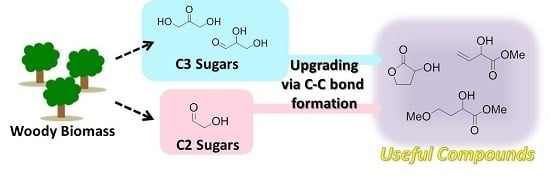
A Novel Strategy for Biomass Upgrade: Cascade Approach to the Synthesis of Useful Compounds via C-C Bond Formation Using Biomass-Derived Sugars as Carbon Nucleophiles
Abstract
:1. Introduction
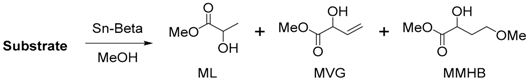
2. Cascade Synthesis Using Biomass-Derived Oxygenates as Carbon Nucleophiles
3. Cascade Synthesis of Useful Four-Carbon Products via an Intermolecular Aldol Reaction between Biomass-Derived Triose Sugars and Formaldehyde
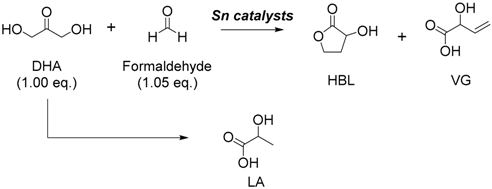
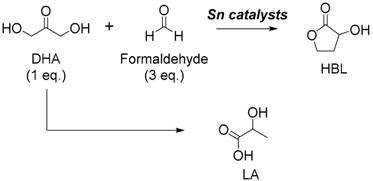
3.1. Tin-Catalyzed Conversion of the Biomass-Derived Triose Sugar 1,3-Dihydroxyacetone (DHA) to α-Hydroxy-γ-butyrolactone (HBL) in the Presence of Formaldehyde
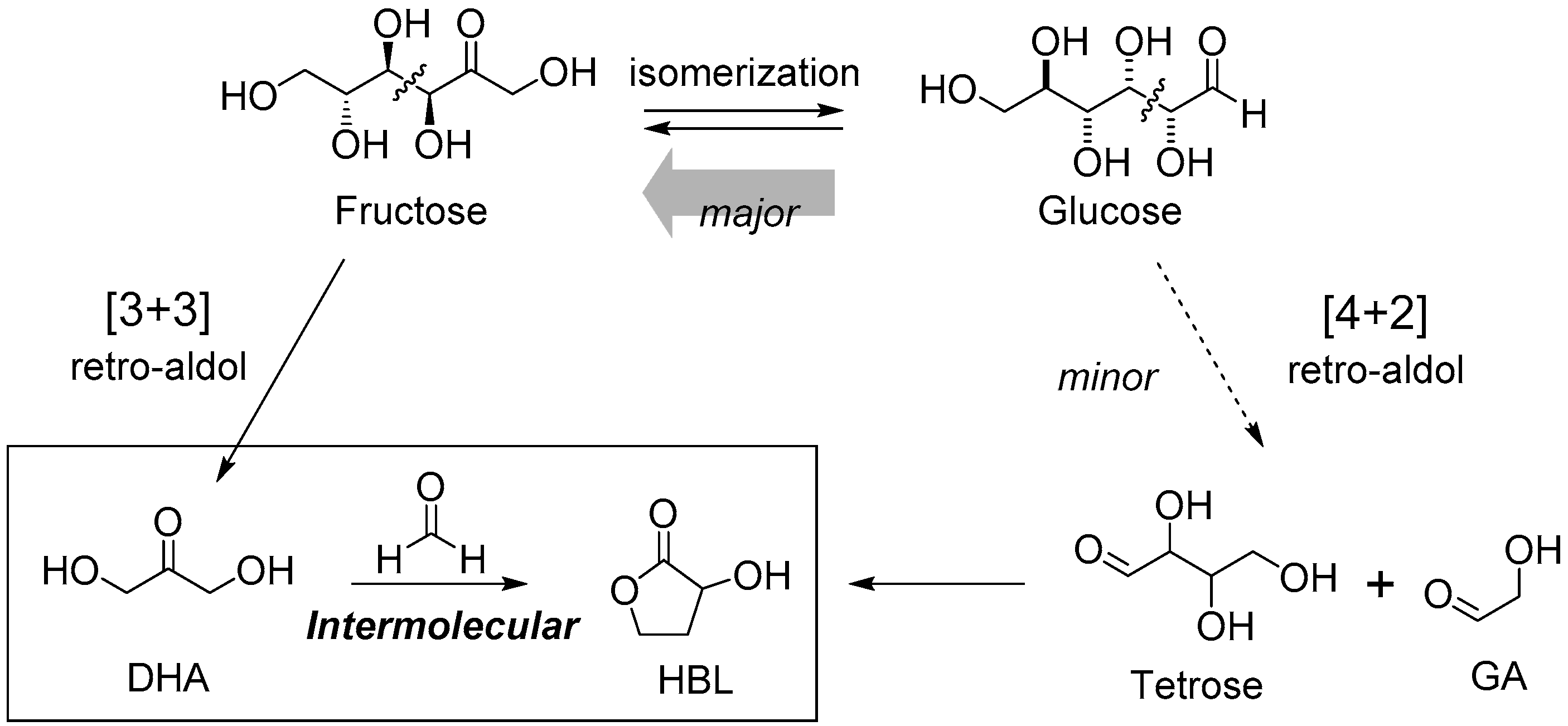
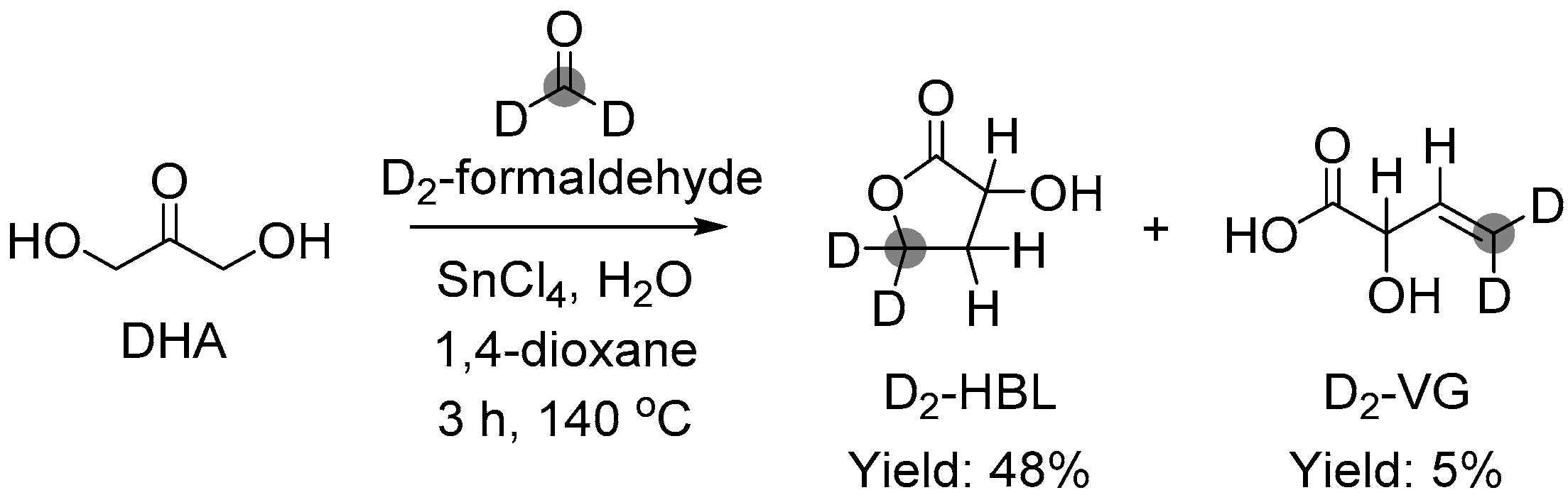
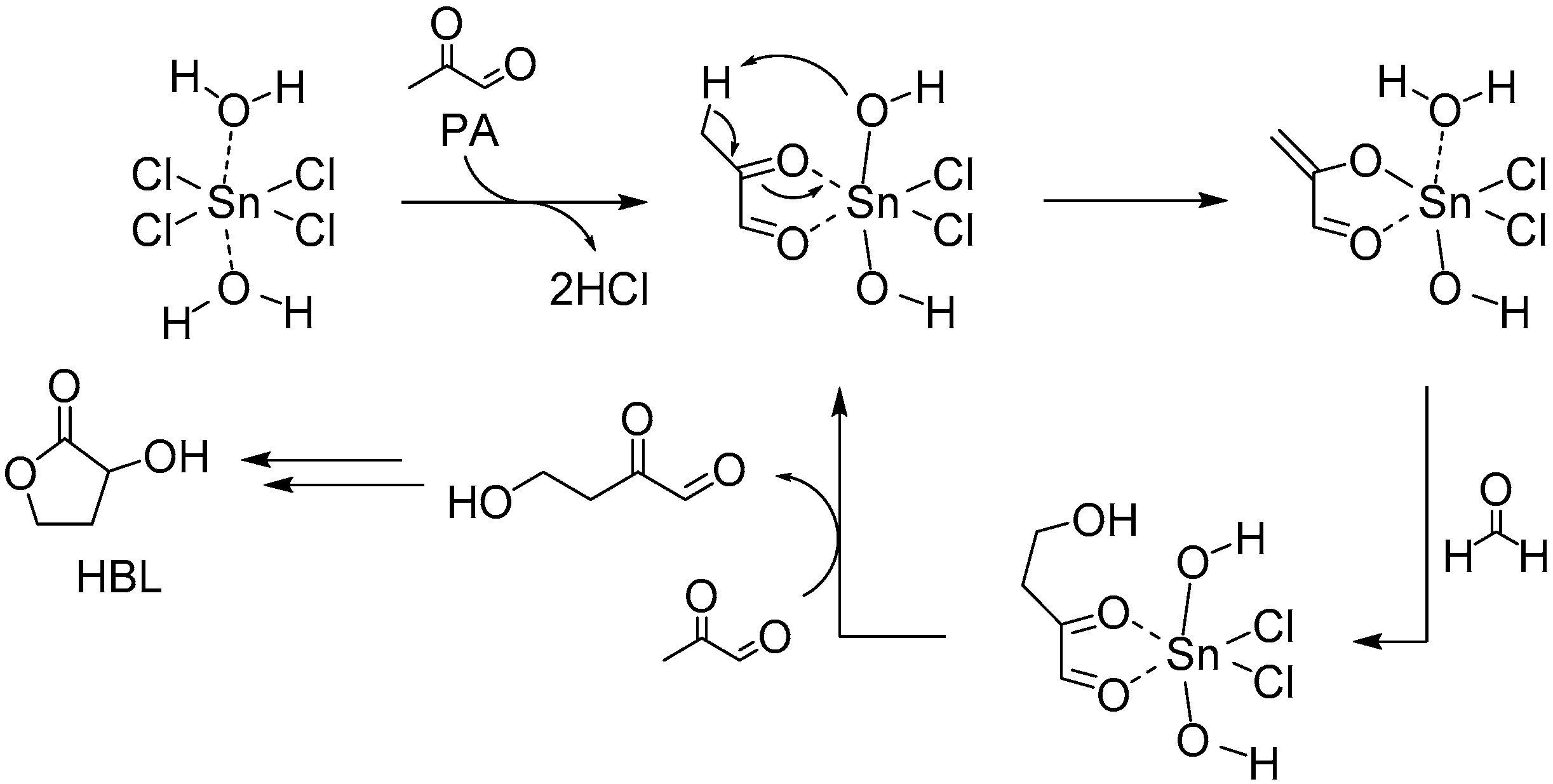
3.1.1. Cascade Reaction Mechanism
3.1.2. Specific Catalysis by Sn Halides in the Synthesis of HBL
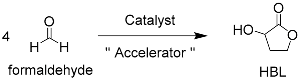
3.2. A Sugar-Accelerated Tin-Catalyzed Cascade Synthesis of α-Hydroxy-γ-butyrolactone from Formaldehyde
3.3. Application of Heterogeneous Catalysts in the Intermolecular Aldol Reaction between 1,3-Dihydroxyacetone and Formaldehyde
4. Cascade Synthesis of 5-Membered Lactones Using Biomass-Derived Sugars and a Range of Aldehydes
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Bozell, J.J.; Peterson, G.R. Technology Development for the Production of Biobased Products from Biorefinery Carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Marshall, A.L.; Alaimo, P.J. Useful Products from Complex Starting Materials: Common Chemicals from Biomass Feedstocks. Chem. Eur. J. 2010, 16, 4970–4980. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Dumont, M.; Raghavan, V. Sustainable Production of Hydroxymethylfurfural and Levulinic Acid: Challenges and Opportunities. Biomass Bioenergy 2015, 72, 143–183. [Google Scholar] [CrossRef]
- Pileidis, F.; Titirici, M. Levulinic Acid Biorefineries: New Challenges for Efficient Utilization of Biomass. ChemSusChem 2016, 9, 562–582. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Zhao, C.; Luo, C.; Dyson, P.J.; Liu, H.C.; Kou, Y. One-Step Conversion of Cellobiose to C6-Alcohols Using a Ruthenium Nanocluster Catalyst. J. Am. Chem. Soc. 2006, 128, 8714–8715. [Google Scholar]
