Chemical Composition, Antioxidant, and Antibacterial Activity of Wood Vinegar from Litchi chinensis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Profiles of Wood Vinegar From GC-MS
2.2. Antibacterial Ability
2.3. Antioxidant Assay
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Instrument
3.3. Methods
3.3.1. Phytochemical Composition Analysis
3.3.2. Antibacterial Activity Assay
Disc Inhibitory Assay
Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
3.3.3. Determination of Total Phenolic Content
3.3.4. Determination of Flavonoids Content
3.3.5. Antioxidant Activity Assay
DPPH Free Radical Scavenging Ability
Reducing Power
Trolox Equivalent Antioxidant Capacity
3.3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Amen-Chen, C.; Pakdel, H.; Roy, C. Separation of phenols from Eucalyptus wood tar. Biomass Bioenerg. 1977, 13, 25–37. [Google Scholar]
- Pimenta, A.S.; Bayana, J.M.; Garcia, M.T.; Splanas, A.M. Evaluation of acute toxicity and genotoxicity of liquid products from pyrolysis of Eucalyptus grandis wood. Arch. Environ. Contam. Toxic. 2000, 38, 169–175. [Google Scholar]
- Guillen, M.D.; Manzanos, M.J. Study of the volatile composition of an aqueous oak smoke preparation. Food Chem. 2002, 79, 283–292. [Google Scholar]
- Mu, J.; Uehara, T.; Furuno, T. Effect of bamboo vinegar on regulation of germination and radicle growth of seed plants II: Composition of moso bamboo vinegar at different collection temperature and its effects. J. Wood Sci. 2004, 50, 470–476. [Google Scholar]
- Loo, A.Y.; Jain, K.; Darah, I. Antioxidant and radical scavenging activities of the pyroligneous acid from a mangrove plant, Rhizophora apiculate. Food Chem. 2007, 104, 300–307. [Google Scholar]
- Loo, A.Y.; Jain, K.; Darah, I. Antioxidant activity of compounds isolated from the pyroligneous acid, Rhizophora apiculate. Food Chem. 2008, 107, 1151–1160. [Google Scholar]
- Wititsiri, S. Production of wood vinegars from coconut shells and additional materials for control of termite workers, Odontotermes sp. and striped mealy bugs, Ferrisia virgate. Songklanakarin J. Sci. Technol. 2011, 33, 349–354. [Google Scholar]
- Amen-Chen, C.; Pakdel, H.; Roy, C. Production of monomeric phenols by thermochemical conversion of biomass: A review. Bioresour. Technol. 2001, 79, 277–299. [Google Scholar]
