Anti-Fatigue Effects of the Unique Polysaccharide Marker of Dendrobium officinale on BALB/c Mice
Abstract
:1. Introduction
2. Results
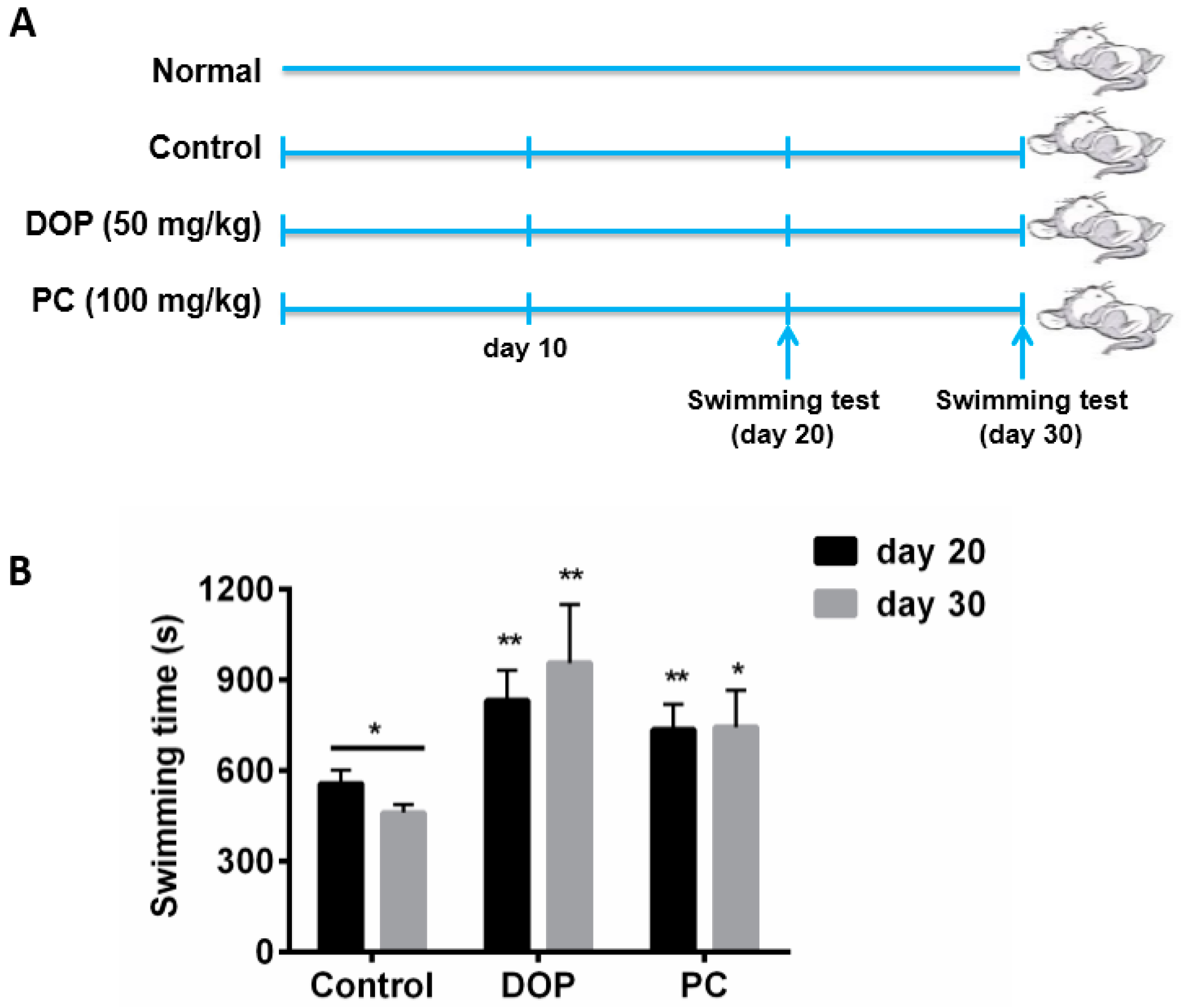
2.1. Effects of DOP and Rhodiola Extract on Weight-Loaded Forced Swimming Endurance Time
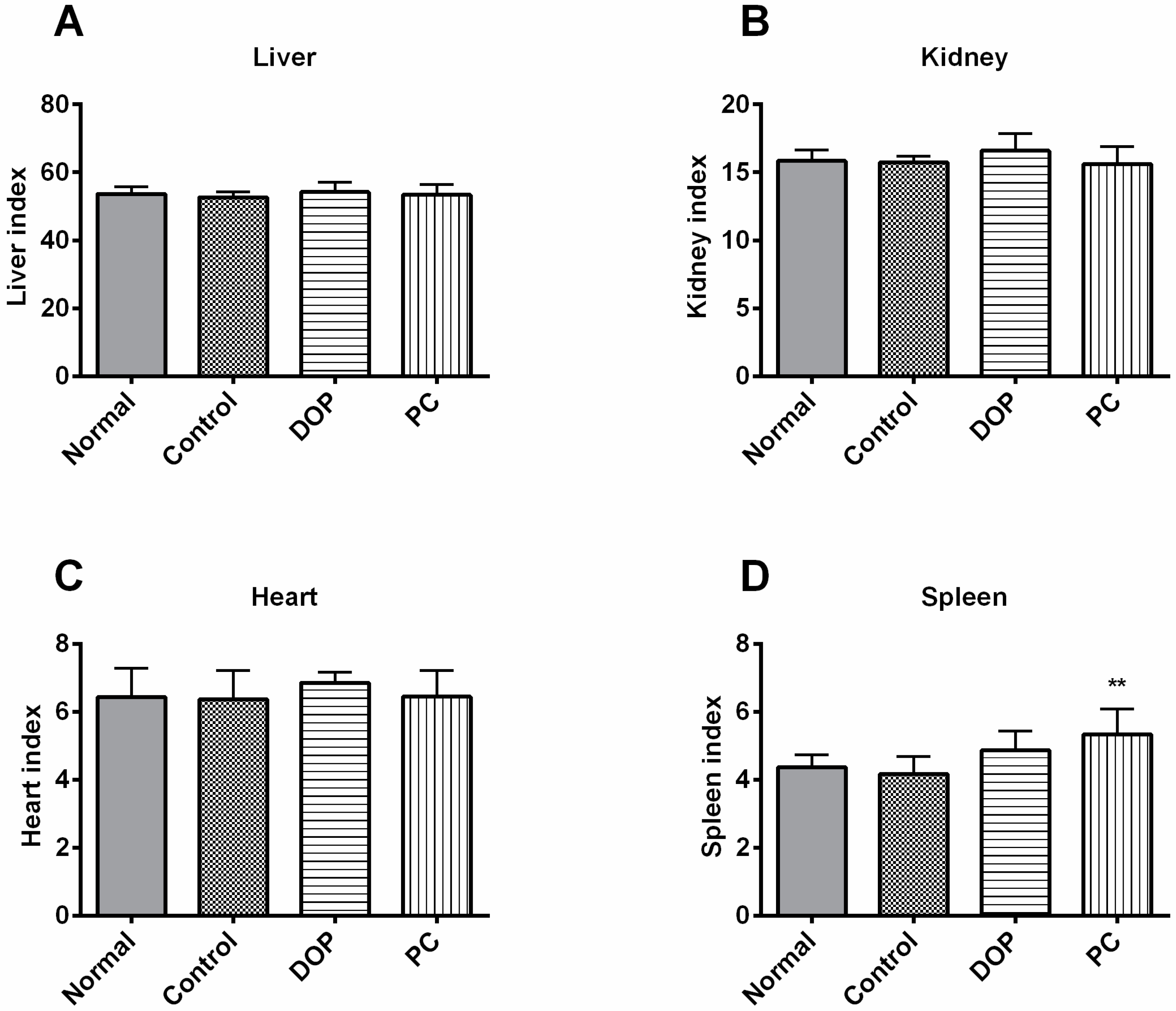
2.2. Effects of DOP and Rhodiola Extract on Body Weight and Organ Indexes
2.3. Effects of DOP and Rhodiola Extract on Serum Biochemical Parameters
2.4. Effects of DOP and Rhodiola Extract on Glycogen in Liver and Gastrocnemius Muscle
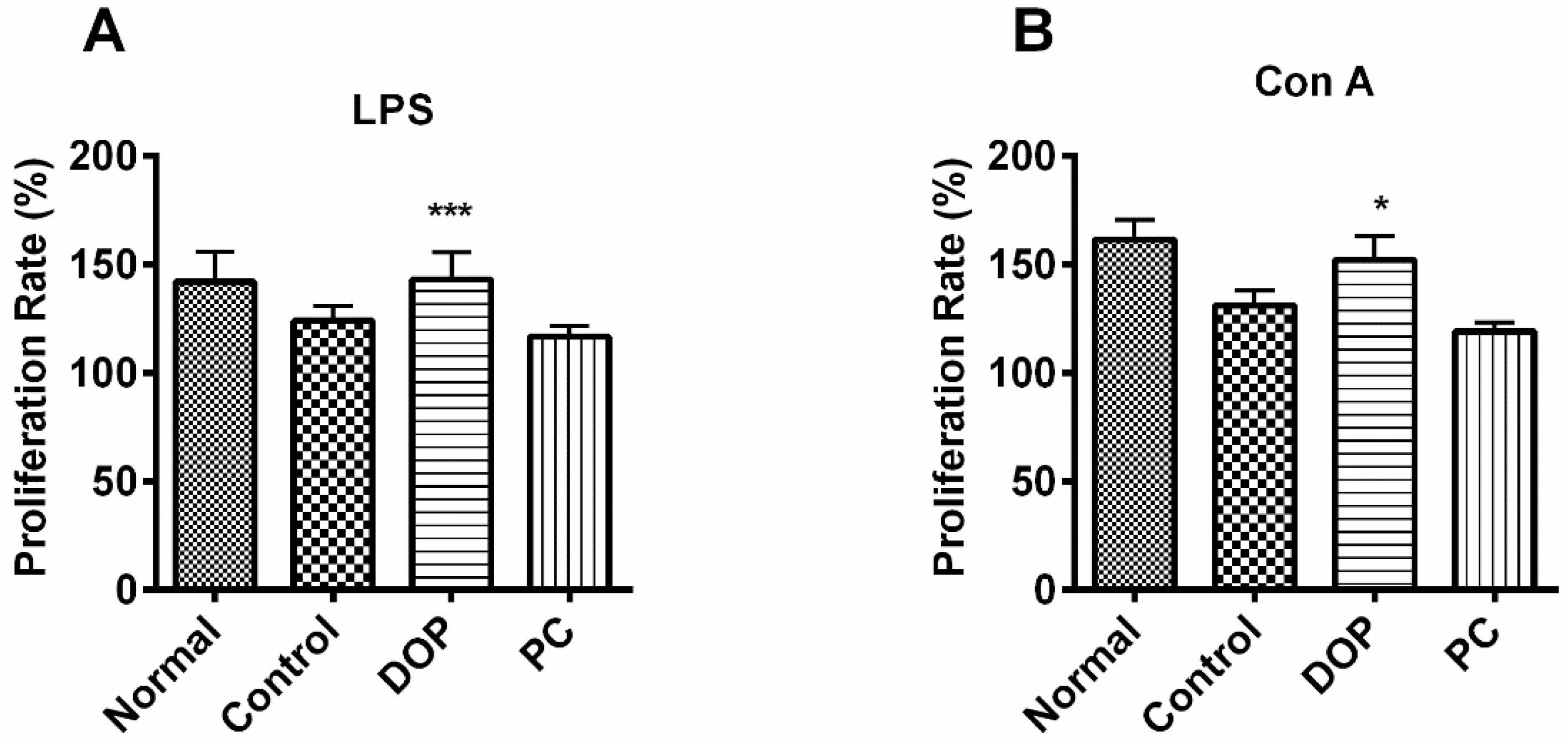
2.5. DOP’s Effect on Proliferation of Mouse Lymphocytes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals and Experimental Design
4.3. Weight-Loaded Swimming Endurance Time
4.4. Biochemical Analysis
4.5. Analysis of Tissue Glycogen Contents
4.6. Lymphocyte Proliferation Assays
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DOP | Dendrobium officinale polysaccharide |
| LDH | Lactic dehydrogenase |
| CK | Creatine phosphokinase |
| TG | Triglyceride |
| BUN | Blood urea nitrogen |
| SOD | Superoxide dismutase |
| MDA | Malondialdehyde |
| LD | Lactic acid |
| GSH-Px | Glutathione peroxidase |
| AIDS | Acquired Immune Deficiency Syndrome |
| LPS | Lipopolysaccharide |
| Con A | Concanavalin A |
| ROS | Reactive oxygen species |
References
- Chaudhuri, A.; Behan, P.O. Fatigue in neurological disorders. Lancet 2004, 363, 978–988. [Google Scholar] [CrossRef]
- Kroenke, K.; Wood, D.R.; Mangelsdorff, A.D.; Meier, N.J.; Powell, J.B. Chronic fatigue in primary care: Prevalence, patient characteristics, and outcome. JAMA 1988, 260, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Papanicolaou, D.A.; Amsterdam, J.D.; Levine, S.; McCann, S.M.; Moore, R.C.; Newbrand, C.H.; Allen, G.; Nisenbaum, R.; Pfaff, D.W.; Tsokos, G.C. Neuroendocrine