- Binder, J.B.; Raines, R.T. Simple Chemical Transformation of Lignocellulosic Biomass into Furans for Fuels and Chemicals. J. Am. Chem. Soc. 2009, 131, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Holm, M.S.; Saravanamurugan, S.; Taarning, E. Conversion of Sugars to Lactic Acid Derivatives Using Heterogeneous Zeotype Catalysts. Science 2010, 328, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Nikolla, E.; Román-Leshkov, Y.; Moliner, M.; Davis, M.E. “One-Pot” Synthesis of 5-(Hydroxymethyl)furfural from Carbohydrates using Tin-Beta Zeolite. ACS Catal. 2011, 1, 408–410. [Google Scholar] [CrossRef]
- Holm, M.S.; Pagán-Torres, Y.J.; Saravanamurugan, S.; Riisager, A.; Dumesic, J.A.; Taarning, E. Sn-Beta Catalysed Conversion of Hemicellulosic Sugars. Green Chem. 2012, 14, 702–706. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, Q.; Wang, Y. Catalytic Transformations of Cellulose and Its Derived Carbohydrates into 5-Hydroxymethylfurfural, Levulinic Acid, and Lactic Acid. Sci. China Chem. 2015, 58, 29–46. [Google Scholar] [CrossRef]
- Dusselier, M.; Clercq, R.; Cornelis, R.; Sels, B.F. Tin Triflate-Catalyzed Conversion of Cellulose to Valuable (α-Hydroxy-) Esters. Catal. Today 2016. [Google Scholar] [CrossRef]
- Huber, G.W.; Chheda, J.N.; Barrett, C.J.; Dumesic, J.A. Production of Liquid Alkanes by Aqueous-Phase Processing of Biomass-Derived Carbohydrates. Science 2005, 308, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Torre, O.; Renz, M.; Villandier, N. Production of High-Quality Diesel from Biomass Waste Products. Angew. Chem. Int. Ed. 2011, 50, 2375–2378. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, E. Integrated Catalytic Process for Biomass Conversion and Upgrading to C12 Furoin and Alkane Fuel. ACS Catal. 2014, 4, 1302–1310. [Google Scholar] [CrossRef]
- Dusselier, M.; Wouwe, P.; Clippel, F.; Dijkmans, J.; Gammon, D.W.; Sels, B.F. Mechanistic Insight into the Conversion of Tetrose Sugars to Novel α-Hydroxy Acid Platform Molecules. ChemCatChem 2013, 5, 569–575. [Google Scholar] [CrossRef]
- Dusselier, M.; Wouwe, P.; Smet, S.; Clercq, R.; Verbelen, L.; Puyvelde, P.; Prez, F.E.; Sels, B.F. Toward Functional Polyester Building Blocks from Renewable Glycolaldehyde with Sn Cascade Catalysis. ACS Catal. 2013, 3, 1786–1800. [Google Scholar] [CrossRef]
