Antioxidative, Antibacterial, and Food Functional Properties of the Half-Fin Anchovy Hydrolysates-Glucose Conjugates Formed via Maillard Reaction
Abstract
:1. Introduction
2. Results and Discussion
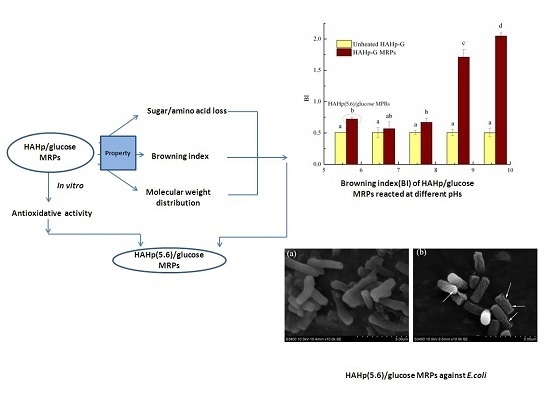
2.1. Characteristics of Sugar and Amino Acid Contents and Browning Index of HAHp-G MRPs
2.2. Molecular Weight Distribution of HAHp-G MRPs
2.3. Antioxidative Activity of HAHp-G MRPs
2.4. Antibacterial Activity of HAHp(5.6)-G MRPs
2.4.1. Antibacterial Spectra and the Minimal Inhibitory Concentration (MIC)
2.4.2. Scanning Electron Microscopy (SEM)
2.5. Functional Properties
3. Materials and Methods
3.1. Materials
3.2. Preparation of HAHp-G MRPs
3.3. Sugar and Amino Acid Content of HAHp-G MRPs
3.4. Measurement of BI
3.5. Molecular Weight Distribution of HAHp-G MRPs
3.6. Determination of Antioxidative Activity of HAHp-G MRPs
3.6.1. Reducing Power Assay
3.6.2. DPPH Radical Scavenging Activity Assay
3.7. Antibacterial Activity
3.7.1. Determination of Antibacterial Spectra and MIC
3.7.2. Scanning Electron Microscopy (SEM)
3.8. Determination of Food Functional Properties
3.8.1. Foaming Capacity and Stability
3.8.2. Emulsifying property
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MR | Maillard reaction |
| MRPs | Maillard reaction products |
| DPPH | 1,1-diphenyl-2-picrylhydrazayl |
| HAHp | the peptic hydrolysates of half-fin anchovy |
| HAHp-G MRPs | the half-fin anchovy hydrolysates-glucose Maillard reaction products |
| BI | browning intensity |
| BSA | bovine serum albumin |
| GSSG | oxidized glutathione |
| GSH | reduced glutathione |
| MIC | the minimal inhibitory concentration |
| SEM | scanning electron microscopy |
| FC | foaming capacity |
| FS | foaming stability |
| EAI | emulsifying activity index |
| ESI | emulsion stability index |
References
- Hodge, J.E. Dehydrated foods, chemistry of browning reactions in model systems. J. Agric. Food Chem. 1953, 1, 928–943. [Google Scholar] [CrossRef]
- Acquistucci, R. Influence of Maillard reaction on protein modification and colour development in Pasta. Comparison of different drying conditions. LWT-Food Sci. Technol. 2000, 33, 48–52. [Google Scholar] [CrossRef]
- Andrewes, P. Changes in Maillard reaction products in ghee during storage. Food Chem. 2012, 135, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Chobert, J.M.; Gaudin, J.C.; Dalgalarrondo, M.; Haertlé, T. Impact of Maillard type glycation on properties of beta-lactoglobulin. Biotechnol Adv. 2006, 24, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, M.; He, C.; Song, H.; Chen, F. Effect of thermal treatment on the flavor generation from Maillard reaction of xylose and chicken peptide. LWT-Food Sci. Technol. 2015, 64, 316–325. [Google Scholar] [CrossRef]
- Franzke, C.; Iwainsky, H. Zurantioxidativen Wirksamkeit der melanoidine. Dtsch. Lebensm. Rundsch. 1954, 50, 251–254. [Google Scholar]
- Chawla, S.P.; Chander, R.; Sharma, A. Antioxidant formation by γ-irradiation of glucose–amino acid model systems. Food Chem. 2007, 103, 1297–1304. [Google Scholar] [CrossRef]
- Virág, D.; Kiss, A.; Forgó, P.; Csutorás, C.; Molnár, S. Study on Maillard-reaction driven transformations and increase of antioxidant activity in lysine fortified biscuits. Microchem. J. 2013, 107, 172–177. [Google Scholar] [CrossRef]
- Gu, F.L.; Abbas, S.; Zhang, X.M. Optimization of Maillard reaction products from casein–glucose using response surface methodology. LWT-Food Sci. Technol. 2009, 42, 1374–1379. [Google Scholar] [CrossRef]