aspects of chronic fatigue syndrome. Neuroimmunomodulation 2004, 11, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Donglian, C.; Huaixing, L.; Bende, T.; Lihua, S.; Ying, W. Anti-fatigue effects of salidroside in mice. J. Med. Coll. PLA 2008, 23, 88–93. [Google Scholar] [CrossRef]
- Darbinyan, V.; Kteyan, A.; Panossian, A.; Gabrielian, E.; Wikman, G.; Wagner, H. Rhodiola rosea in stress induced fatigue—A double blind cross-over study of a standardized extract SHR-5 with a repeated low-dose regimen on the mental performance of healthy physicians during night duty. Phytomedicine 2000, 7, 365–371. [Google Scholar] [CrossRef]
- Levine, P.H.; Whiteside, T.L.; Friberg, D.; Bryant, J.; Colclough, G.; Herberman, R.B. Dysfunction of natural killer activity in a family with chronic fatigue syndrome. Clin. Immunol. Immunopathol. 1998, 88, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Ojo-Amaize, E.A.; Conley, E.J.; Peter, J.B. Decreased natural killer cell activity is associated with severity of chronic fatigue immune dysfunction syndrome. Clin. Infect. Dis. 1994, 18, S157–S159. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, D.; Wener, M.; Pearlman, T.; Kith, P. Markers of inflammation and immune activation in chronic fatigue and chronic fatigue syndrome. J. Rheumatol. 1997, 24, 372–376. [Google Scholar] [PubMed]
- Landay, A.; Lennette, E.; Jessop, C.; Levy, J. Chronic fatigue syndrome: Clinical condition associated with immune activation. Lancet 1991, 338, 707–712. [Google Scholar] [CrossRef]
- Mawle, A.C.; Nisenbaum, R.; Dobbins, J.G.; Gary, H.E.; Stewart, J.A.; Reyes, M.; Steele, L.; Schmid, D.S.; Reeves, W.C. Immune responses associated with chronic fatigue syndrome: A case-control study. J. Infect. Dis. 1997, 175, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B.; Liu, J.; Wong, J.H.; Ye, X.; Sze, S.C.W.; Tong, Y.; Zhang, K.Y. Review of research on dendrobium, a prized folk medicine. Appl. Microbiol. Biotechnol. 