- Clercq, R.; Dusselier, M.; Christiaens, C.; Dijkmans, J.; Iacobescu, R.; Pontikes, Y.; Sels, B.F. Confinement Effects in Lewis Acid-Catalyzed Sugar Conversion: Steering Toward Functional Polyester Building Blocks. ACS Catal. 2015, 5, 5803–5811. [Google Scholar] [CrossRef]
- Butlerov, A.M. Formation Synthétique D’une Substance Sucrée. C. R. Acad. Sci. 1861, 53, 145–147. [Google Scholar]
- Loew, O. Ueber Formaldehyd Und Dessen Condensation. J. Prakt. Chem. 1886, 33, 321–351. [Google Scholar] [CrossRef]
- Breslow, R. On the Mechanism of the Formose Reaction. Tetrahedron Lett. 1959, 1, 22–26. [Google Scholar] [CrossRef]
- Socha, R.F.; Weiss, A.H.; Sakharov, M.M. Homogeneously Catalyzed Condensation of Formaldehyde to Carbohydrates: VII. An Overall Formose Reaction Model. J. Catal. 1981, 67, 207–217. [Google Scholar] [CrossRef]
- Deng, J.; Pam, T.; Xu, Q.; Chen, M.Y.; Zhang, Y.; Guo, Q.X.; Fu, Y. Linked Strategy for the Production of Fuels via Formose Reaction. Sci. Rep. 2013, 3, 1244. [Google Scholar] [CrossRef] [PubMed]
- Delidovich, I.V.; Simonov, A.N.; Taran, O.P.; Parmon, V.N. Catalytic Formation of Monosaccharides: From the Formose Reaction towards Selective Synthesis. ChemSusChem 2014, 7, 1833–1846. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Motokura, K.; Sakamoto, Y.; Miyaji, A.; Baba, T. Tin-Catalyzed Conversion of Biomass-Derived Triose Sugar and Formaldehyde to α-Hydroxy-γ-Butyrolactone. Chem. Commun. 2014, 50, 4600–4602. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Matsuo, T.; Motokura, K.; Sakamoto, Y.; Miyaji, A.; Baba, T. Mechanistic Studies on the Cascade Conversion of 1,3-Dihydroxyacetone and Formaldehyde into α-Hydroxy-γ-Butyrolactone. ChemSusChem 2015, 8, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Shiury, S.; Partridge, J.; Uskokovic, M. Triply Convergent Synthesis of lar,25-Dihydroxy-24(R)-fluorocholecalciferol. J. Org. Chem. 1988, 53, 1040–1046. [Google Scholar]
- Green, D.; Kiddle, J.; Thompson, C. Stereochemistry of remote dianion addition to imines. Application to the synthesis of (1S, 8aS)-1-hydroxyindolizidine. Tetrahedron 1995, 51, 2865–2874. [Google Scholar] [CrossRef]
- Schinzer, D.; Bauer, A.; Schieber, J. Syntheses of (−)-Epothilone B. Chem. Eur. J. 1999, 5, 2492–2500. [Google Scholar] [CrossRef]
