Time-Resolved Fluorescence Spectroscopy and Fluorescence Lifetime Imaging Microscopy for Characterization of Dendritic Polymer Nanoparticles and Applications in Nanomedicine
Abstract
:1. Introduction
1.1. Time-Resolved Fluorescence Spectroscopy and Imaging in Relation to Nanomedicine and Pharmaceutics
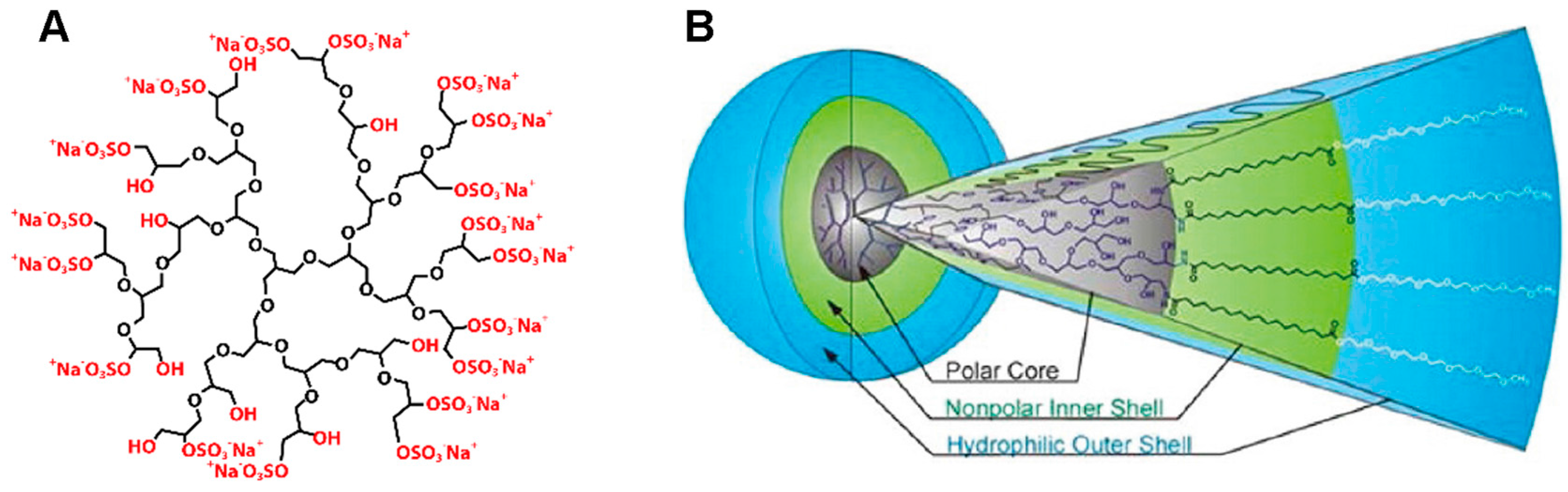
1.2. Dendritic Nanoparticles
2. Basic Concepts of Time-Resolved Fluorescence
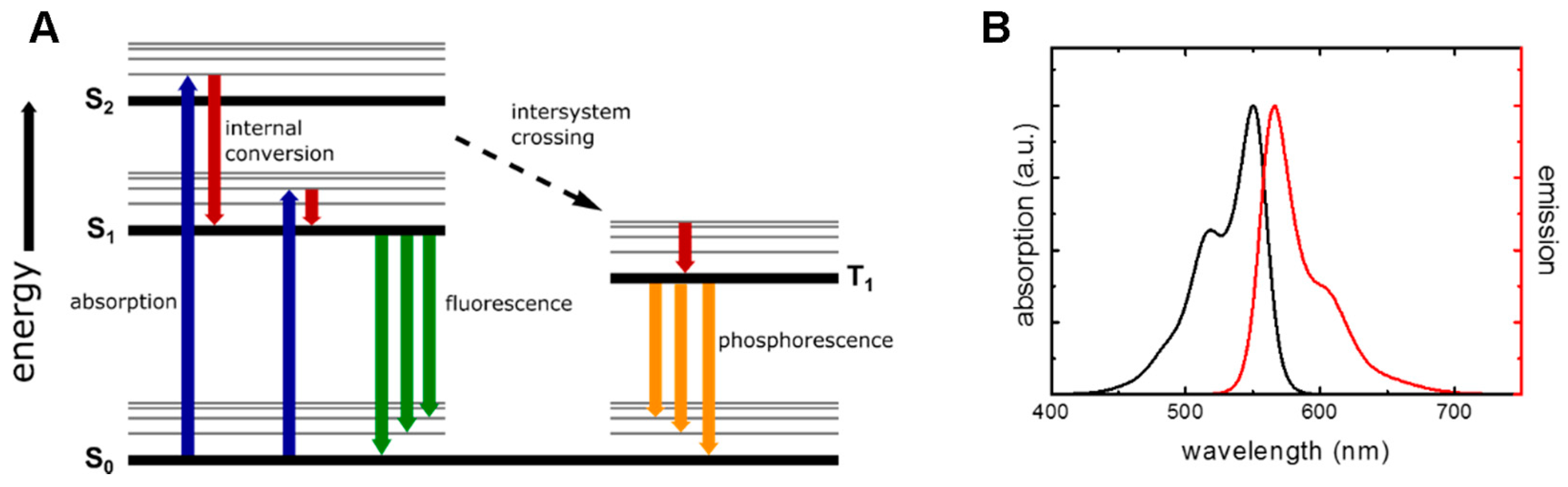
2.1. Fluorescence Properties and Lifetime
2.2. Fluorescence Quenching and Energy Transfer Mechanisms
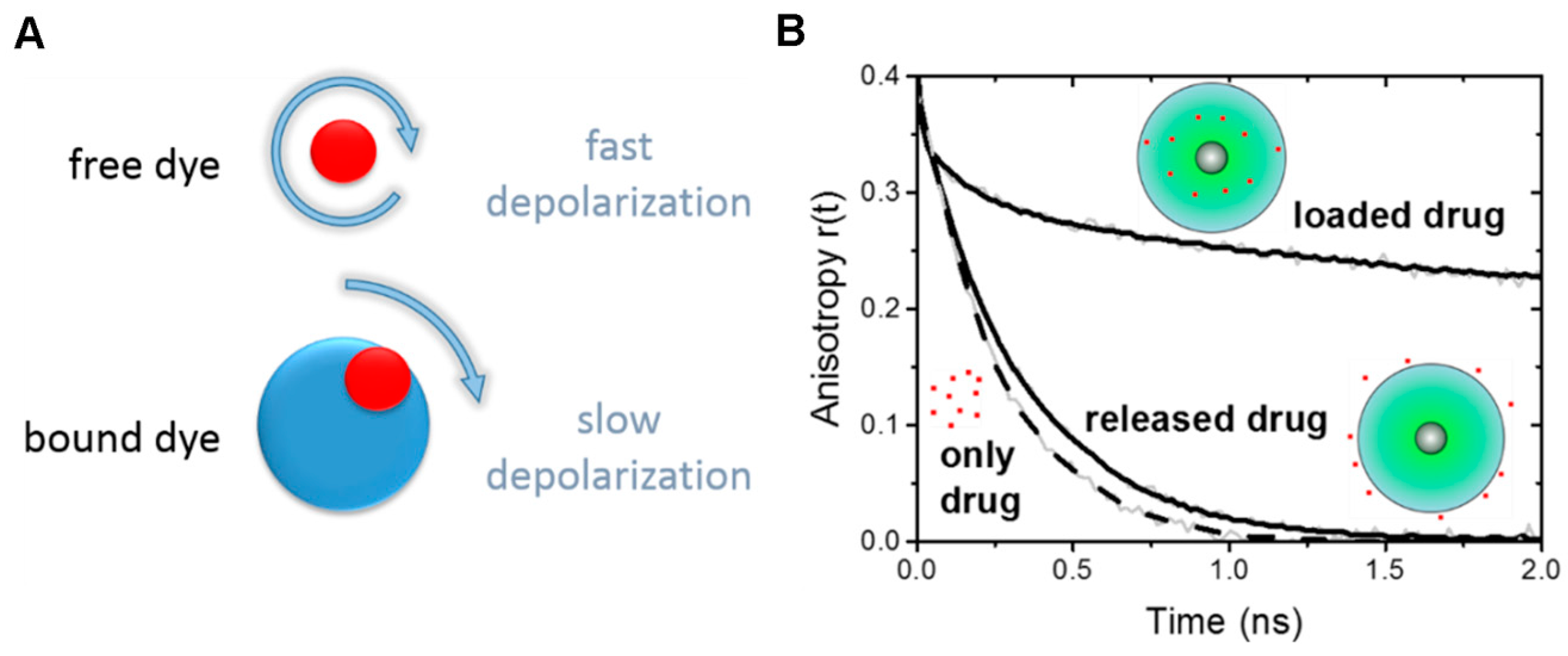
