Preparation of Thermo-Responsive and Cross-Linked Fluorinated Nanoparticles via RAFT-Mediated Aqueous Polymerization in Nanoreactors
Abstract
:1. Introduction
2. Results and Discussion
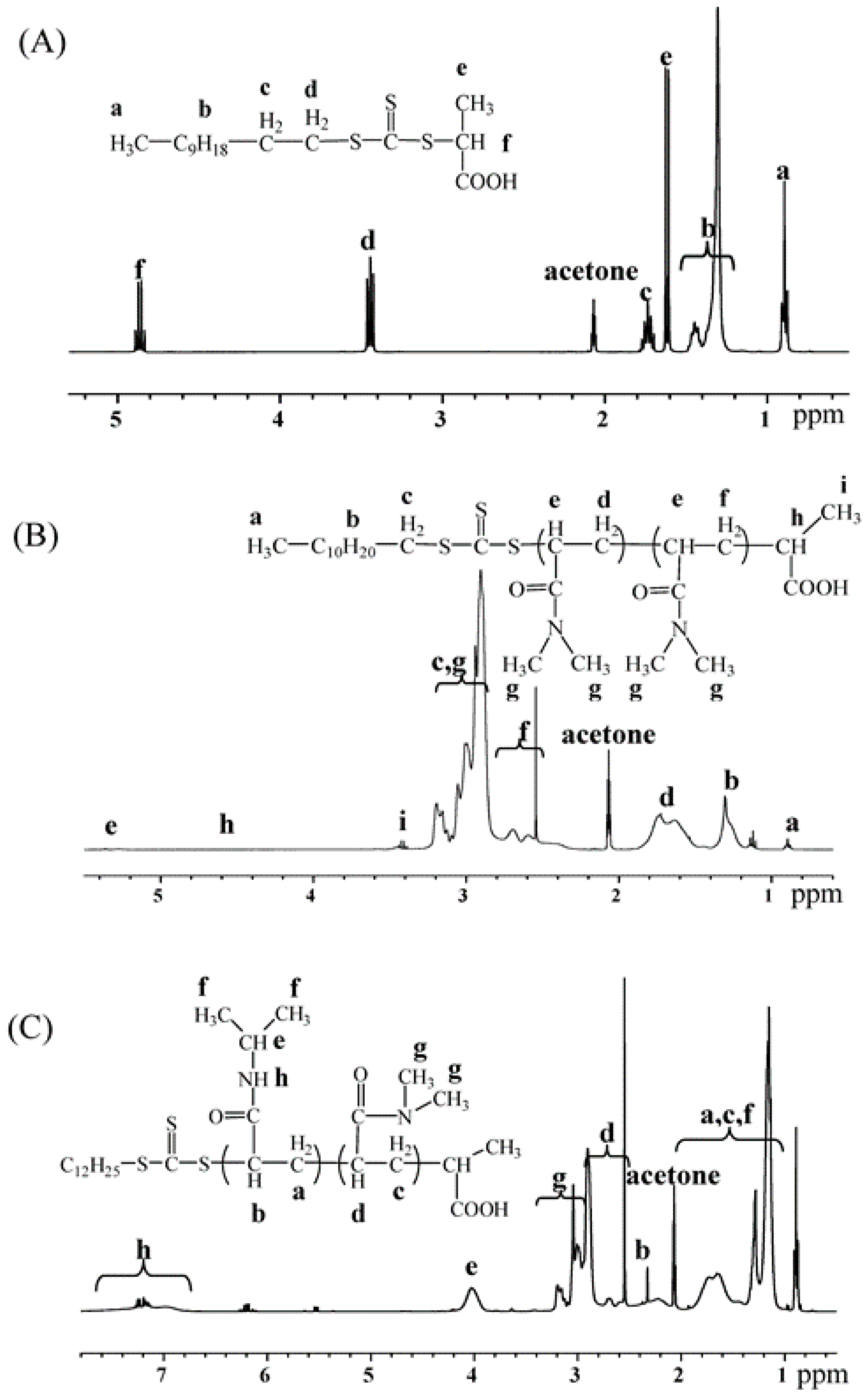
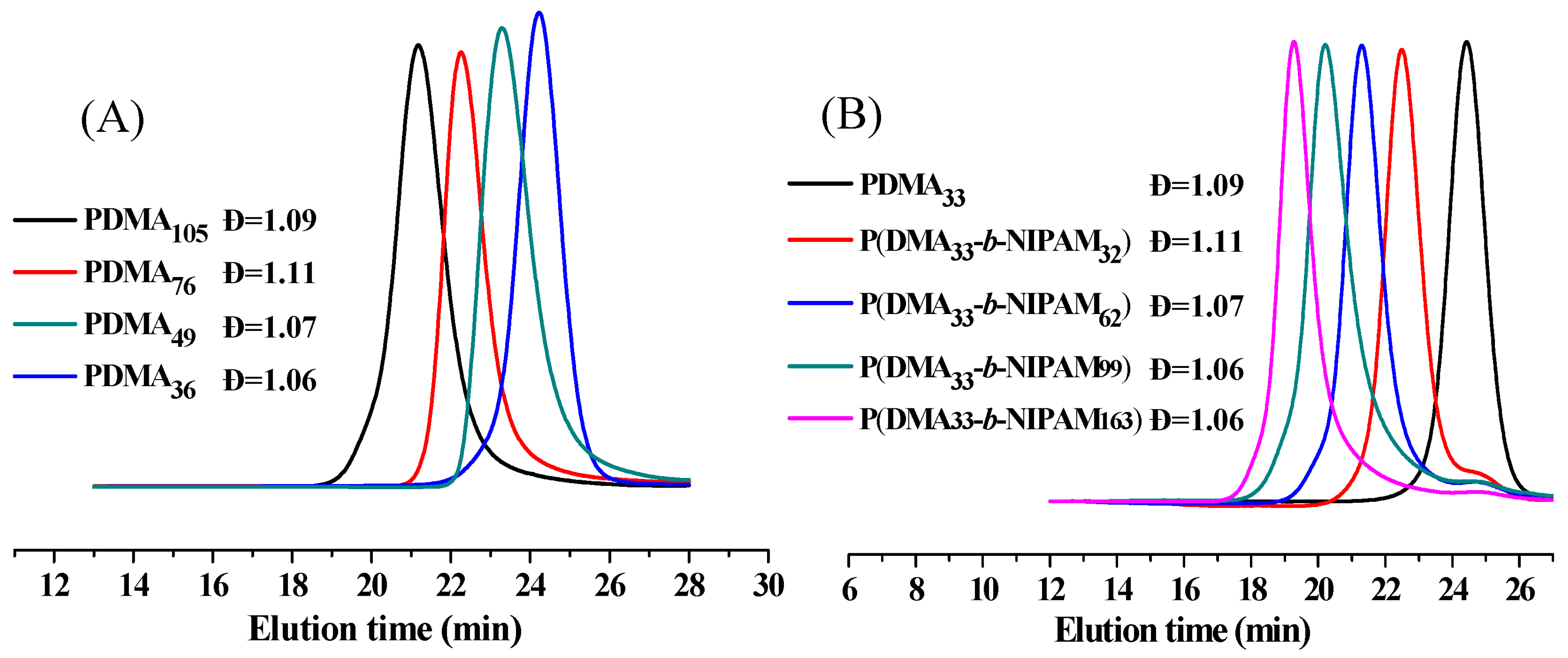
2.1. Synthesis and Characterization of PDMA-S-(C=S)-S-C12H25 and P(DMA-b-NIPAM)-S-(C=S)-S-C12H25
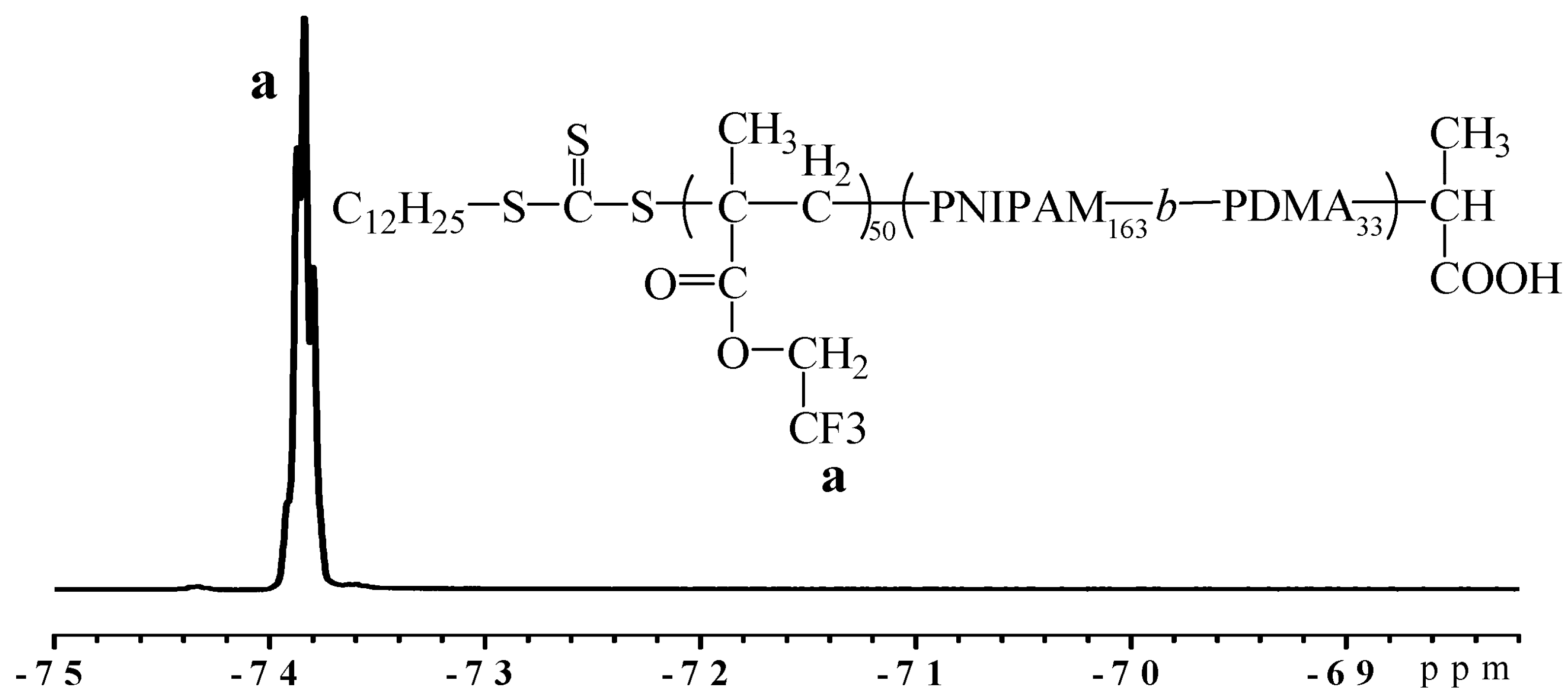
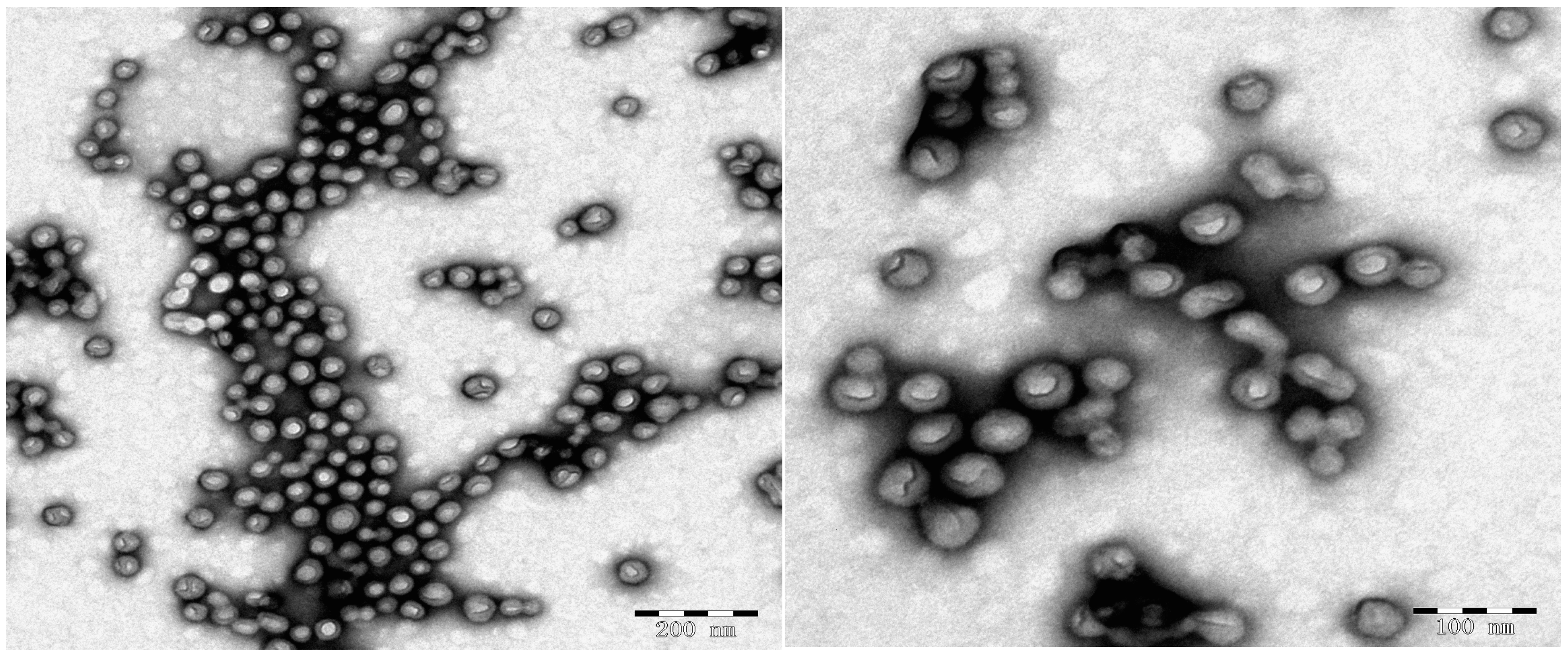
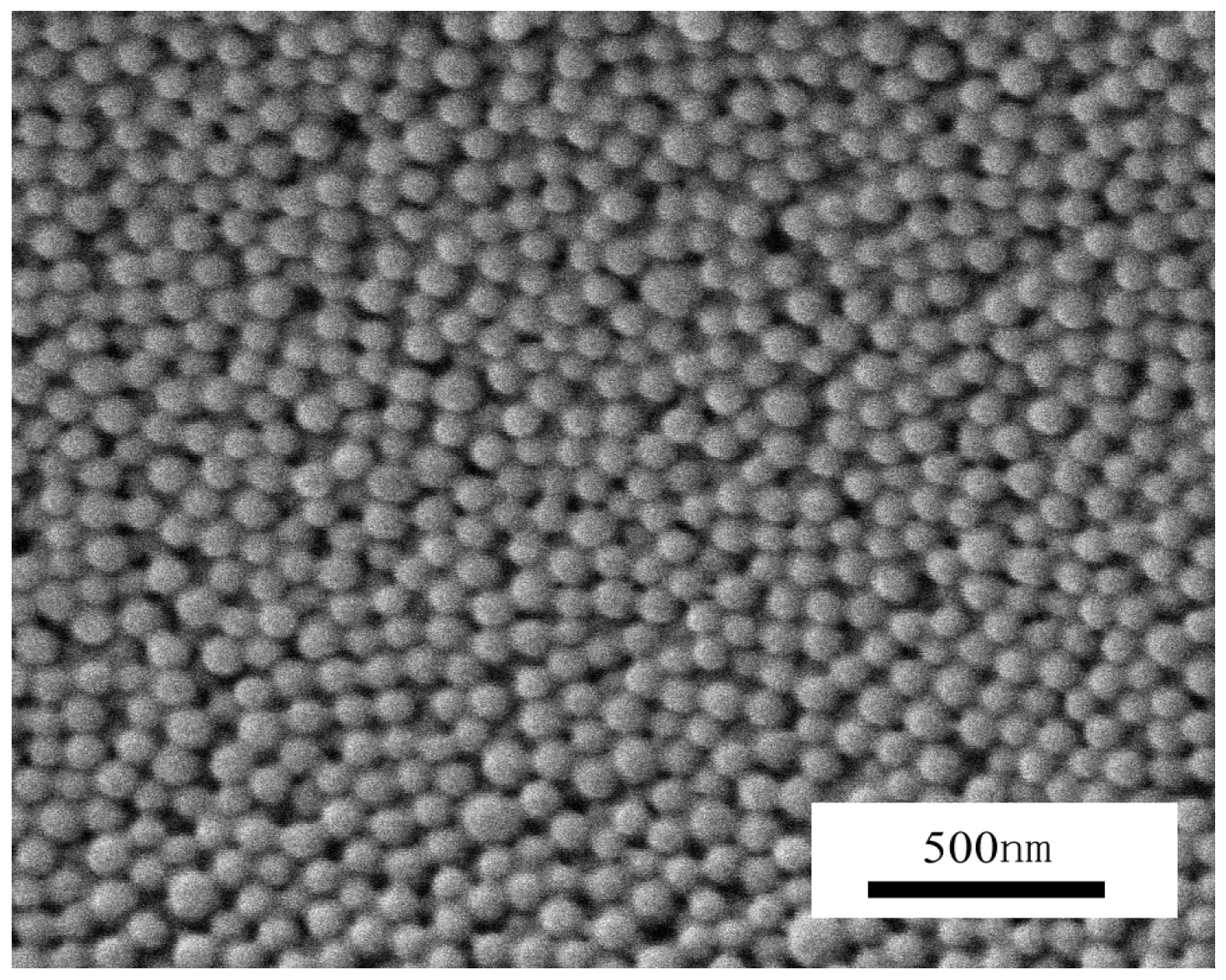
2.2. Crosslinking Polymerization of TFEMA in Nanoreactors Formed by P(DMA-b-NIPAM)-S-(C=S)-S-C12H25
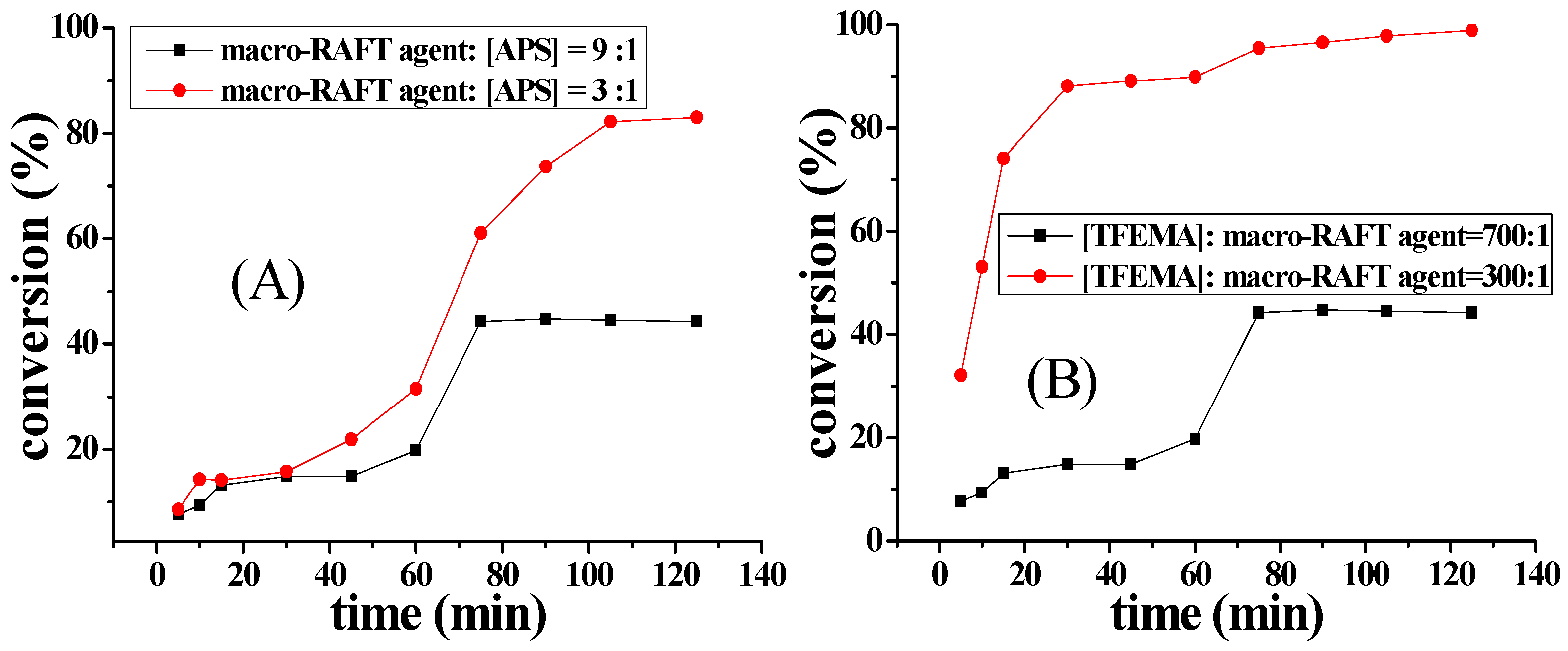
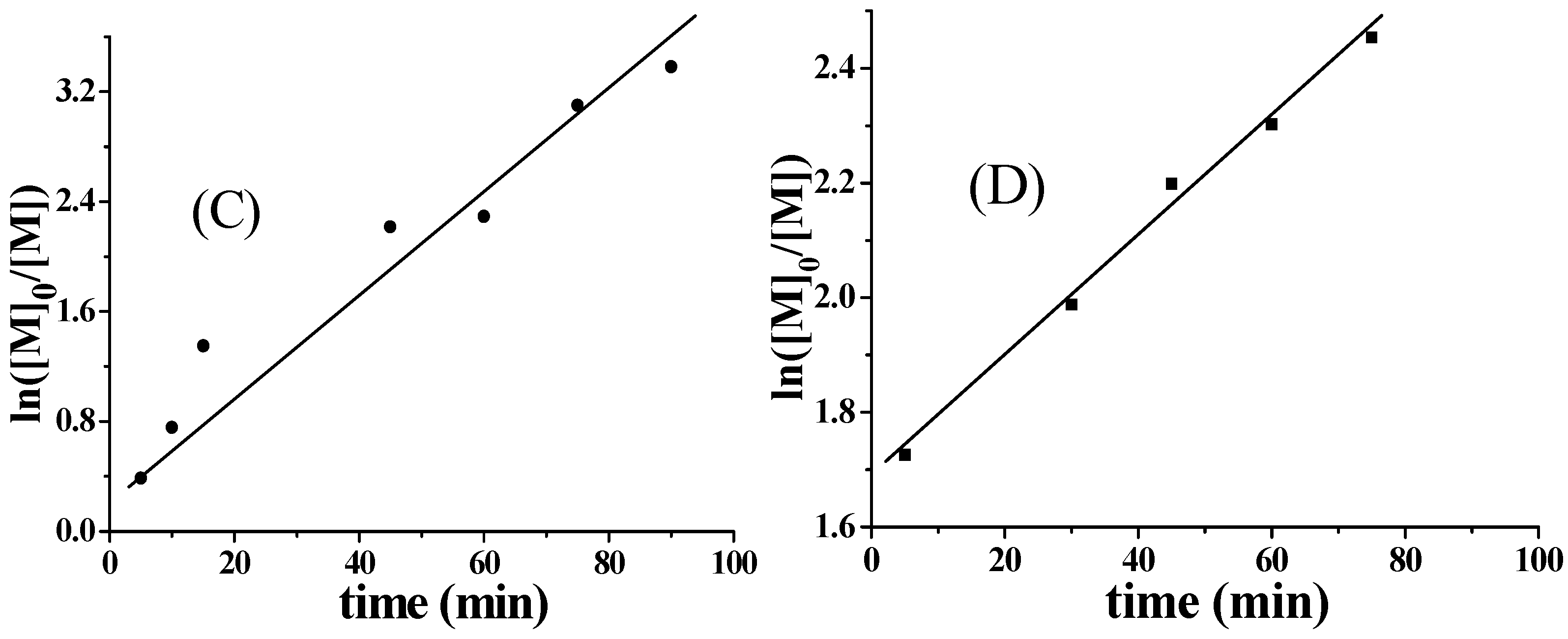
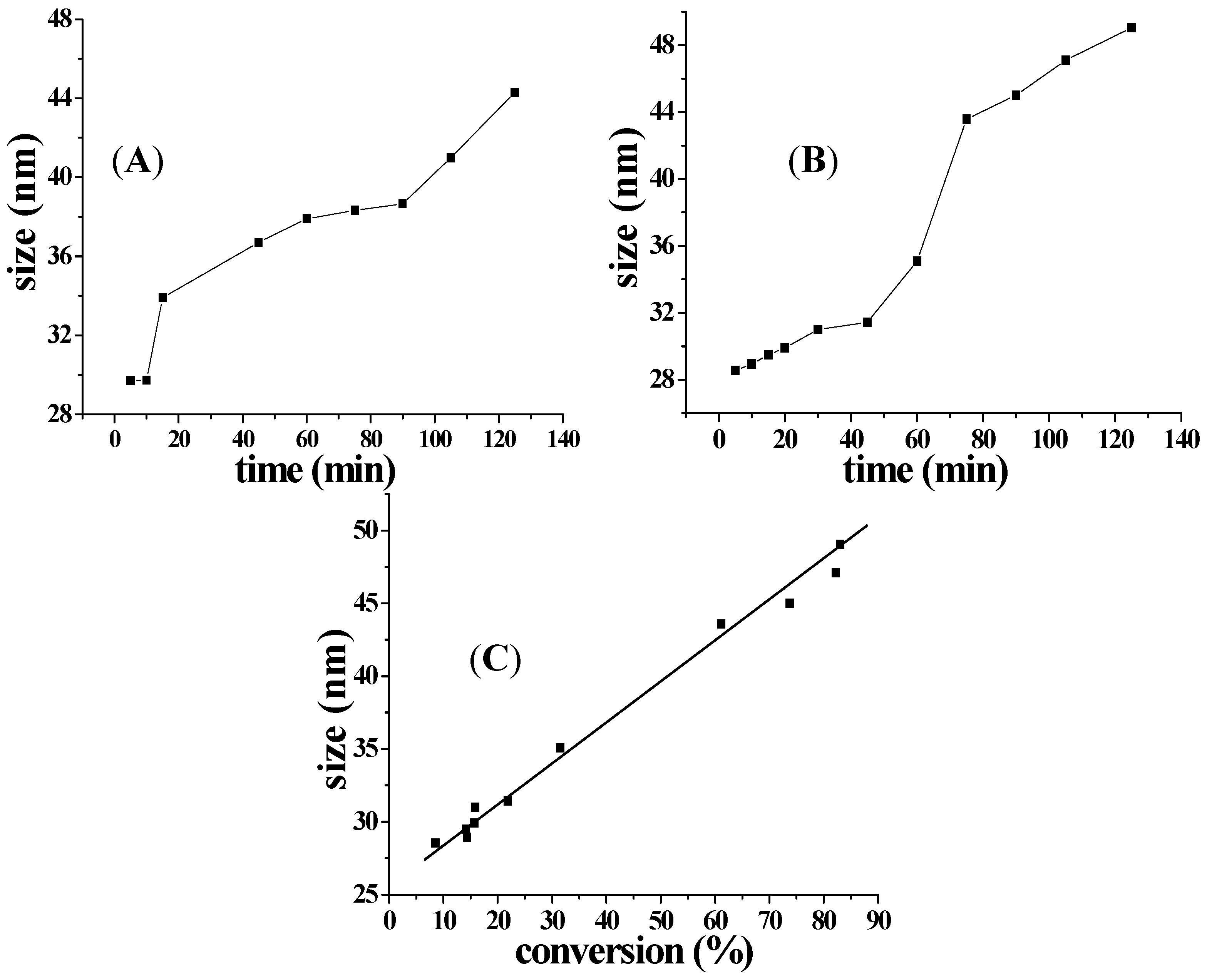
2.3. Influence of the Amount of APS on Aqueous Polymerization
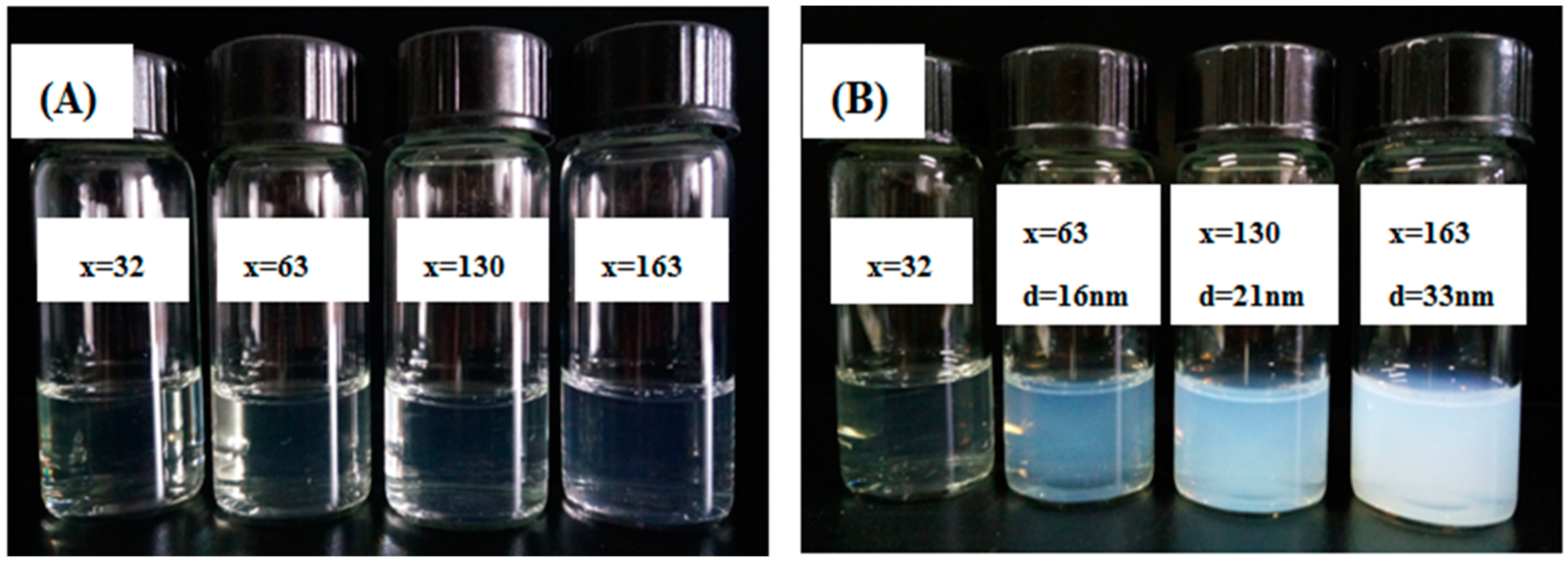
2.4. Influence of Amount of Cross-Linker on Nanoparticles
2.5. Influence of Amount of TFEMA on the Nanoparticles
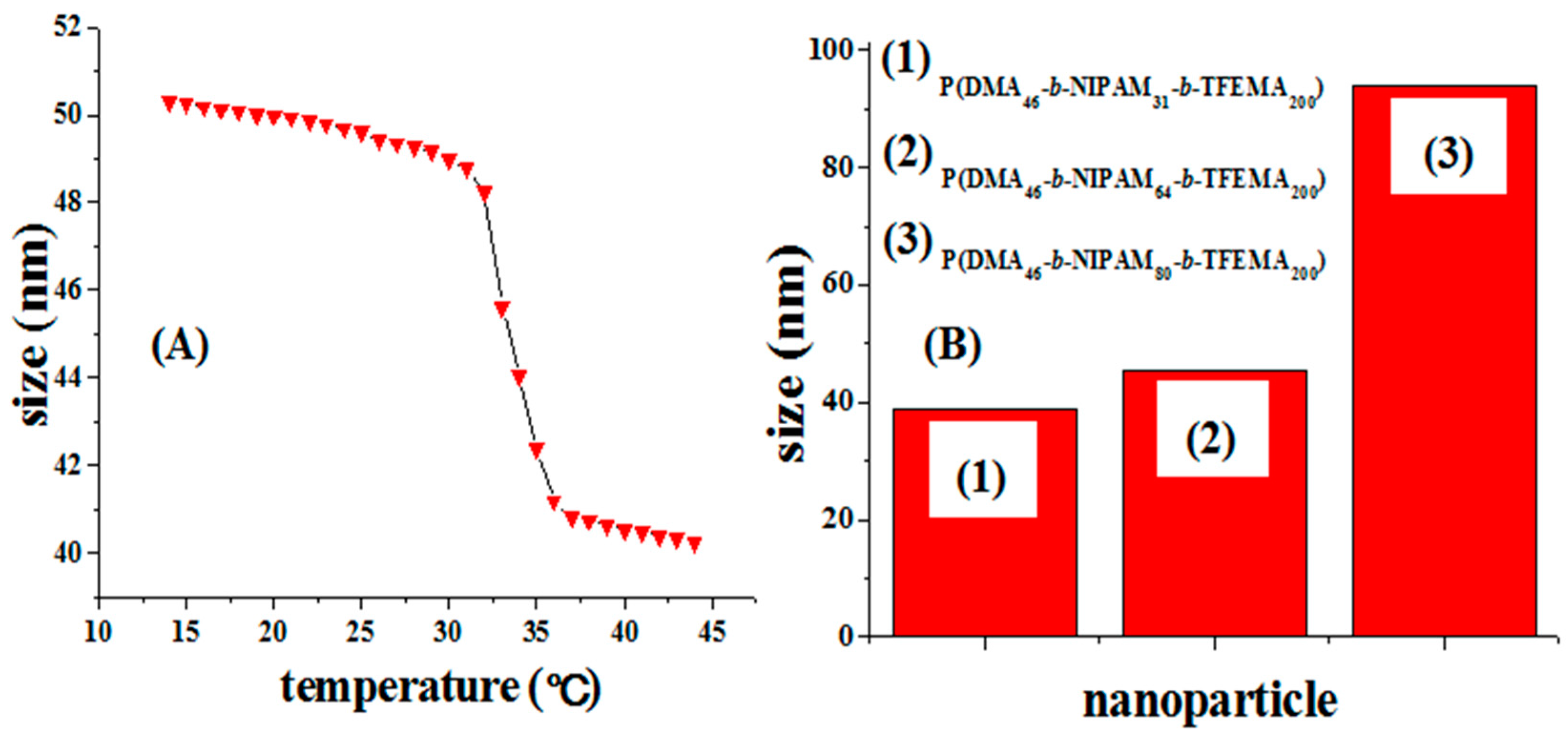
2.6. Thermo-Responsive Characterization of Cross-Linked Nanoparticles
3. Materials and Methods
3.1. Materials and Reagents
3.2. NMR Analysis
3.3. GPC Analysis