- Mulzer, J.; Mantoulidis, A.; Öhler, E. Total Syntheses of Epothilones B and D. J. Org. Chem. 2000, 65, 7456–7467. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Hrnciar, P. Synthesis of Polyhydroxylated Pyrrolizidine Alkaloids of the Alexine Family by Tandem Ring-Closing Metathesis-Transannular Cyclization. (+)-Australine. J. Org. Chem. 2000, 65, 9129–9142. [Google Scholar] [CrossRef] [PubMed]
- Phukan, P.; Sasmal, S.; Maier, M. Flexible Routes to the 5-Hydroxy Acid Fragment of the Cryptophycins. Eur. J. Org. Chem. 2003, 9, 1733–1740. [Google Scholar] [CrossRef]
- Mereyala, H.B.; Joe, M.; Gadikota, R.R. Synthesis of Harzialactone A and Its Isomers from D-Glucose and Assignment of Absolute Stereochemistry. Tetrahedron Asymmetry 2000, 11, 4071–4081. [Google Scholar] [CrossRef]
- Movassaghi, M.; Jacobsen, E.N. A Direct Method for the Conversion of Terminal Epoxides into γ-Butanolides. J. Am. Chem. Soc. 2002, 124, 2456–2457. [Google Scholar] [CrossRef] [PubMed]
- Hoge, G. Synthesis of Both Enantiomers of a P-Chirogenic 1,2-Bisphospholanoethane Ligand via Convergent Routes and Application to Rhodium-Catalyzed Asymmetric Hydrogenation of CI-1008 (Pregabalin). J. Am. Chem. Soc. 2003, 125, 10219–10227. [Google Scholar] [CrossRef] [PubMed]
- Kagayama, T.; Sakaguchi, S.; Ishii, Y. Synthesis of α-Hydroxy-γ-butyrolactones from Acrylates and 1,3-Dioxolanes Using N-Hydroxyphthalimide (NHPI) as a Key Catalyst. Tetrahedron Lett. 2005, 46, 3687–3689. [Google Scholar] [CrossRef]
- Jian, Y.; Wu, Y.; Li, L.; Lu, J. An Expeditious Route to the Antipode of Harzialactone A. Tetrahedron Asymmetry 2005, 16, 2649–2651. [Google Scholar] [CrossRef]
- Kotkar, S.; Suryavanshi, G.; Sudalai, A. A Short Synthesis of (+)-Harzialactone A and (R)-(+)-4-Hexanolide via Proline-catalyzed Sequential α-Aminooxylation and Horner–Wadsworth–Emmons Olefination of Aldehydes. Tetrahedron Asymmetry 2007, 18, 1795–1798. [Google Scholar] [CrossRef]
- Sabitha, G.; Srinivas, R.; Das, S.; Yadav, J. Synthesis of (3R,5R)-Harzialactone A and Its (3R,5S)-Isomer. Beilstein J. Org. Chem. 2010, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cui, L.; Wang, Y.; Zhang, L. Homogeneous Gold-Catalyzed Oxidative Carboheterofunctionalization of Alkenes. J. Am. Chem. Soc. 2010, 132, 1474–1475. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, S.; Wu, Y.; Li, Y. Synthesis of (−)-Harzialactone A from a Readily Accessible Epoxy Chiral Building Block. Chin. J. Chem. 2011, 29, 2664–2668. [Google Scholar] [CrossRef]