- Gu, F.L.; Kim, J.M.; Abbas, S.; Zhang, X.M.; Xia, S.Q.; Chen, Z.X. Structure and antioxidant activity of high molecular weight Maillard reaction products from casein–glucose. Food Chem. 2010, 120, 505–511. [Google Scholar] [CrossRef]
- Joubran, Y.; Mackie, A.; Lesmes, U. Impact of the Maillard reaction on the antioxidant capacity of bovine lactoferrin. Food Chem. 2013, 141, 3796–3802. [Google Scholar] [CrossRef] [PubMed]
- Lertittikul, W.; Benjakul, S.; Tanaka, M. Characteristics and antioxidative activity of Maillard reaction products from a porcine plasma protein–glucose model system as influenced by pH. Food Chem. 2007, 100, 669–677. [Google Scholar] [CrossRef]
- Wang, W.Q.; Bao, Y.H.; Chen, Y. Characteristics and antioxidant activity of water-soluble Maillard reaction products from interactions in a whey protein isolate and sugars system. Food Chem. 2013, 139, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Tan, C.; Huang, M.; Liu, P.; Eric, K.; Zhang, X.; Xia, S.; Jia, C. Transglutaminase cross-linking effect on sensory characteristics and antioxidant activities of Maillard reaction products from soybean protein hydrolysates. Food Chem. 2013, 136, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.A.; Farah, A.; Silva, D.A.; Nunan, E.A.; Gloria, M.B. Antibacterial activity of coffee extracts and selected coffee chemical compounds against enterobacteria. J. Agric. Food Chem. 2006, 54, 8738–8743. [Google Scholar] [CrossRef] [PubMed]
- Rufian-Henares, J.A.; de la Cueva, S.P. Antimicrobial activity of coffee melanoidins – A study of their metal-chelating properties. J. Agric. Food Chem. 2009, 57, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Rufian-Henares, J.A.; Morales, F.J. Antimicrobial activity of melanoidins against Escherichia coli is mediated by a membrane-damage mechanism. J. Agric. Food Chem. 2008, 56, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kato, A.; Kobayashi, K. Bifunctional lysozyme-galactomannan conjugate having excellent emulsifying properties and bactericidal effect. J. Agric. Food Chem. 1992, 40, 735–739. [Google Scholar] [CrossRef]
- Song, Y.; Babiker, E.E.; Usui, M.; Saito, A.; Kato, A. Emulsifying properties and bactericidal action of chitosan-lysozyme conjugates. Food Res. Int. 2002, 35, 459–466. [Google Scholar] [CrossRef]
- Liang, C.; Yuan, F.; Liu, F.; Wang, Y.; Gao, Y. Structure and antimicrobial mechanism of ε-polylysine–chitosan conjugates through Maillard reaction. Int. J. Biol. Macromol. 2014, 70, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, F.; Chobert, J.M.; Popineau, Y.; Nicolas, M.G.; Haertlé, T. Improvement of the functional properties of β-lactoglobulin glycated through the Maillard reaction is related to the sugar nature. Int. Dairy J. 2001, 11, 145–152. [Google Scholar] [CrossRef]
- Corzo-Martínez, M.; Sánchez, C.C.; Moreno, F.J.; Patino, J.M.P.; Villamie, M. Interfacial and foaming properties of bovine b-lactoglobulin: Galactose Maillard conjugates. Food Hydrocolloid. 2012, 27, 438–447. [Google Scholar] [CrossRef] [Green Version]
- Nacka, F.; Chobert, J.M.; Burova, T.; Léonil, J.; Haertlé, T. Induction of new physicochemical and functional properties by the glycosylation of whey proteins. J. Protein Chem. 1988, 17, 495–503. [Google Scholar] [CrossRef]