2012, 93, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Huang, M.K.; Ye, J.B.; Ning, L.; Zhong, W.G. The lowest doses of Dendrobium officinale for improving sports ability, anti-fatigue and immune ability of mice. J. South. Agric. 2014, 45, 1089–1093. [Google Scholar]
- Xu, J.; Li, S.-L.; Yue, R.-Q.; Ko, C.-H.; Hu, J.-M.; Liu, J.; Ho, H.-M.; Yi, T.; Zhao, Z.-Z.; Zhou, J. A novel and rapid hpgpc-based strategy for quality control of saccharide-dominant herbal materials: Dendrobium officinale, a case study. Anal. Bioanal. Chem. 2014, 406, 6409–6417. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.-L.; Huang, X.-J.; Nie, S.-P.; Xie, M.-Y.; Phillips, G.O.; Cui, S.W. Study on Dendrobium officinale o-acetyl-glucomannan (dendronan®): Part III—Immunomodulatory activity in vitro. Bioact. Carbohydr. Diet. Fibre 2015, 5, 99–105. [Google Scholar] [CrossRef]
- Liu, X.-F.; Zhu, J.; Ge, S.-Y.; Xia, L.-J.; Yang, H.-Y.; Qian, Y.-T.; Ren, F.-Z. Orally administered Dendrobium officinale and its polysaccharides enhance immune functions in balb/c mice. Nat. Prod. Commun. 2011, 6, 867–870. [Google Scholar] [PubMed]
- Xia, L.; Liu, X.; Guo, H.; Zhang, H.; Zhu, J.; Ren, F. Partial characterization and immunomodulatory activity of polysaccharides from the stem of Dendrobium officinale (tiepishihu) in vitro. J. Funct. Foods 2012, 4, 294–301. [Google Scholar] [CrossRef]
- Wei, W.; Feng, L.; Bao, W.-R.; Ma, D.-L.; Leung, C.-H.; Nie, S.-P.; Han, Q.-B. Structure characterization and immunomodulating effects of polysaccharides isolated from dendrobium officinale. J. Agric. Food Chem. 2016, 64, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Dubovik, B.V.; Bogomazov, S.D. Multifactorial method for assessing the physical work capacity of mice. Farmakol. Toksikol. 1987, 50, 116–121. [Google Scholar] [PubMed]