- Oramahi, H.A.; Yoshimura, T. Antifungal and antitermitic activities of wood vinegar from Vitex pubescens Vahl. J. Wood Sci. 2013, 59, 344–350. [Google Scholar]
- Velmurugan, N.; Han, S.S.; Lee, Y.S. Antifungal activity of neutralized wood vinegar with water extracts of Pinus densiflora and Quercus serrata saw dusts. Int. J. Environ. Res. 2009, 3, 167–176. [Google Scholar]
- Baimark, Y.; Niamsa, N. Study on wood vinegars for use as coagulating and antifungal agents on the production of natural rubber sheets. Biomass Bioenerg. 2009, 33, 994–998. [Google Scholar]
- Ma, X.H.; Wei, Q.; Zhang, S.S.; Shi, L.; Zhao, Z. Isolation and bioactivities of organic acids and phenols from walnut shell pyroligneous acid. J. Anal. Appl. Pyrolysis 2011, 91, 338–343. [Google Scholar]
- Hwang, Y.H.; Matsushita, Y.I.; Sugamoto, K.; Matsui, T. Antimicrobial effect of the wood vinegar from Cryptomeria japonica Sapwood on Plant Pathogenic Microorganisms. J. Microbiol. Biotechnol. 2005, 15, 1106–1109. [Google Scholar]
- Ronald, H.A. Gas Chromatography Mass Spectroscopy. In Handbook of Instrumental Techniques for Analytical Chemistry; Prentice Hall PTR: Upper Saddle River, NJ, USA, 1997; pp. 609–611. [Google Scholar]
- Van Bergen, P.F.; Poole, I.; Ogilvie, T.M.; Caple, C.; Evershed, R.P. Evidence for demethylation of syringyl moieties in archaeological wood using pyrolysis-gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2000, 14, 71–79. [Google Scholar] [CrossRef]
- Martone, P.T.; Estevez, J.M.; Lu, F.; Ruel, K.; Denny, M.W.; Somerville, C.; Ralph, J. Discovery of lignin in seaweed reveals convergent evolution of cell-wall architecture. Curr. Biol. 2009, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Wittkowski, R.; Ruther, J.; Drinda, H.; Rafiei-Taghanaki, F. Formation of smoke flavor compounds by thermal lignin degradation. ACS Symp Ser. 1992, 490, 232–243. [Google Scholar]
- Chan, E.W.; Tan, Y.P.; Chin, S.J.; Gan, L.Y. Antioxidant and anti-tyrosinase properties of wood vinegar from Matang mangroves, Malaysia. ISME/G LOMIS Electron. J. 2012, 10, 19–21. [Google Scholar]
- Chan, E.W.; Fong, C.H.; Kang, K.X.; Chong, H.H.; Chong, H.H. Potent antibacterial activity of wood vinegar from Matang mangroves, Malaysia. SME/G LOMIS Electron. J. 2012, 10, 10–12. [Google Scholar]
- Vijayakumar, S.; Rajenderan, I.; Laishram, I.; Anandan, I.; Balaji, I.; Biswas, I. Biofilm Formation and Motility Depend on the Nature of the Acinetobacter baumannii Clinical Isolates Front. Public Health 2016. [Google Scholar] [CrossRef]