3.4. TEM Characterization
3.5. SEM Characterization
3.6. DLS Characterization
3.7. Synthesis of Macro-RAFT Agent of PDMA77-S-(C=S)-S-C12H25
3.8. Synthesis of Thermo-Responsive Diblock P(DMA77-b-NIPAM73)-S-(C=S)-S-C12H25
3.9. Aqueous RAFT-Mediated Polymerization of Cross-Linked TFEMA Nanoparticles in the Presence of P(DMA-b-NIPAM)-S-(C=S)-S-C12H25
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Varma, S.; McLachlan, J.; Leclair, A.M.; Galarreta, B.C.; Norton, P.R.; Lagugné-Labarthet, F. Positionally controlled growth of cells using a cytophobic fluorinated polymer. Anal. Bioanal. Chem. 2010, 396, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Riess, J.G. Blood substitutes and other potential biomedical applications of fluorinated colloids. J. Fluorine Chem. 2002, 114, 119–126. [Google Scholar] [CrossRef]
- Krafift, M.P. Fluorocarbons and fluorinated amphiphiles in drug delivery and biomedical research. Adv. Drug Deliv. Rev. 2001, 47, 209–228. [Google Scholar] [CrossRef]
- Hansen, K.B.; Hsiao, Y.; Xu, F.; Rivera, N.; Clausen, A.; Kubryk, M.; Krska, S.; Rosner, T.; Simmons, B.; Balsells, J.; et al. Highly Efficient Asymmetric Synthesis of Sitagliptin. J. Am. Chem. Soc. 2009, 131, 8798–8804. [Google Scholar] [CrossRef] [PubMed]