- Öztürk, N.; Başer, K.H.C.; Aydin, S.; Öztürk, Y.; Çaliş, I. Effects of gentiana lutea ssp. Symphyandra on the central nervous system in mice. Phytother. Res. 2002, 16, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.X. Method for Preparing Compound Micro Dendrobium officinale Kimura et Migo Granule and Capsule for Nourishing, Reducing Blood Sugar and Anti-Fatigue. Chinese Patents CN1709402 A, 21 December 2005. [Google Scholar]
- Langdon, W.B. Performance of genetic programming optimised bowtie2 on genome comparison and analytic testing (GCAT) benchmarks. BioData Min. 2015, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Yan, M.; Chen, S. Review of pharmacological activities of Dendrobium officinale based on traditional functions. China J. Chin. Mater. Med. 2013, 38, 489–493. [Google Scholar]
- Wakayoshi, K.; Yoshida, T.; Udo, M.; Kasai, T.; Moritani, T.; Mutoh, Y.; Miyashita, M. A simple method for determining critical speed as swimming fatigue threshold in competitive swimming. Int. J. Sports Med. 1992, 13, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Yu, K.; Kang, D.; Suh, H. Anti-stress and anti-fatigue effect of fermented rice bran. Phytother. Res. 2002, 16, 700–702. [Google Scholar] [CrossRef] [PubMed]
- Selsby, J.T.; Beckett, K.D.; Kern, M.; Devor, S.T. Swim performance following creatine supplementation in division iii athletes. J. Strength Cond. Res. 2003, 17, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Hauger, R.L.; Millan, M.A.; Lorang, M.; Harwood, J.P.; Aguilera, G. Corticotropin-releasing factor receptors and pituitary adrenal responses during immobilization stress. Endocrinology 1988, 123, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, R.; Siwicki, A.; Skopinska-Rózewska, E.; Wasiutynski, A.; Sommer, E.; Furmanowa, M. The effect of chinese medicinal herb rhodiola kirilowii extracts on cellular immunity in mice and rats. Pol. J. Vet. Sci. 2009, 12, 399–405. [Google Scholar] [PubMed]