- Hiller, B.; Lorenzen, P.C. Functional properties of milk proteins as affected by Maillard reaction induced oligomerisation. Food Res. Int. 2010, 43, 1155–1166. [Google Scholar] [CrossRef]
- Honda, A.; Huroda, N. Functional improvement in dried egg white through the Maillard reaction. J. Agric. Food Chem. 1999, 47, 1845–1850. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, G.; Zhao, M.; Ren, J.; Yang, B. Improvement of functional properties of peanut protein isolate by conjugation with dextran through Maillard reaction. Food Chem. 2012, 131, 901–906. [Google Scholar] [CrossRef]
- Chung, Y.C.; Kuo, C.L.; Chen, C.C. Preparation and important functional properties of water-soluble chitosan produced through Maillard reaction. Bioresour. Technol. 2005, 96, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, G.B.; Malec, L.S.; Vigo, M. Reducing sugars effect on available lysine loss of casein by moderate heat treatment. Food Chem. 1998, 62, 309–313. [Google Scholar] [CrossRef]
- Somoza, V. Five years of research on health risks and benefits of Maillard reaction products: An update. Mol. Nutr. Food Res. 2005, 49, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Qian, H.; Yao, W.R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Pan, G.G.; Melton, L.D. Nonenzymatic browning of lactose and caseinate during dry heating at different relative humidities. J. Agric. Food Chem. 2007, 55, 10036–10042. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.S.; Park, M.R.; Lee, K.W.; Kim, S.H.; Kim, Y. Dietary Maillard reaction products and their fermented products reduce cardiovascular risk in an animal model. J. Dairy Sci. 2015, 98, 5102–5012. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, L.; Li, F.; Wang, C.; Yuan, D.; Chen, J.; Tan, L.; Jin, Z.; Ma, W. Acute and sub-chronic toxicity of glucose–cysteine Maillard reaction products in Sprague-Dawley rats. Food Chem. Toxicol. 2015, 80, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Wei, R.B.; Zhang, B.; Yang, Z.S.; Wang, D.F. Antioxidant and antiproliferative activities of heated sterilized pepsin hydrolysate derived from half-fin anchovy (Setipinna taty). Mar. Drugs 2011, 9, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Wei, R.B.; Zhang, B.; Wang, D.F. Optimization of the antibacterial activity of half-fin anchovy (Setipinna taty) hydrolysates. Food Bioprocess Tech. 2012, 5, 1979–1989. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Seiquer, I.; Haro, A.; Castellano, R.; Navaro, M.P. Development of the Maillard reaction in foods cooked by different techniques. Intake of Maillard-derived compounds. Food Chem. 2010, 122, 145–153. [Google Scholar] [CrossRef]
- Miranda, L.T.; Rakovski, C.; Were, L.M. Effect of Maillard reaction products on oxidation products in ground chicken breast. Meat Sci. 2012, 90, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.; Ames, J.M.; Mac Dougall, D.B.; Taylor, P.C. Laboratory reaction cell to model Maillard reaction colour development in a starch–glucose–lysine system. J. Food Sci. 1998, 63, 1991–1996. [Google Scholar]
- Ajandouz, E.H.; Desseaux, V.; Tazi, S.; Puigserver, A. Effects of temperature and pH on the kinetics of caramelisation, protein cross-linking and Maillard reactions in aqueous model systems. Food Chem. 2008, 107, 1244–1252. [Google Scholar] [CrossRef]