2.3. Time-Resolved Fluorescence Anisotropy
2.4. Measurement of Fluorescence Lifetime—Spectroscopy
2.5. Measurement of Fluorescence Lifetime—Microscopy
3. Molecular Properties of Dendritic Nanoparticles
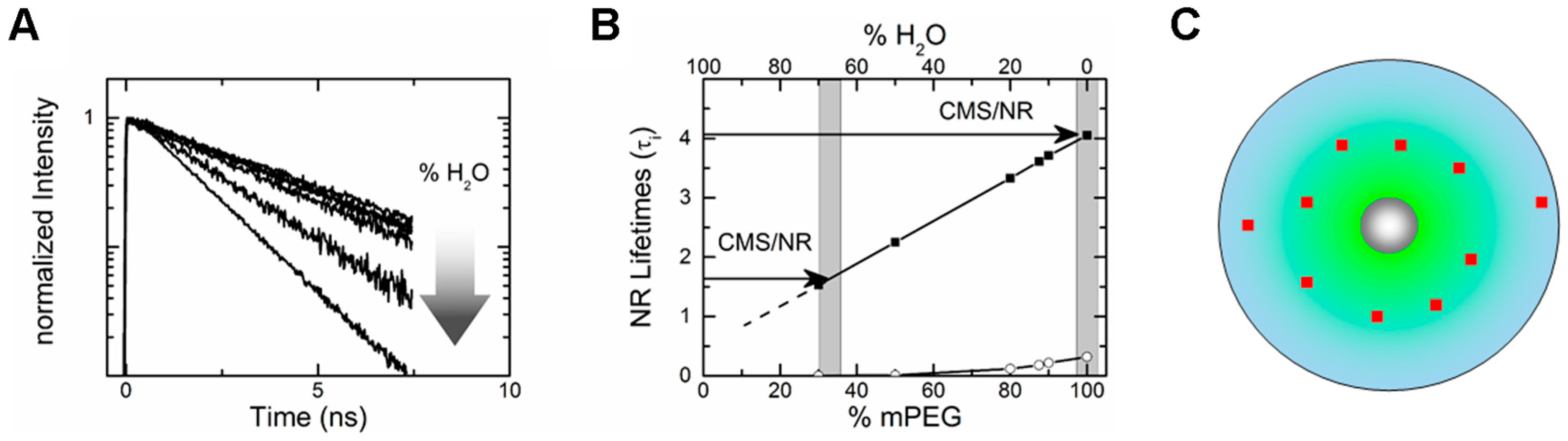
3.1. Drug Binding and Release from the Nanoparticle (via Time-Resolved Anisotropy)
