Essential Oil Variation from Twenty Two Genotypes of Citrus in Brazil—Chemometric Approach and Repellency Against Diaphorina citri Kuwayama
Abstract
:1. Introduction
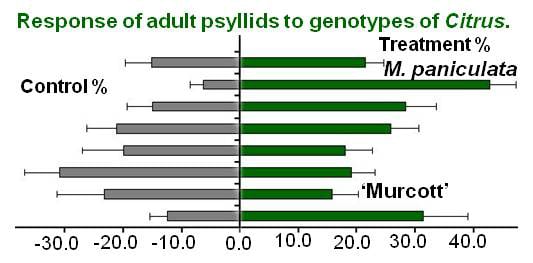
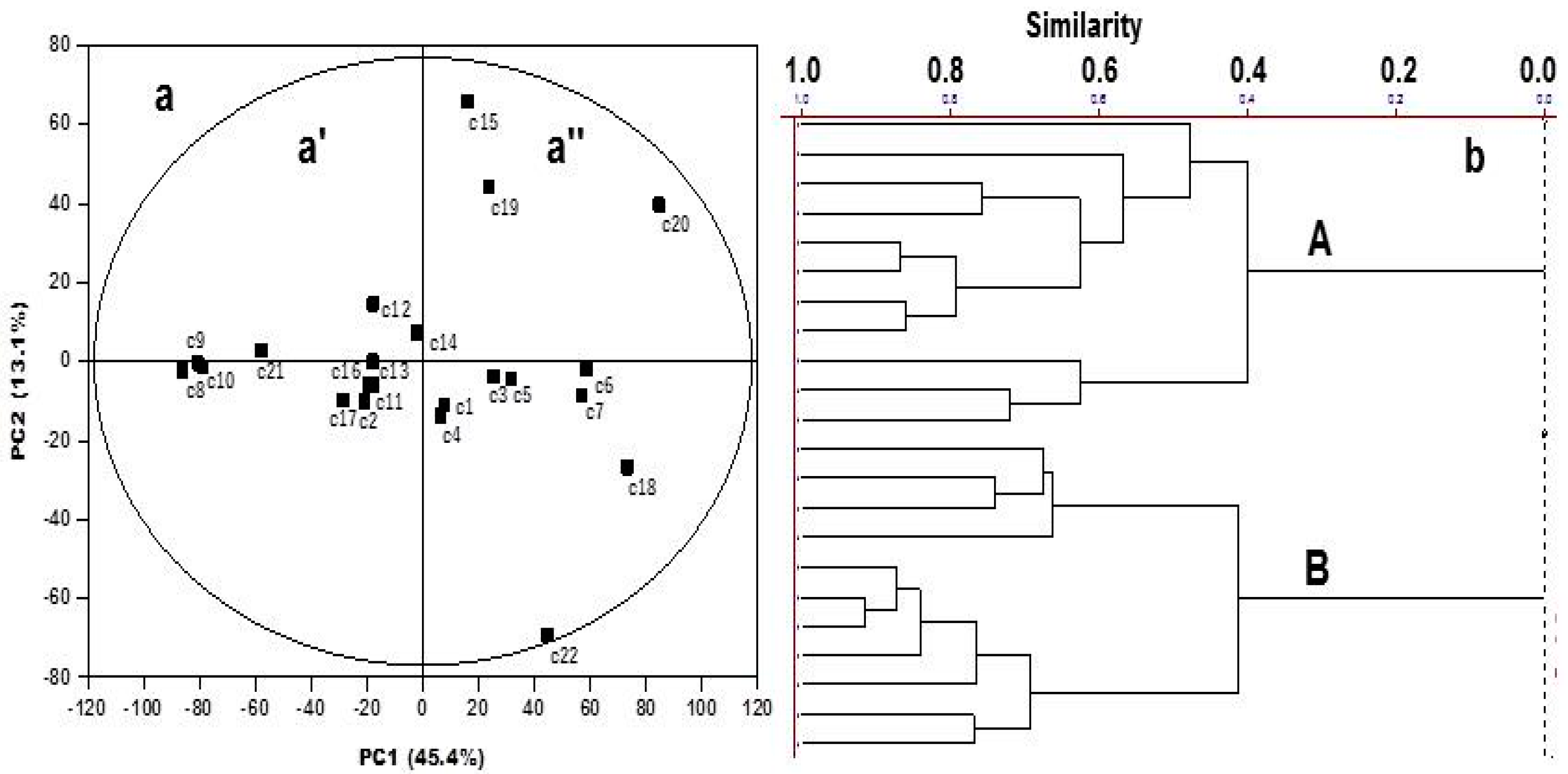
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material
3.2. Diaphorina Citri Rearing
3.3. Isolation and Analysis of Essential Oils
3.4. Multivariate and Statistical Analysis
3.5. Olfactory Bioassays with the 22 Genotypes of Citrus
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| GC-MS | Gas Chromatography-Mass Spectrometry |
| PCA | Principal Component Analysis |
| PCs | Principal Components |
References
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of Citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Johnson, E.G.; Wu, J.; Bright, D.B.; Graham, J.H. Association of ‘Candidatus Liberibacter asiaticus’ root infection, but not phloem plugging with root loss on huanglongbing-affected trees prior to appearance of foliar symptoms. Plant Pathol. 2014, 63, 290–298. [Google Scholar] [CrossRef]
- Bassanezi, R.B.; Montesino, L.H.; Gasparoto, M.C.G.; Bergamin, A.; Amorim, L. Yield loss caused by huanglongbing in different sweet orange cultivars in Sao Paulo, Brazil. Eur. J. Plant Pathol. 2011, 130, 577–586. [Google Scholar] [CrossRef]
- Yamamoto, P.T.; Gravena, S. Espécies e abundância de cigarrinhas e psilídeos em pomares cítricos. Ann. Soc. Entomol. Brasil 2000, 29, 169–176. [Google Scholar] [CrossRef]
- Coletta-Filho, H.D.; Targon, M.L.P.N.; Takita, M.A.; de Negri, J.D.; Pompeu jr., J.; Machado, M.A. First report of the causal agent of Huanglongbing (“Candidatus Liberibacter asiaticus”) in Brazil. Plant Dis. 2004, 88, 1382. [Google Scholar] [CrossRef]
- Chakravarthi, V.P.; Savithri, P.; Prasad, P.R.; Naidu, V.G.; Reddy, P.P.; Kumar, N.K.K.; Verghese, A. Relative susceptibility of Citrus germplasm to Citrus psylla, Diaphorina citri Kuwayama (Homoptera: Psyllidae). In Advances in IPM for horticultural crops. Proceedings of the First National Symposium on Pest Management in Horticultural Crops: Environmental implications and thrusts, Bangalore, India; 1998; pp. 30–31. [Google Scholar]
- Tsai, J.H.; Liu, Y.H. Biology of Diaphorina citri (Homoptera: Psyllidae) on four host plants. J. Econ. Entomol. 2000, 93, 1721–1725. [Google Scholar] [CrossRef] [PubMed]