- Sommer, E.; Mielcarek, S.; Buchwald, W.; Krajewska-Patan, A. The influence of Rhodiola rosea extracts on non-specific and specific cellular immunity in pigs, rats and mice. Cent. Eur. J. Immunol. 2007, 32, 84–91. [Google Scholar]
- Guan, S.; He, J.; Guo, W.; Wei, J.; Lu, J.; Deng, X. Adjuvant effects of salidroside from Rhodiola rosea L. on the immune responses to ovalbumin in mice. Immunopharmacol. Immunotoxicol. 2011, 33, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Skopińska-Różewska, E.; Bychawska, M.; Białas-Chromiec, B.; Sommer, E. The in vivo effect of Rhodiola rosea and rhodiola quadrifida hydro-alcoholic extracts on chemokinetic activity of spleen lymphocytes in mice. Cent. Eur. J. Immunol. 2009, 34, 42–45. [Google Scholar]
- Dorchy, H. Sports and type I diabetes: Personal experience. Rev. Med. Brux. 2002, 23, A211–A217. [Google Scholar] [PubMed]
- Singh, A.; Naidu, P.S.; Gupta, S.; Kulkarni, S.K. Effect of natural and synthetic antioxidants in a mouse model of chronic fatigue syndrome. J. Med. Food 2002, 5, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, L.; Mikhaylova, S.V.; Capelli, E.; Ferrari, D.; Ngonga, G.K.; Ricevuti, G. Immunological aspects of chronic fatigue syndrome. Autoimmun. Rev. 2009, 8, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Nie, S.; Cai, H.; Zhang, G.; Cui, S.W.; Xie, M.; Phillips, G.O. Study on Dendrobium officinale O-acetyl-glucomannan (dendronan): Part iv. Immunomodulatory activity in vivo. J. Funct. Foods 2015, 15, 525–532. [Google Scholar] [CrossRef]