- Riess, G.; Labbe, C. Block copolymers in emulsion and dispersion polymerization. Macromol. Rapid Commun. 2004, 25, 401–435. [Google Scholar] [CrossRef]
- Urbani, C.N.; Monteiro, M.J. Nanoreactors for Aqueous RAFT-Mediated Polymerizations. Macromolecules 2009, 42, 3884–3886. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K.; Xia, J. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Moad, G.; Chen, M.; Haussler, M.; Postma, A.; Rizzardo, E.; Thang, S.H. Functional polymers for optoelectronic applications by RAFT polymerization. Polym. Chem. 2011, 2, 492–519. [Google Scholar] [CrossRef]
- Monteiro, M.J.; Hodgson, M.; de Brouwer, H.J. The influence of RAFT on the rates and molecular weight distributions of styrene in seeded emulsion polymerizations. Polym. Sci. Part A Polym. Chem. 2000, 38, 3864–3874. [Google Scholar] [CrossRef]
- Brouwer, H.; Tsavalas, J.G.; Schork, F.J.; Monteiro, M.J. Living radical polymerization in miniemulsion using reversible addition-fragmentation chain transfer. Macromolecules 2000, 33, 9239–9246. [Google Scholar] [CrossRef]
- Ferguson, C.J.; Hughes, R.J.; Pham, B.T.T.; Hawkett, B.S.; Gilbert, R.G.; Serelis, A.K.; Such, C.H. Effective ab initio emulsion polymerization under RAFT Control. Macromolecules 2002, 35, 9243–9245. [Google Scholar] [CrossRef]
- Stoffelbach, F.; Tibiletti, L.; Rieger, J.; Charleux, B. Surfactant-free, controlled/living radical emulsion polymerization in batch conditions using a low molar mass, surface-active reversible addition-fragmentation chain-transfer (RAFT) agent. Macromolecules 2008, 41, 7850–7856. [Google Scholar] [CrossRef]
- Zetterlund, P.B.; Kagawa, Y.; Okubo, M. Controlled/living radical polymerization in dispersed systems. Chem. Rev. 2008, 108, 3747–3794. [Google Scholar] [CrossRef] [PubMed]
- Pepels, M.P.F.; Holdsworth, C.I.; Pascual, S.; Monteiro, M.J. RAFT-mediated emulsion polymerization of styrene with low reactive xanthate agents: Microemulsion-like behavior. Macromolecules 2010, 43, 7565–7576. [Google Scholar] [CrossRef]