- Su, G.; Zheng, L.; Cui, C.; Yang, B.; Ren, J.; Zhao, M. Characterization of antioxidant activity and volatile compounds of Maillard reaction products derived from different peptide fractions of peanut hydrolysate. Food Res. Int. 2011, 44, 3250–3258. [Google Scholar] [CrossRef]
- Jing, H.; Yap, M.; Wong, P.Y.Y.; Kitts, D.D. Comparison of physicochemical and antioxidant properties of egg-white proteins and fructose and inulin Maillard reaction products. Food Bioprocess Tech. 2011, 4, 1489–1496. [Google Scholar] [CrossRef]
- Mastrocola, D.; Munari, M. Progress of the Maillard reaction and antioxidant action of Maillard reaction products in preheated model systems during storage. J. Agric. Food Chem. 2000, 48, 3555–3559. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Kitts, D.D. Chemical and biochemical properties of casein–sugar Maillard reaction products. Food Chem. Toxicol. 2000, 40, 1007–1015. [Google Scholar] [CrossRef]
- Yanagimoto, K.; Lee, K.G.; Ochi, H.; Shibamoto, T. Antioxidative activity of heterocyclic compounds formed in Maillard reaction products. Int. Congress Series 2002, 1245, 335–340. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Iijima, T.; Watanabe, T.; Nakazawa, H. Antioxidative effect of Maillard reaction products using glucose–glycine model system. J. Agric. Food Chem. 1997, 45, 4106–4109. [Google Scholar] [CrossRef]
- Liardon, R.; Friedman, M. Effect of peptide bond cleavage on the racemization of amino acid residues in proteins. J. Agric. Food Chem. 1987, 35, 661–667. [Google Scholar] [CrossRef]
- Damodaran, S. Amino acids, peptides and proteins. In Food Chemistry, 3nd ed.; Fennema, O.R., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1996; pp. 321–430. [Google Scholar]
- Gautieri, A.; Redaelli, A.; Buehler, M.J.; Vesentini, S. Age- and diabetes-related nonenzymatic crosslinks in collagen fibrils: Candidate amino acids involved in Advanced Glycation End-products. Matrix Biol. 2014, 34, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srikanth, V.; Maczurek, A.; Phan, T.; Steele, M.; Westcott, B.; Juskiw, D.; Münch, G. Advanced glycation endproducts and their receptor RAGE in Alzheimer's disease. Neurobiol. Aging 2011, 32, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.L.; Chen, Y.C.; Tan, F.J. Antioxidative properties of a chitosan–glucose Maillard reaction product and its effect on pork qualities during refrigerated storage. Food Chem. 2011, 124, 589–595. [Google Scholar] [CrossRef]
- Gao, P.S.; Zhu, Z.Q.; Zhang, P. Effects of chitosan–glucose complex coating on postharvest quality and shelf life of table grapes. Carbohydr. Polym. 2013, 95, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.J.; Feng, L.F.; Li, J.R. Changes in microbial and postharvest quality of shiitake mushroom (Lentinus edodes) treated with chitosan–glucose complex coating under cold storage. Food Chem. 2012, 131, 780–786. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chander, R.; Sharma, A. Chitosan glucose complex—A novel food preservative. Food Chem. 2008, 106, 521–528. [Google Scholar] [CrossRef]
- Hauser, C.; Müller, U.; Sauer, T.; Augner, K.; Pischetsrieder, M. Maillard reaction products as antimicrobial components for packaging films. Food Chem. 2014, 145, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Mutilangi, W.A.M.; Panyam, D.; Kilara, A. Biochemical properties of hydrolysates from proteolysis of heat-denatured whey protein isolate. J. Food Sci. 1996, 61, 270–274, 303. [Google Scholar] [CrossRef]