- Kumar, D.N.; Reddy, C.R.; Das, B. Stereoselective Synthesis of Cytotoxic Marine Metabolite Harzialactone A by Three Different Routes. Synthesis 2011, 19, 3190–3194. [Google Scholar]
- Uchiro, H.; Shinozaki, N.; Kobayakawa, Y.; Nakagawa, H.; Makino, K. A novel convergent method for the synthesis of α-acyl-γ-hydroxylactams and its application in the total synthesis of PI-090 and 091. Bioorg. Med. Chem. Lett. 2012, 22, 4765–4768. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Liu, M.; Sun, Y.; Ye, L. Efficient Synthesis of γ-Lactones via Gold-Catalyzed Tandem Cycloisomerization/Oxidation. Org. Lett. 2012, 4, 4958–4961. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.N.; Bhatt, S.; Chattopadhyay, S. Chemoenzymatic Asymmetric Synthesis of Harzialactone A Stereomers. Tetrahedron Asymmetry 2009, 20, 205–209. [Google Scholar] [CrossRef]
- Chen, B.; Yin, H.; Wang, Z.; Xu, J.; Fan, L.; Zhao, J. Facile Synthesis of Enantiopure 4-Substituted 2-Hydroxy-4-butyrolactones using a Robust Fusarium Lactonase. Adv. Synth. Catal. 2009, 351, 2959–2966. [Google Scholar] [CrossRef]
- Chen, B.; Yin, H.; Wang, Z.; Xu, J. New Synthesis of Harzialactone A via Kinetic Resolution Using Recombinant Fusarium Proliferatum Lactonase. Tetrahedron Asymmetry 2010, 21, 237–240. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Matsuo, T.; Motokura, K.; Sakamoto, Y.; Miyaji, A.; Baba, T. Mechanistic Insight into a Sugar-accelerated Tin-catalyzed Cascade Synthesis of α-Hydroxy-γ-Butyrolactone from Formaldehyde. ChemSusChem 2015, 8, 3661–3667. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Matsuo, T.; Motokura, K.; Miyaji, A.; Baba, T. Influence of the Interaction between Tin Catalyst and Accelerator on the Formose-inspired Synthesis of α-Hydroxy-γ-Butyrolactone. ChemCatChem 2016, 8, 331–335. [Google Scholar] [CrossRef]
- Matsumoto, T.; Inoue, S. Selective Formation of Triose from Formaldehyde catalyzed by Ethylbenzothiazolium Bromide. J. Chem. Soc. Chem. Commun. 1983, 4, 171–172. [Google Scholar] [CrossRef]
- Vyver, S.; Odermatt, C.; Romero, K.; Prasomsri, T.; Román-Leshkov, Y. Solid Lewis Acids Catalyze the Carbon–Carbon Coupling between Carbohydrates and Formaldehyde. ACS Catal. 2015, 5, 972–977. [Google Scholar] [CrossRef]