- Nava, D.E.; Torres, M.L.; Rodrigues, G.M.D.L.; Bento, J.M.S.; Parra, J.R.P. Biology of Diaphorina citri (Hem., Psyllidae) on different hosts and at different temperatures. J App. Entomol. 2007, 131, 709–715. [Google Scholar] [CrossRef]
- Ikeda, K.; Ashihara, W. Preference of adult Asian Citrus psyllid, Diaphorina citri (Homoptera: Psyllidae) for Murraya paniculata and Citrus unshiu. Jpn. J. Appl. Entomol. Zool 2008, 52, 27–30. [Google Scholar] [CrossRef]
- Westbrook, C.J.; Hall, D.G.; Stover, E.; Duan, Y.P.; Lee, R.F. Colonization of Citrus-related germplasm by Diaphorina citri (Hemiptera: Psyllidae). Hort Sci. 2011, 46, 997–1005. [Google Scholar]
- Richardson, M.L.; Hall, D.G. Resistance of Poncirus and Citrus × Poncirus germplasm to the Asian Citrus psyllid. Crop. Sci. 2013, 53, 183–188. [Google Scholar] [CrossRef]
- Miresmailli, S.; Isman, M.B. Efficacy and persistence of rosemary oil as an acaricide against two spotted spider mite (Acari: Tetranychidae) on greenhouse tomato. J. Econ. Entomol. 2006, 99, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Borgoni, P.C.; Vendramim, J.D.; Lourencão, A.L.; Machado, M.A. Resistance of Citrus and related genera to Diaphorina citri Kuwayama (Hemiptera: Liviidae). Neotrop. Entomol. 2014, 43, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Sétamou, M.; Flores, D.; French, J.V.; Hall, D.G. Dispersion patterns and sampling plans for Diaphorina citri (Hemiptera: Psyllidae) in Citrus. J. Econ. Entomol. 2008, 101, 1478–1487. [Google Scholar] [CrossRef]
- Padín, S.B.; Fusé, C.; Urrutia, M.I.; Dal Bello, G.M. Toxicity and repellency of nine medicinal plants against Tribolium castaneum in stored wheat. Bull. Insectology 2013, 66, 45–49. [Google Scholar]
- Nishida, R.; Ohsugi, T.; Kokubo, S.; Fukami, H. Oviposition stimulants of a Citrus-feeding swallowtail butterfly, Papilio xuthus L. Experientia 1987, 43, 342–344. [Google Scholar] [CrossRef]
- Lopes, S.A.; Frare, G.F. Graft transmission and cultivar reaction of Citrus to ‘Candidatus Liberibacter americanus’. Plant Dis. 2008, 92, 21–24. [Google Scholar] [CrossRef]
- Van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; p. 804. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.