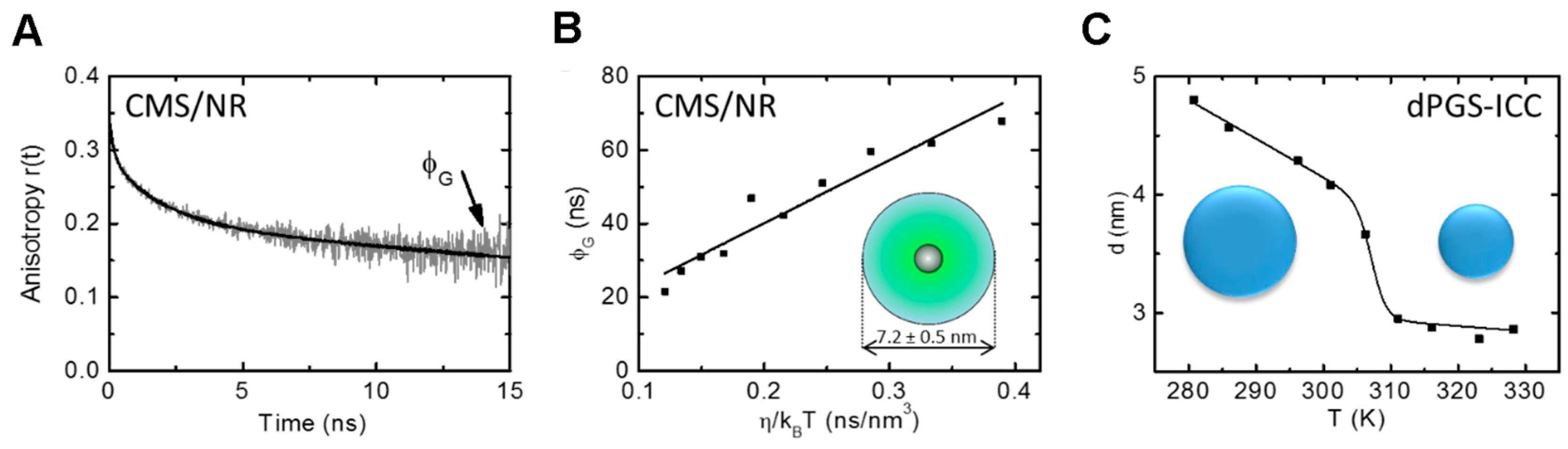
3.2. Nanoparticle Size—Unimer (via Time-Resolved Anisotropy)
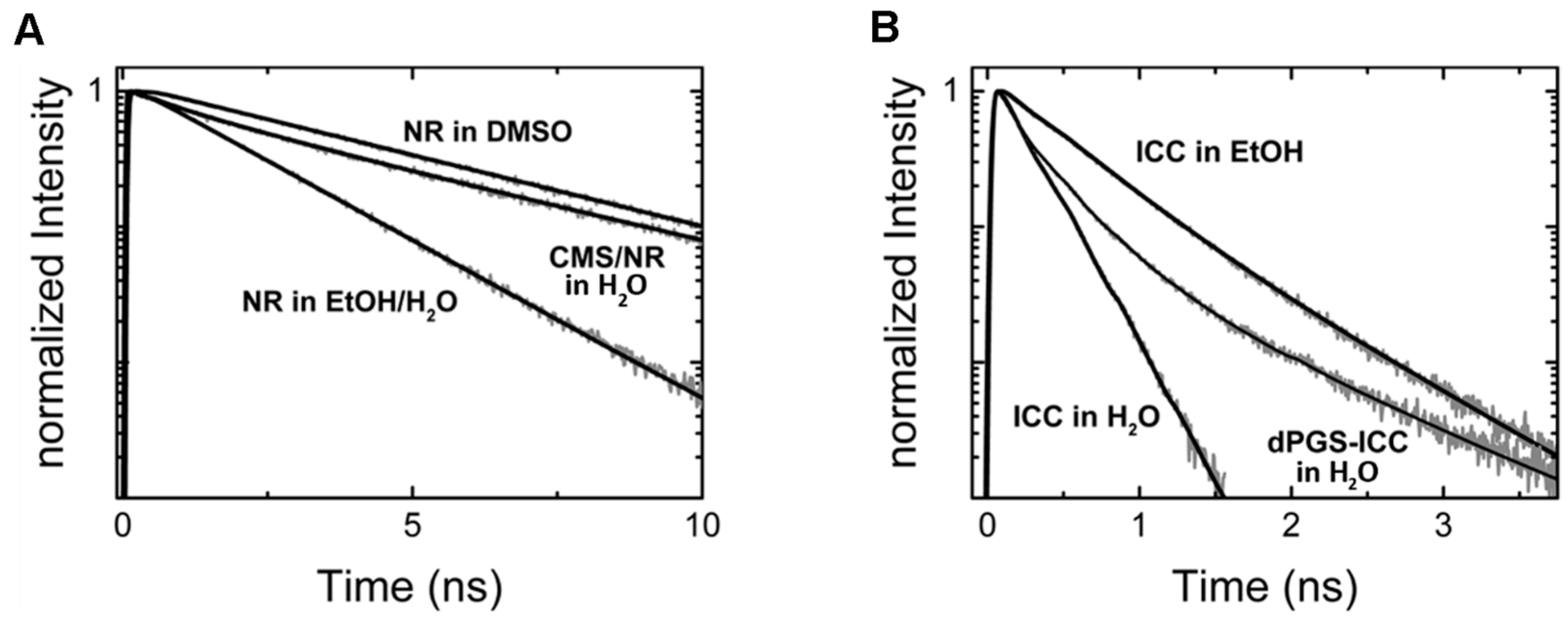
3.3. Drug Partitioning in Nanocarrier (via Fluorescence Lifetime)
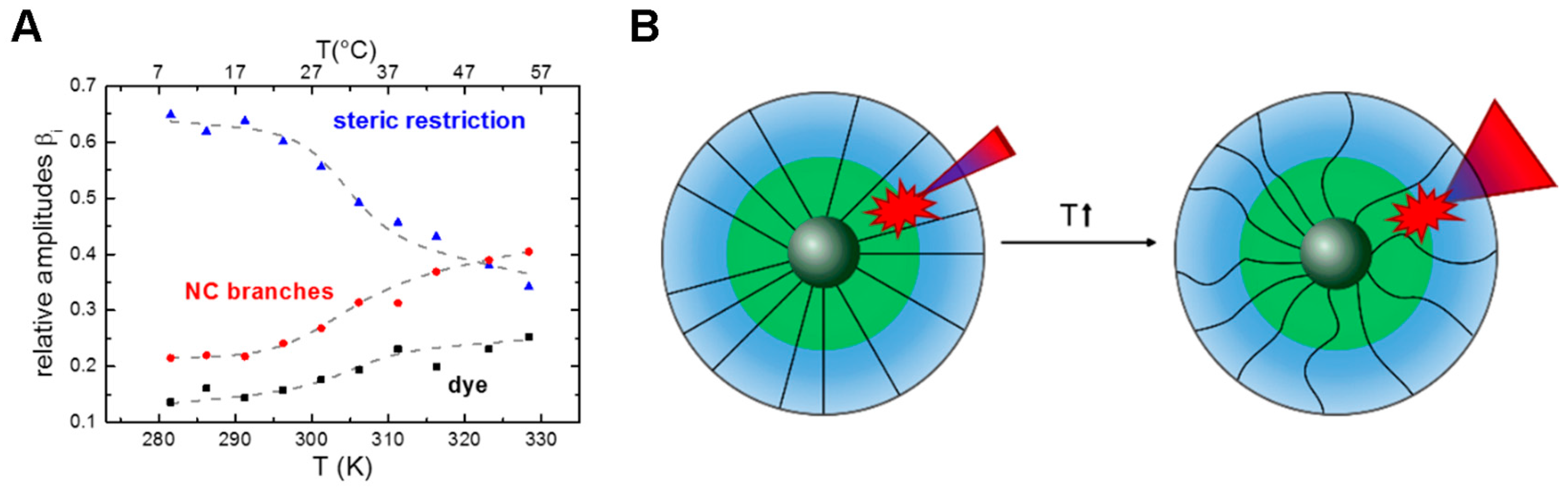