- Vyver, S.; Román-Leshkov, Y. Metalloenzyme-Like Zeolite as Lewis Acid Catalysts for C-C Bond Formation. Angew. Chem. Int. Ed. 2015, 54, 12554–12561. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, N.; Shibasaki, M. Recent Advances in Direct Catalytic Asymmetric Transformations under Proton-Transfer Conditions. Angew. Chem. Int. Ed. 2011, 50, 4760–4772. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, M.; Yoshikawa, N. Lanthanide Complexes in Multifunctional Asymmetric Catalysis. Chem. Rev. 2002, 102, 2187–2210. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Shibasaki, M. Multimetallic Bifunctional Asymmetric Catalysis Based on Proximity Effect Control. Bull. Chem. Soc. Jpn. 2008, 81, 60–75. [Google Scholar] [CrossRef]
- Motokura, K.; Tada, M.; Iwasawa, Y. Heterogeneous Organic Base-Catalyzed Reactions Enhanced by Acid Supports. J. Am. Chem. Soc. 2007, 129, 9540–9541. [Google Scholar] [CrossRef] [PubMed]
- Motokura, K.; Tada, M.; Iwasawa, Y. Cooperative Catalysis of Primary and Tertiary Amines on Oxide Support Surface for One-Pot C-C Bond Forming Reactions. Angew. Chem. Int. Ed. 2008, 47, 9230–9235. [Google Scholar] [CrossRef] [PubMed]
- Motokura, K.; Tada, M.; Iwasawa, Y. Layered Materials with Coexisting Acidic and Basic Sites for Catalytic One-Pot Reaction Sequences. J. Am. Chem. Soc. 2009, 131, 7944–7945. [Google Scholar] [CrossRef] [PubMed]
- Noda, H.; Motokura, K.; Miyaji, A.; Baba, T. Heterogeneous Synergistic Catalysis by a Palladium Complex and an Amine on a Silica Surface for Acceleration of the Tsuji-Trost Reaction. Angew. Chem. Int. Ed. 2012, 51, 8017–8020. [Google Scholar] [CrossRef] [PubMed]
- Noda, H.; Motokura, K.; Wakabayashi, Y.; Sasaki, K.; Tajiri, H.; Miyaji, A.; Yamaguchi, S.; Baba, T. Direct Estimation of the Surface Location of Immobilized Functional Groups for Concerted Catalysis Using a Probe Molecule. Chem. Eur. J. 2016, 22, 5113–5117. [Google Scholar] [CrossRef] [PubMed]
- Motokura, K.; Ito, Y.; Noda, H.; Miyaji, A.; Yamaguchi, S.; Baba, T. Surface Functionalization for Synergistic Catalysis: Silica-Alumina-Supported Cationic Indium and Organic Base for Cyanoethoxycarbonylation. ChemPlusChem 2014, 79, 1053–1058. [Google Scholar] [CrossRef]
- Noda, H.; Motokura, K.; Chun, W.; Miyaji, A.; Yamaguchi, S.; Baba, T. Heterogeneous Double-activation Catalysis: Rh Complex and Tertiary Amine on the Same Solid Surface for the 1,4-Addition Reaction of Aryl- and Alkylboronic Acids. Catal. Sci. Technol. 2015, 5, 2714–2727. [Google Scholar] [CrossRef]