- Schönfelder, D.; Fischer, K.; Schmidt, M.; Nuyken, O.; Weberskirch, R. Poly(2-oxazoline) functionalized with palladium carbene complexes: Soluble, amphiphilic polymer supports for C–C coupling reactions in water. Macromolecules 2005, 38, 254–262. [Google Scholar] [CrossRef]
- Schönfelder, D.; Nuyken, O.; Weberskirch, R.J. Heck and Suzuki coupling reactions in water using poly(2-oxazoline)s functionalized with palladium carbene complexes as soluble, amphiphilic polymer supports. Organomet. Chem. 2005, 690, 4648–4655. [Google Scholar] [CrossRef]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Garnier, S.; Laschewsky, A. New amphiphilic diblock copolymers: Surfactant properties and solubilization in their micelles. Langmuir 2006, 22, 4044–4053. [Google Scholar] [CrossRef] [PubMed]
- Save, M.; Manguian, M.; Chassenieux, C.; Charleux, B. Synthesis by RAFT of amphiphilic block and comblike cationic copolymers and their use in emulsion polymerization for the electrosteric stabilization of latexes. Macromolecules 2005, 38, 280–289. [Google Scholar] [CrossRef]
- Rieger, J.; Stoffelbach, F.; Bui, C.; Alaimo, D.; Jérôme, C.; Charleux, B. Amphiphilic poly(ethylene oxide) macromolecular RAFT agent as a stabilizer and control agent in ab initio batch emulsion polymerization. Macromolecules 2008, 41, 4065–4068. [Google Scholar] [CrossRef]