- Chalermsan, Y.; Peerapan, S. Wood vinegar: By-product from rural charcoal kiln and its role in plant protection. Asain J. Food Agro-Ind. 2009, S189–S195. [Google Scholar]
- Ma, C.; Li, W.; Zu, Y.; Yang, L.; Li, J. Antioxidant Properties of Pyroligneous Acid Obtained by Thermochemical Conversion of Schisandra chinensis Baill. Molecules 2014, 19, 20821–20838. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.H.; Xue, Q.C.; Li, Z.C. Handbook of Synthetic Flavour for Food; Chinese Light Industry Press: Beijing, China, 1993; pp. 123–145. [Google Scholar]
- Kim, A.R.; Zou, Y.; Kim, H.S.; Choi, J.S.; Chang, G.Y.; Kim, Y.J.; Chung, H.Y. Selective peroxynitrite scavenging activity of 3-methyl-1,2-cyclopentanedione from coffee extract. J. Pharm. Pharmacol. 2002, 54, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Su, P.W.; Yang, C.H.; Yang, J.F.; Su, P.Y.; Chuang, L.Y. Antibacterial Activities and Antibacterial Mechanism of Polygonum cuspidatum Extracts against Nosocomial Drug-Resistant Pathogens. Molecules 2015, 20, 11119–11130. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.F.; Yang, C.H.; Wu, C.C.; Chuang, L.Y. Antioxidant and antimicrobial activities of the extracts from Sophora flavescens. J. Pharm. Phytochem. 2015, 3, 26–31. [Google Scholar]
- Sample Availability: Samples of the wood vinegar are available from the Bu-Quang charcoal company (Kaohsiung, Taiwan).
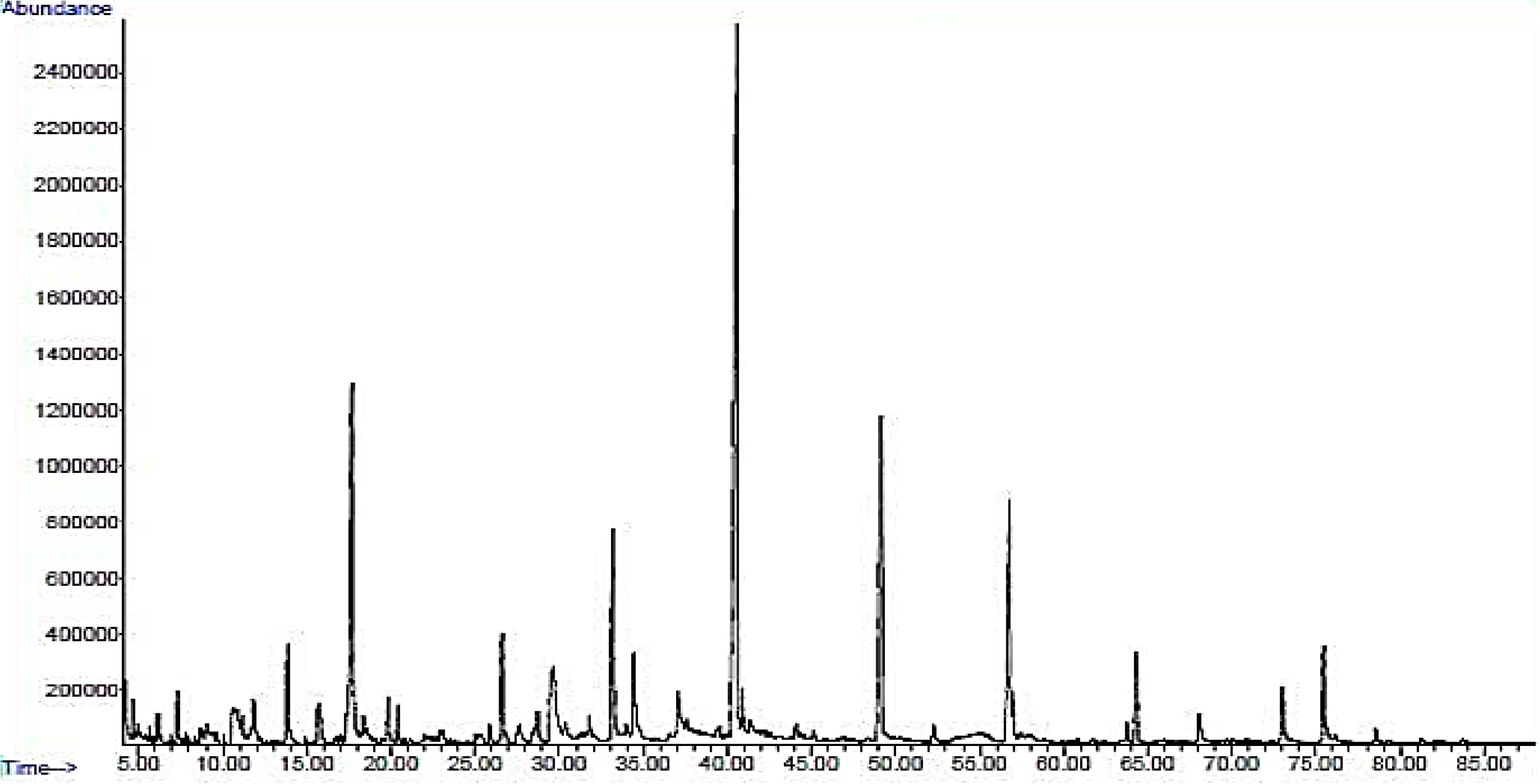
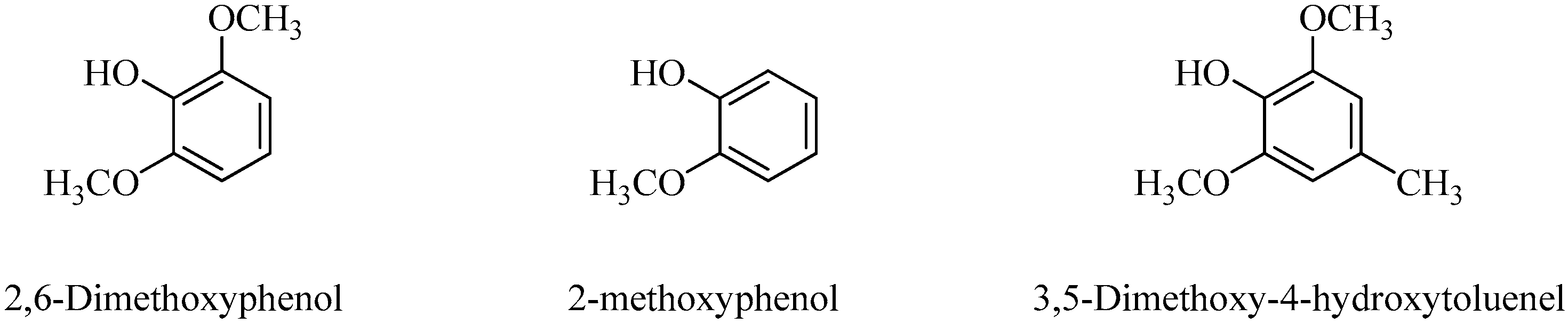

| No. | RT (min) | Name of the Compounds | Molecular Formula | MolecularWeight | % Area |
|---|---|---|---|---|---|
| 1 | 4.644 | 2-methyl-pyridine | C6H7N | 93.06 | 0.23 |
| 2 | 10.664 | Phenol | C6H6O | 94.04 | 2.03 |
| 3 | 13.869 | 3-methyl-1,2-cyclopentanedione | C6H8O2 | 112.05 | 2.65 |
| 4 | 15.682 | 2-methylphenol | C7H8O | 108.06 | 1.54 |
| 5 | 17.663 | 2-methoxyphenol (Guaiacol) | C7H8O2 | 124.05 | 12.36 |
| 6 | 19.838 | Maltol | C6H6O3 | 126.03 | 1.02 |
| 7 | 20.416 | 3-ethyl-2-hydroxy-2-cyclopenten-1-one | C7H10O2 | 126.07 | 0.74 |
| 8 | 26.601 | Creosol | C8H10O2 | 138.07 | 3.15 |
| 9 | 29.641 | Catechol | C6H6O2 | 110.04 | 5.17 |
| 10 | 33.199 | 3-methoxy-1,2-benzenediol | C7H8O3 | 140.05 | 6.12 |
| 11 | 34.415 | 4-ethyl-2-methoxyphenol | C9H12O2 | 152.08 | 3.09 |
