of glutathione derivatives. J. Med. Chem. 2001, 44, 2976–2983. [Google Scholar] [CrossRef] [PubMed]
- Caldas, J.P.A. Investigação da Atividade Antileishmania E Citotóxica das Neolignanas Burchelina, Grandisina E Licarina A. Master’s Thesis, Universidade Federal da Paraíba, João Pessoa, Brazil, 2010. [Google Scholar]
- Bernardes, L.S.C.; Kato, M.J.; Albuquerque, S.; Carvalho, I. Synthesis and trypanocidal activity of 1,4-bis-(3,4,5-trimethoxy-phenyl)-1,4-butanediol and 1,4-bis-(3,4-dimethoxyphenyl)-1,4-butanediol. Bioorg. Med. Chem. 2006, 14, 7075–7082. [Google Scholar] [CrossRef] [PubMed]
- Konishi, T.; Konoshima, T.; Daikonya, A.; Kitanaka, S. Neolignans from Piper futokadsura and their inhibition of nitric oxide production. Chem. Pharm. Bull. 2005, 53, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Konishi, H.; Aritomi, K.; Okano, T.; Kiji, J. A mild selective monobromination reagent system for alkoxybenzenes; N-Bromosuccinimide–Silica Gel. Bull. Chem. Soc. Jpn. 1989, 62, 591–593. [Google Scholar] [CrossRef]
- Vuligonda, V.; Thacher, S.M.; Chandraratna, R.A.S. Enantioselective synthesis of potent retinoid X receptor ligands: Differential biological activities of individual antipodes. J. Med. Chem. 2001, 44, 2298–2303. [Google Scholar] [CrossRef] [PubMed]
- Dabdoub, M.J.; Baroni, A.C.M.; Lenardão, E.J.; Thiago, R.; Gianeti, T.R.; Hurtado, G.R. Synthesis of (Z)-1-phenylseleno-1,4-diorganyl-1-buten-3-ynes: Hydroselenation of symmetrical and unsymmetrical 1,4-diorganyl-1,3-butadiynes. Tetrahedron 2001, 57, 4271–4276. [Google Scholar] [CrossRef]
- Dabdoub, M.J.; Dabdoub, V.B.; Lenardão, E.J.; Hurtado, G.R.; Barbosa, S.L.; Guerrero, P.G., Jr.; Nazário, C.D.; Viana, L.H.; Santana, A.S.; Baroni, A.C.M. Synthesis of (Z)-1-Organylthiobut-1-en-3-ynes: Hydrothiolation of symmetrical and unsymmetrical buta-1,3-diynes. Synlett 2009, 6, 986–990. [Google Scholar] [CrossRef]
- Burroughs, L.; Ritchie, J.; Ngwenya, M.; Khan, D.; Lewis, W.; Woodward, S. Anionic sigmatropic-electrocyclic-Chugaev cascades: Accessing 12-aryl-5-(methylthiocarbonylthio)tetracenes and a related anthra[2,3-b]thiophene. Beilstein J. Org. Chem. 2015, 11, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Barral, K.; Moorhouse, A.D.; Moses, J.E. Efficient conversion of aromatic amines into azides: A one-pot synthesis of triazole linkages. Org. Lett. 2007, 9, 1809–1811. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Park, S.R.; Jeon, H.B.; Kim, K.S. A new solvent system for efficient synthesis of 1,2,3-triazoles. Tetrahedron Lett. 2006, 47, 5105–5109. [Google Scholar] [CrossRef]
- Viswanadhan, V.N.; Ghose, A.K.; Revankar, G.R.; Robins, R.K. Atomic physicochemical parameters for three dimensional structure directed quantitative structure-activity relationships. 4. Additional parameters for hydrophobic and dispersive interactions and their application for an automated superposition of certain naturally occurring nucleoside antibiotics. J. Chem. Inf. Comput. Sci. 1989, 29, 163–172. [Google Scholar]
- Rizk, Y.S.; Fischer, A.; Cunha, M.C.; Rodrigues, P.O.; Marques, M.C.S.; Matos, M.F.C.; Kadri, M.C.T.; Carollo, C.A.; Arruda, C.C.P. In vitro activity of the hydroethanolic extract and biflavonoids isolated from Selaginella sellowii on Leishmania (Leishmania) amazonensis. Mem. Inst. Oswaldo Cruz 2014, 109, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Paladi, C.S.; Pimentel, I.A.S.; Katz, S.; Cunha, R.L.O.R.; Judice, W.A.S.; Caires, A.C.F.; Barbieri, C.L. In vitro and in vivo activity of a palladacycle complex on Leishmania (Leishmania) amazonensis. PLoS Negl. Trop. Dis. 2012, 6, e1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, A.H.; Nathan, C.F.; Stuehr, D.J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 1988, 141, 2407–2412. [Google Scholar] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.G.F.; Silva, A.C.; Citó, A.M.; Borges, A.R.; Lima, S.G.; Lopes, J.A.; Fiqueiredo, R.C. In vitro antileishmanial activity and cytotoxicity of essential oil from Lippiasidoides Cham. Parasitol Int. 2011, 60, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 4–19 are available from the authors.