- Ganeva, D.E.; Sprong, E.; Bruyn, H.D.; Warr, G.G.; Such, C.H.; Hawkett, B.S. Particle formation in ab initio RAFT mediated emulsion polymerization systems. Macromolecules 2007, 40, 6181–6189. [Google Scholar] [CrossRef]
- Winnik, F.M. Fluorescence studies of aqueous solutions of poly(N-isopropylacrylamide) below and above their LCST. Macromolecules 1990, 23, 233–242. [Google Scholar] [CrossRef]
- Valade, D.; Jeon, Y.; Kessel, S.; Monteiro, M.J. Influence of the Z-group on the RAFT-mediated polymerizations in nanoreactors. Polym. Sci. Part A Polym. Chem. 2012, 50, 4762–4771. [Google Scholar] [CrossRef]
- Gupta, M.K.; Martin, J.R.; Werfel, T.A.; Shen, T.W.; Page, J.M.; Duvall, C.L. Cell Protective, ABC Triblock Polymer-Based Thermoresponsive Hydrogels with ROS-Triggered Degradation and Drug Release. J. Am. Chem. Soc. 2014, 136, 14896–14902. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Q.; Duhamel, J. Extraction of Oil from Oil Sands Using Thermoresponsive Polymeric Surfactants. ACS Appl. Mater. Interfaces 2015, 7, 5879–5889. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.H.; Wang, H.L.; Zhang, L.; Ma, J.Z. Ab initio reversible addition-fragmentation chain transfer emulsion polymerization of styrene/butyl acrylate mediated by poly(acrylic acid)-block-polystyrene trithiocarbonate. Polym Int. 2014, 63, 2098–2104. [Google Scholar] [CrossRef]
- Zhou, Y.X. Synthesis of three trithiocarbonates reversible addition-fragmentation chain transfer agents by phase transfer catalysis reaction. Contemp. Chem. Ind. 2013, 42, 152–155. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
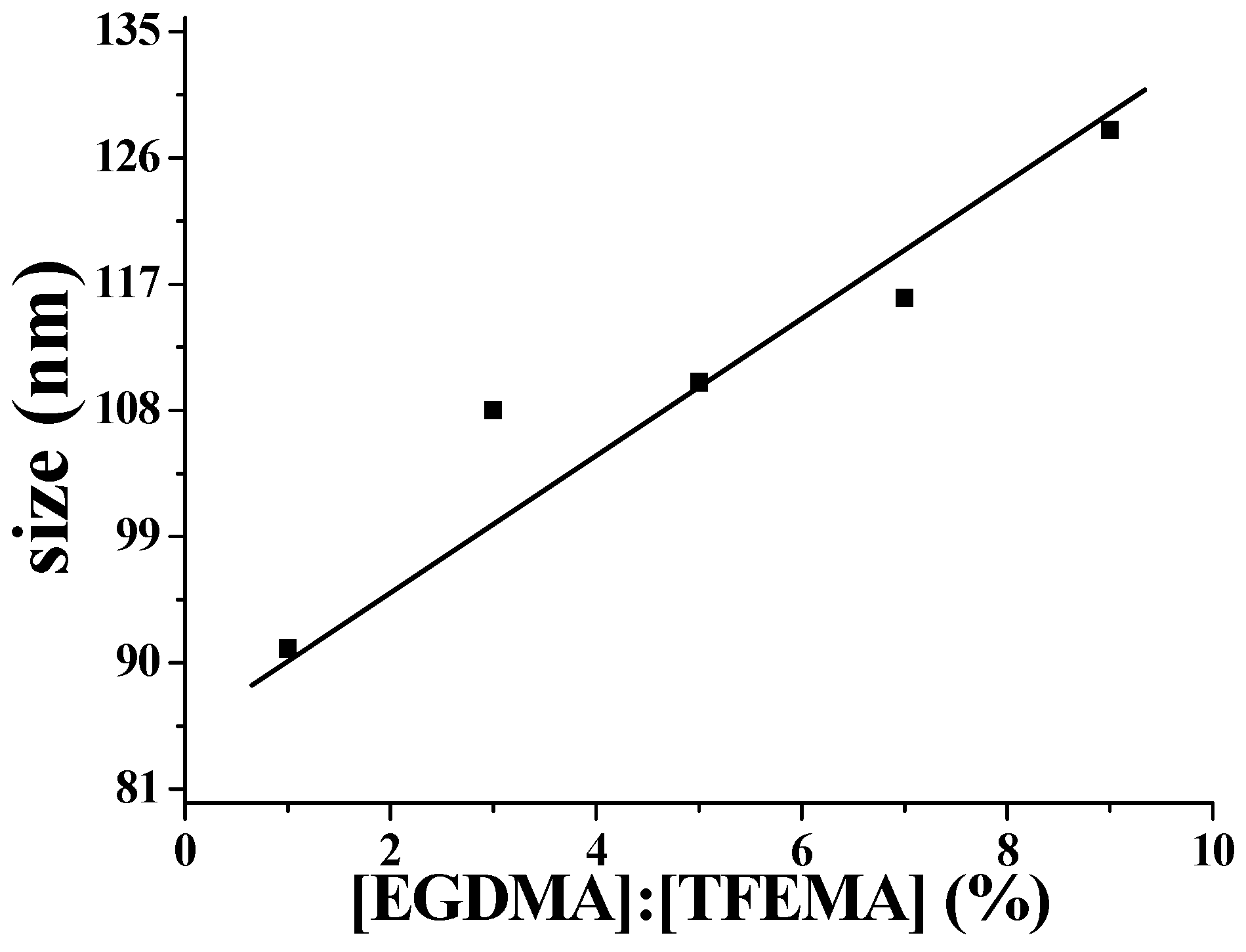
| [DMA]:[DOPAT] | Mn,target a (g/mol) | x b (%) | Mn,theory c (g/mol) | Mn,GPC d | Ð e |
|---|---|---|---|---|---|
| 35:1 | 3800 | 97 | 3700 | 3900 | 1.09 |
| 100:1 | 10,250 | 84 | 8700 | 7500 | 1.11 |
| 150:1 | 15,200 | 73 | 11,200 | 10,700 | 1.09 |
| [NIPAM]:[macro-CTA] | Mn,target a (g/mol) | x b (%) | Mn,theory c (g/mol) | Mn,GPC d | Ð e |
|---|---|---|---|---|---|
| 40:1 | 8100 | 92 | 7700 | 7200 | 1.07 |
| 100:1 | 17,200 | 78 | 12,400 | 14,800 | 1.16 |