- Chen, Y.; Kong, L.D.; Xia, X.; Kung, H.F.; Zhang, L. Behavioral and biochemical studies of total furocoumarins from seeds of psoralea corylifolia in the forced swimming test in mice. J. Ethnopharmacol. 2005, 96, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar] [PubMed]
- Boström, S.; Fahlen, M.; Hjalmarson, A.; Johansson, R. Activities of rat muscle enzymes after acute exercise. Acta Physiol. Scand. 1974, 90, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Busse, C.E.; Czogiel, I.; Braun, P.; Arndt, P.F.; Wardemann, H. Single-cell based high-throughput sequencing of full-length immunoglobulin heavy and light chain genes. Eur. J. Immunol. 2014, 44, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-H.; Lin, K.-C.; Lu, W.-J.; Thomas, P.-A.; Jayakumar, T.; Sheu, J.-R. Astaxanthin, a carotenoid, stimulates immune responses by enhancing ifn-γ and il-2 secretion in primary cultured lymphocytes in vitro and ex vivo. Int. J. Mol. Sci. 2015, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples are not available from the authors.



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, W.; Li, Z.-P.; Zhu, T.; Fung, H.-Y.; Wong, T.-L.; Wen, X.; Ma, D.-L.; Leung, C.-H.; Han, Q.-B. Anti-Fatigue Effects of the Unique Polysaccharide Marker of Dendrobium officinale on BALB/c Mice. Molecules 2017, 22, 155. https://doi.org/10.3390/molecules22010155
Wei W, Li Z-P, Zhu T, Fung H-Y, Wong T-L, Wen X, Ma D-L, Leung C-H, Han Q-B. Anti-Fatigue Effects of the Unique Polysaccharide Marker of Dendrobium officinale on BALB/c Mice. Molecules. 2017; 22(1):155. https://doi.org/10.3390/molecules22010155
Chicago/Turabian StyleWei, Wei, Zhi-Peng Li, Tong Zhu, Hau-Yee Fung, Tin-Long Wong, Xin Wen, Dik-Lung Ma, Chung-Hang Leung, and Quan-Bin Han. 2017. "Anti-Fatigue Effects of the Unique Polysaccharide Marker of Dendrobium officinale on BALB/c Mice" Molecules 22, no. 1: 155. https://doi.org/10.3390/molecules22010155