- Kim, H.Y.; Li, J.-Y.; Oh, K. A Soft Vinyl Enolization Approach to α-Acylvinyl Anions: Direct Aldol/Aldol Condensation Reactions of (E)-β-Chlorovinyl Ketones. Angew. Chem. Int. Ed. 2013, 52, 3736–3740. [Google Scholar] [CrossRef] [PubMed]
- Sauer, S.J.; Garnsey, M.R.; Coltart, D.M. Direct Carbon–Carbon Bond Formation via Reductive Soft Enolization: A Kinetically Controlled syn-Aldol Addition of α-Halo Thioesters and Enolizable Aldehydes. J. Am. Chem. Soc. 2010, 132, 13997–13999. [Google Scholar] [CrossRef] [PubMed]
- Kohler, M.C.; Yost, J.M.; Garnsey, M.R.; Coltart, D.M. A Facile and Efficient Direct Aldol Addition of Simple Thioesters. Org. Lett. 2010, 12, 3376–3379. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.; Van de Vyver, S.; Román-Leshkov, Y. Acid-Base Pairs in Lewis Acidic Zeolites Promote Direct Aldol Reactions by Soft Enolization. Angew. Chem. Int. Ed. 2015, 54, 9835–9838. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Matsuo, T.; Motokura, K.; Miyaji, A.; Baba, T. Cascade Synthesis of Five-membered Lactones using Biomass-derived Sugars as Carbon Nucleophiles. Chem. Asian J. 2016. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Men-nucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]














| Entry | Substrate | Conversion (%) | Yield (%) | ||
|---|---|---|---|---|---|
| ML | MVG | MMHB | |||
| 1 | Xylose | 98 | 42 | 7 | <2 |
| 2 | Ribose | 96 | 38 | 8 | <2 |
| 3 | Glucose | 98 | 51 | 10 | <2 |
| 4 | Fructose | 98 | 54 | 11 | <2 |
| 5 | Mannose | 96 | 47 | 9 | <2 |
| 6 | Galactose | 95 | 45 | 5 | <2 |
| 7 | Sucrose | 92 | 57 | 5 | 0 |
| 8 | Glycolaldehyde | - | 16 | 27 | 6 |
| Entry | Catalyst | Time (h) | Yield (%) | ||
|---|---|---|---|---|---|
| GADMA | MMHB | MVG | |||
| 1 | none | 26 | 47 | 0 | 0 |
| 2 | HCl | 22 | 56 | 0 | 0 |
| 3 | NaOH | 20 | 0 | 0 | 0 |
| 4 | SnCl4·5H2O | 1 | 54 | 10 | <1 |
| 5 | SnCl4·5H2O | 20 | 3 | 58 | 3 |
| 6 | SnCl2·2H2O | 1 | 51 | 14 | <1 |
| 7 | SnCl2·2H2O | 21 | 1 | 55 | 4 |
| 8 | AlCl3·6H2O | 20 | 47 | 7 | 0 |
| 9 | CrCl3·6H2O | 20 | 71 | <1 | 0 |
| 10 2 | SnCl4·5H2O | 1 | - | 50 | 2 |
| Entry | Catalysts | Conversion of DHA (%) | Yield (%) | ||
|---|---|---|---|---|---|
| HBL | VG | LA | |||
| 1 | HCl | >99 | 0 | 0 | 21 |
| 2 | SnCl4·5H2O | >99 | 42 | 6 | 12 |
| 3 | SnCl2·2H2O | >99 | 24 | 3 | 13 |
| 4 2 | SnCl2·2H2O | >99 | 37 | 4 | 16 |