| 250:1 | 31,800 | 64 | 20,300 | 22,000 | 1.17 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Zhang, L.; Geng, B.; Azhar, U.; Xu, A.; Zhang, S. Preparation of Thermo-Responsive and Cross-Linked Fluorinated Nanoparticles via RAFT-Mediated Aqueous Polymerization in Nanoreactors. Molecules 2017, 22, 152. https://doi.org/10.3390/molecules22020152
Ma J, Zhang L, Geng B, Azhar U, Xu A, Zhang S. Preparation of Thermo-Responsive and Cross-Linked Fluorinated Nanoparticles via RAFT-Mediated Aqueous Polymerization in Nanoreactors. Molecules. 2017; 22(2):152. https://doi.org/10.3390/molecules22020152
Chicago/Turabian StyleMa, Jiachen, Luqing Zhang, Bing Geng, Umair Azhar, Anhou Xu, and Shuxiang Zhang. 2017. "Preparation of Thermo-Responsive and Cross-Linked Fluorinated Nanoparticles via RAFT-Mediated Aqueous Polymerization in Nanoreactors" Molecules 22, no. 2: 152. https://doi.org/10.3390/molecules22020152
APA StyleMa, J., Zhang, L., Geng, B., Azhar, U., Xu, A., & Zhang, S. (2017). Preparation of Thermo-Responsive and Cross-Linked Fluorinated Nanoparticles via RAFT-Mediated Aqueous Polymerization in Nanoreactors. Molecules, 22(2), 152. https://doi.org/10.3390/molecules22020152