| Compounds | R1 | R2 | R3 | R4 | R5 | R6 | ClogP 1 | Intracellular Amastigotes IC50 (µM) 2 | J774.A1 Cells IC50 (µM) 3 | SI 4 |
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | -H | -OCH3 | -H | -H | -OCH3 | -H | 3.4 | 13.1 | 877.2 | 66.9 |
| 5 | -OCH3 | -OCH3 | -H | -OCH3 | -OCH3 | -H | 3.1 | 21.3 | 76.4 | 3.6 |
| 6 | -OCH3 | -OCH3 | -OCH3 | -OCH3 | -OCH3 | -OCH3 | 2.8 | 9.4 | >622.8 | >66.2 |
| 7 | -OCH2O- | -H | -OCH2O- | -H | 2.9 | 20.4 | 86.2 | 4.2 | ||
| 8 | -H | -OCH3 | -H | -OCH3 | -OCH3 | -H | 3.3 | 16.0 | 79.2 | 4.9 |
| 9 | -OCH3 | -OCH3 | -H | -H | -OCH3 | -H | 3.3 | 18.3 | 22.6 | 1.2 |
| 10 | -H | -OCH3 | -H | -OCH3 | -OCH3 | -OCH3 | 3.1 | 16.8 | 65.4 | 3.9 |
| 11 | -OCH3 | -OCH3 | -OCH3 | -H | -OCH3 | -H | 3.1 | 16.5 | 67.5 | 4.1 |
| 12 | -H | -OCH3 | -H | -OCH2O- | -H | 3.2 | 14.8 | 96.5 | 6.5 | |
| 13 | -OCH2O- | -H | -H | -OCH3 | -H | 3.2 | 50.7 | 134.6 | 2.6 | |
| 14 | -OCH3 | -OCH3 | -H | -OCH3 | -OCH3 | -OCH3 | 2.9 | 5.6 | 79.1 | 14.1 |
| 15 | -OCH3 | -OCH3 | -OCH3 | -OCH3 | -OCH3 | -H | 2.9 | 29.2 | 76.9 | 2.6 |
| 16 | -OCH3 | -OCH3 | -H | -OCH2O- | -H | 3.0 | 32.7 | >768.5 | >23.5 | |
| 17 | -OCH2O- | -H | -OCH3 | -OCH3 | -H | 3.0 | 29.9 | 9.2 | 0.3 | |
| 18 | -OCH3 | -OCH3 | -OCH3 | -OCH2O- | -H | 2.9 | 29.8 | 66.9 | 2.2 | |
| 19 | -OCH2O- | -H | -OCH3 | -OCH3 | -OCH3 | 2.9 | 4.4 | 46.9 | 10.6 | |
| Doxorubicin 5 | - | 0.5 | - | |||||||
| Amphotericin B 5 | 0.7 | 2.2 | 3.1 | |||||||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, E.C.; Cassamale, T.B.; Carvalho, D.B.; Bosquiroli, L.S.S.; Ojeda, M.; Ximenes, T.V.; Matos, M.F.C.; Kadri, M.C.T.; Baroni, A.C.M.; Arruda, C.C.P. Antileishmanial Activity and Structure-Activity Relationship of Triazolic Compounds Derived from the Neolignans Grandisin, Veraguensin, and Machilin G. Molecules 2016, 21, 802. https://doi.org/10.3390/molecules21060802
Costa EC, Cassamale TB, Carvalho DB, Bosquiroli LSS, Ojeda M, Ximenes TV, Matos MFC, Kadri MCT, Baroni ACM, Arruda CCP. Antileishmanial Activity and Structure-Activity Relationship of Triazolic Compounds Derived from the Neolignans Grandisin, Veraguensin, and Machilin G. Molecules. 2016; 21(6):802. https://doi.org/10.3390/molecules21060802
Chicago/Turabian StyleCosta, Eduarda C., Tatiana B. Cassamale, Diego B. Carvalho, Lauriane S. S. Bosquiroli, Mariáh Ojeda, Thalita V. Ximenes, Maria F. C. Matos, Mônica C. T. Kadri, Adriano C. M. Baroni, and Carla C. P. Arruda. 2016. "Antileishmanial Activity and Structure-Activity Relationship of Triazolic Compounds Derived from the Neolignans Grandisin, Veraguensin, and Machilin G" Molecules 21, no. 6: 802. https://doi.org/10.3390/molecules21060802