| 12 | 37.068 | 4-methyl-1,2-benzenediol | C7H8O2 | 124.05 | 1.66 |
| 13 | 40.568 | 2,6-dimethoxyphenol (Syringol) | C8H10O3 | 154.06 | 29.54 |
| 14 | 40.895 | 3,4-dimethoxyphenol | C8H10O3 | 154.06 | 0.93 |
| 15 | 49.159 | 3,5-dimethoxy-4-hydroxytoluene | C9H12O3 | 168.08 | 11.07 |
| 16 | 56.966 | 1-(4-hydroxy-3-methoxyphenyl)-2-propanone | C10H12O3 | 180.08 | 1.10 |
| 17 | 73.001 | 1-(4-hydroxy-3,5-dimethoxyphenyl)-ethanone | C10H12O4 | 196.07 | 1.59 |
| Strains * | Disc Inhibition Zone (mm) # | MIC (μL/100 μL) | MBC (μL/100 μL) | |
|---|---|---|---|---|
| Wood Vinegar | Tetracycline (7.5 mg/mL) | |||
| Ec 25257 | 15.20 ± 0.40 | 27.90 ± 0.00 | 2.38–2.86 | 2.86 |
| Ab 814 | 17.50 ± 0.20 | 12.73 ± 0.53 | 1.90 | 2.38 |
| Sa 985 | 19.00 ± 1.00 | 17.09 ± 0.09 | 0.95–1.90 | 1.90 |
| Pa 717 | 17.70 ± 0.20 | 23.34 ± 1.91 | 0.95–1.90 | 1.90 |
| ORSa 220 | 16.30 ± 1.10 | 21.31 ± 0.11 | 2.38–3.80 | 3.80 |
| Samples | DPPH IC50 (ppm) | TPC (g Gallic acid/100 g DW) | TFC (g Quercetin/100 g DW) | TEAC (g Trolox Equivalent/100 g DW) | Reducing Power (abs/10−3 ppm) |
|---|---|---|---|---|---|
| Wood vinegar | 36.53 ± 1.57 | 37.34 ± 0.07 | 4.42 ± 0.01 | 38.38 ± 0.12 | 67.9 |
| BHT | 175.12 ± 19.92 | - | - | 35.64 ± 0.35 | 2.2 |
| Vitamin C | 7.01 ± 0.61 | - | - | 38.47 ± 0.04 | 7.3 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.-F.; Yang, C.-H.; Liang, M.-T.; Gao, Z.-J.; Wu, Y.-W.; Chuang, L.-Y. Chemical Composition, Antioxidant, and Antibacterial Activity of Wood Vinegar from Litchi chinensis. Molecules 2016, 21, 1150. https://doi.org/10.3390/molecules21091150
Yang J-F, Yang C-H, Liang M-T, Gao Z-J, Wu Y-W, Chuang L-Y. Chemical Composition, Antioxidant, and Antibacterial Activity of Wood Vinegar from Litchi chinensis. Molecules. 2016; 21(9):1150. https://doi.org/10.3390/molecules21091150
Chicago/Turabian StyleYang, Jyh-Ferng, Cheng-Hong Yang, Ming-Tsai Liang, Zi-Jie Gao, Yuh-Wern Wu, and Li-Yeh Chuang. 2016. "Chemical Composition, Antioxidant, and Antibacterial Activity of Wood Vinegar from Litchi chinensis" Molecules 21, no. 9: 1150. https://doi.org/10.3390/molecules21091150
APA StyleYang, J. -F., Yang, C. -H., Liang, M. -T., Gao, Z. -J., Wu, Y. -W., & Chuang, L. -Y. (2016). Chemical Composition, Antioxidant, and Antibacterial Activity of Wood Vinegar from Litchi chinensis. Molecules, 21(9), 1150. https://doi.org/10.3390/molecules21091150