- Fechner, A.; Knoth, A.; Scherze, I.; Muschiolik, G. Stability and release properties of double-emulsions stabilised by caseinate–dextran conjugates. Food Hydrocolloid. 2007, 21, 943–952. [Google Scholar] [CrossRef]
- Wooster, T.J.; Augustin, M.A. β-Lactoglobulin–dextran–Maillard conjugates: Their effect on interfacial thickness and emulsion stability. J. Colloid Interfaces Sci. 2006, 303, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.T.; Kilara, A. Studies on whey protein concentrates. 2. Foaming and emulsifying properties and their relationships with physicochemical properties. J. Dairy Sci. 1990, 73, 2731–2740. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, X.J.; Li, H.; Gao, W.Y. Determination of steady-state kinetic parameters of 1-deoxy-D-xylulose-5-phosphate synthase by pre-column derivatization high performance liquid chromatography using 2,4-dinitrophenylhydrazine as derivative reagent. Chinese J. Anal. Chem. 2012, 40, 1859–1864. [Google Scholar] [CrossRef]
- Vhangani, L.N.; Wyk, J.V. Antioxidant activity of Maillard reaction products (MRPs) derived from fructose–lysine and ribose–lysine model systems. Food Chem. 2013, 137, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Samaras, T.S.; Camburn, P.A.; Chandra, S.X.; Gordon, M.H. Antioxidant properties of kilned and roasted malts. J. Agric. Food Chem. 2005, 53, 8068–8074. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Friedrich, C.L.; Moyles, D.; Beveridge, T.J.; Hancock, R.E.W. Antibacterial action of structurally diverse cationic peptides on Gram-positive bacteria. Antimicrob. Agents Chemother. 2000, 44, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Sathe, S.K.; Salunkhe, D.K. Biochemical properties of the Great Northern Bean (Phaseolus vulgaris L.) proteins: emulsion, foaming, viscosity and gelation properties. J. Food Sci. 1981, 46, 71–74, 81. [Google Scholar] [CrossRef]
- Song, R.; Wei, R.B.; Luo, H.Y. Biochemical properties and stability of antioxidative activity of half-fin anchovy (Setipinna taty) fermented product. J. Aquat. Food Prod. Technol. 2015, 24, 397–410. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the prepared half-fin anchovy MRPs are available from the authors.





| Bacterial Strains | Inhibition Zone Diameter (mm) | MIC (μg/mL) |
|---|---|---|
| Gram-negative | ||
| E. coli | 28.2 ± 0.06 | 8.3 |
| P. aeruginosa | 13.1 ± 0.00 | 16.7 |
| P. vulgaris | 22.1 ± 0.01 | 8.3 |
| P. fluorescens | 21.8 ± 0.30 | 8.3 |
| Gram-positive | ||
| B. subtilis | 22.8 ± 0.30 | 8.3 |
| B. megaterium | 12.5 ± 0.09 | 16.7 |
| S. lutea | 11.9 ± 0.01 | 16.7 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, R.; Yang, P.; Wei, R.; Ruan, G. Antioxidative, Antibacterial, and Food Functional Properties of the Half-Fin Anchovy Hydrolysates-Glucose Conjugates Formed via Maillard Reaction. Molecules 2016, 21, 795. https://doi.org/10.3390/molecules21060795
Song R, Yang P, Wei R, Ruan G. Antioxidative, Antibacterial, and Food Functional Properties of the Half-Fin Anchovy Hydrolysates-Glucose Conjugates Formed via Maillard Reaction. Molecules. 2016; 21(6):795. https://doi.org/10.3390/molecules21060795
Chicago/Turabian StyleSong, Ru, Peiyu Yang, Rongbian Wei, and Guanqiang Ruan. 2016. "Antioxidative, Antibacterial, and Food Functional Properties of the Half-Fin Anchovy Hydrolysates-Glucose Conjugates Formed via Maillard Reaction" Molecules 21, no. 6: 795. https://doi.org/10.3390/molecules21060795