| 5 | nBu3SnCl | >99 | 25 | 3 | 12 |
| 6 | SnO2 | 95 | 0 | 0 | <1 |
| 7 3 | SnCl4 | >99 | 40 | 4 | 17 |
| 8 | SnCl4 | >99 | 63 | 8 | 20 |
| 9 4 | SnCl4 | >99 | 70 | 5 | 5 |
| 10 | AlCl3 | >99 | 0 | 0 | 5 |
| 11 | TiCl4 | >99 | 0 | 0 | 2 |
| 12 | ZrCl4 | >99 | 0 | 0 | 18 |
| 13 | Sn(OAc)2 | >99 | 0 | 0 | 5 |
| 14 | Sn(OTf)2 | >99 | 0 | 0 | 36 |
| 15 | none | 79 | 0 | 0 | 4 |
| Entry | Catalysts | Accelerator | HBL (mmol) 2 | Yield of HBL (%) 2 |
|---|---|---|---|---|
| 1 | SnCl4·5H2O | - | 0.02 | 1 |
| 2 | SnCl2·2H2O | - | 0.17 | 11 |
| 3 | SnCl4·5H2O | Glucose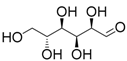 | 0.33 | - |
| 4 | SnCl2·2H2O | Glucose | 0.53 (0.41) 3 | 26 |
| 5 | 1,3-Dihydroxyacetone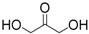 | 0.52 (0.39) 3 | 25 | |
| 6 | Sorbitol | 0.16 | 10 | |
| 7 | Glucosamine | <0.01 | <1 | |
| 8 | Hydroxyacetone | 0.34 | 22 | |
| 9 | Hydroxyacetphenone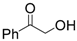 | 0.39 | 25 | |
| 10 | Acetoin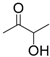 | 0.06 | 4 | |
| 11 | Benzoin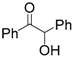 | 0.07 | 5 |
| Entry | Catalysts | DHA Conversion (%) | Yield (%) 2 | |
|---|---|---|---|---|
| HBL | LA | |||
| 1 | Sn-Beta | 98 | 60 | 9 |
| 2 | Sn-Beta 3 | 98 | 68 | 8 |
| 3 | Zr-Beta | 99 | 3 | 2 |
| 4 | Hf-Beta | 99 | 7 | 3 |
| 5 | Ti-Beta | 96 | 6 | 3 |
| 6 | Al-Beta | 95 | 11 | 6 |
| 7 | Sn-MCM-41 | 99 | 64 | 15 |
| 8 | Sn-MFI | 90 | 61 | 6 |
| 9 | SnO2/Si-Beta | 53 | 3 | <1 |
| 10 | none | 12 | <1 | <1 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, S.; Baba, T. A Novel Strategy for Biomass Upgrade: Cascade Approach to the Synthesis of Useful Compounds via C-C Bond Formation Using Biomass-Derived Sugars as Carbon Nucleophiles. Molecules 2016, 21, 937. https://doi.org/10.3390/molecules21070937
Yamaguchi S, Baba T. A Novel Strategy for Biomass Upgrade: Cascade Approach to the Synthesis of Useful Compounds via C-C Bond Formation Using Biomass-Derived Sugars as Carbon Nucleophiles. Molecules. 2016; 21(7):937. https://doi.org/10.3390/molecules21070937
Chicago/Turabian StyleYamaguchi, Sho, and Toshihide Baba. 2016. "A Novel Strategy for Biomass Upgrade: Cascade Approach to the Synthesis of Useful Compounds via C-C Bond Formation Using Biomass-Derived Sugars as Carbon Nucleophiles" Molecules 21, no. 7: 937. https://doi.org/10.3390/molecules21070937