| Citrus Genotype | Index of Repellency (IR) |
|---|---|
| C. sinensis (sweet orange) cv. ‘Pera’ (C-1) | 0.92 ± 0.17 |
| C. sinensis (sweet orange) cv. ‘Natal’ (C-2) | 1.29 ± 0.17 |
| C. sinensis (sweet orange) cv. ‘Valencia’ (C-3) | 1.20 ± 0.16 |
| C. sinensis (sweet orange) cv. ‘Washington Navel’ (‘Bahia’) (C-4) | 1.25 ± 0.20 |
| C. sinensis (sweet orange) cv. ‘Hamlin’ (C-5) | 1.04 ± 0.18 |
| C. reticulata Blanco (tangerine or mandarin) cv. ‘Cravo’ (C-6) | 1.11 ± 0.15 |
| C. reticulata Blanco (tangerine or mandarin) cv. ‘Ponkan’ (C-7) | 1.58 ± 0.17 |
| C. deliciosa Tenore (mandarin) cv. ‘Mexerica-do-rio’ (C-8) | 1.23 ± 0.08 |
| C. limettioides Tanaka (sweet lime) cv ‘Palestine’ (C-9) | 1.22 ± 0.24 |
| C. latifolia Tanaka (lime) cv. ‘Tahiti’ (C-10) | 1.29 ± 0.17 |
| C. paradisi Mcf. (grapefruit) cv. ‘Marsh Seedless’ (C-11) | 1.39 ± 0.14 |
| C. limon (L.) Burm. F. (Sicilian lemon) (C-12) | 1.32 ± 0.14 |
| C. aurantium L. (sour orange) (C-13) | 1.16 ± 0.21 |
| C. grandis Osbeck (sweet pummel) (C-14) | 1.15 ± 0.19 |
| C. medica L. (citron) (C-15) | 1.43 ± 0.18 |
| C. reticulata L. × C. sinensis L. (tangor) ‘Murcott’ (C-16) | 0.81 ± 0.16 |
| C. paradisi × P. trifoliata (citrumelo) cv. ‘Swingle’ (C-17) | 0.76 ± 0.19 |
| P. trifoliata × C. sinensis (citrange) cv. ‘Troyer’ (C-18) | 0.95 ± 0.25 |
| C. sunki hort. ex Tanaka × P. trifoliata L. Raf. (citrandarin) ‘English’ (C-19) | 1.10 ± 0.20 |
| P. trifoliata L. Raf. (poncirus) cv ‘Rubidoux’ (C-20) | 1.31 ± 0.21 |
| M. paniculata L. Jack (orange jasmine) (C-21) | 1.75 ± 0.07 |
| F. margarita Lour. (kumquat) (C-22) | 1.18 ± 0.20 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, M.D.S.; Ribeiro, L.D.P.; Borgoni, P.C.; Silva, M.F.d.G.F.d.; Forim, M.R.; Fernandes, J.B.; Vieira, P.C.; Vendramin, J.D.; Machado, M.A. Essential Oil Variation from Twenty Two Genotypes of Citrus in Brazil—Chemometric Approach and Repellency Against Diaphorina citri Kuwayama. Molecules 2016, 21, 814. https://doi.org/10.3390/molecules21060814
Andrade MDS, Ribeiro LDP, Borgoni PC, Silva MFdGFd, Forim MR, Fernandes JB, Vieira PC, Vendramin JD, Machado MA. Essential Oil Variation from Twenty Two Genotypes of Citrus in Brazil—Chemometric Approach and Repellency Against Diaphorina citri Kuwayama. Molecules. 2016; 21(6):814. https://doi.org/10.3390/molecules21060814
Chicago/Turabian StyleAndrade, Moacir Dos Santos, Leandro Do Prado Ribeiro, Paulo Cesar Borgoni, Maria Fátima das Graças Fernandes da Silva, Moacir Rossi Forim, João Batista Fernandes, Paulo Cezar Vieira, José Djair Vendramin, and Marcos Antônio Machado. 2016. "Essential Oil Variation from Twenty Two Genotypes of Citrus in Brazil—Chemometric Approach and Repellency Against Diaphorina citri Kuwayama" Molecules 21, no. 6: 814. https://doi.org/10.3390/molecules21060814