3.4. Conformational Flexibility of Nanocarriers (via Time-Resolved Anisotropy)
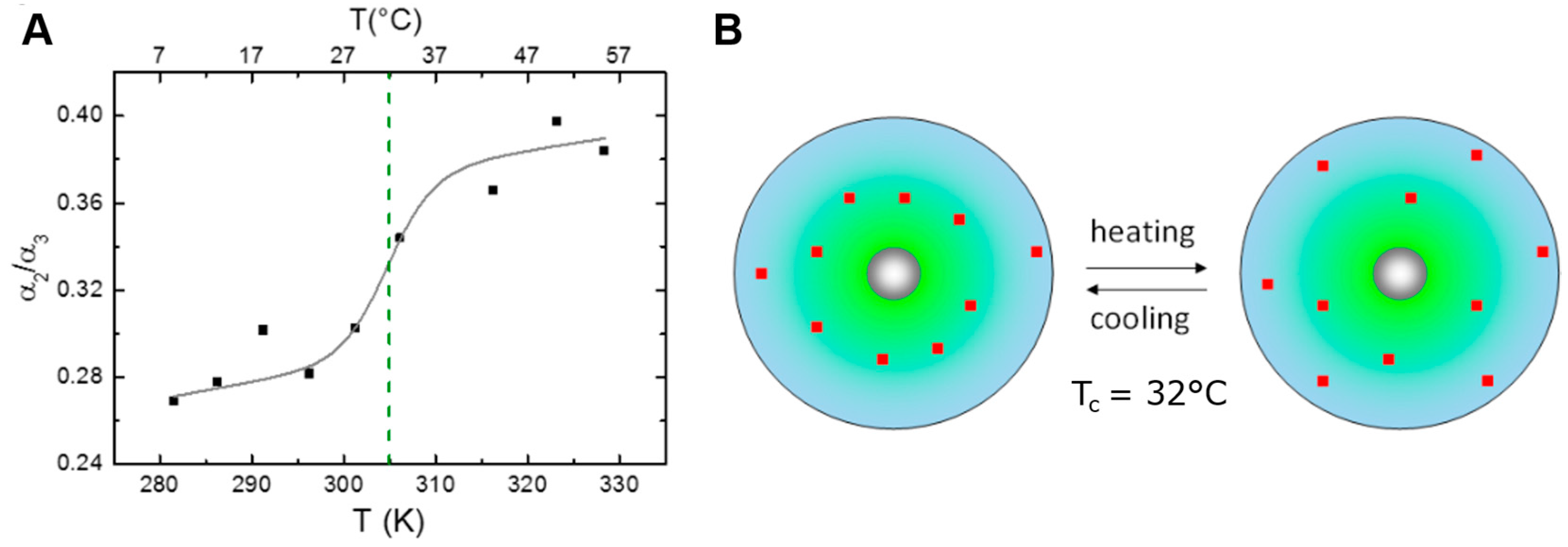
3.5. Conformational Transitions Affecting Drug Partitioning (Temperature Dependent Anisotropy, Temperature Dependent Lifetime, Two-State Transitions)
4. From Molecular to Cellular/Tissue Insight
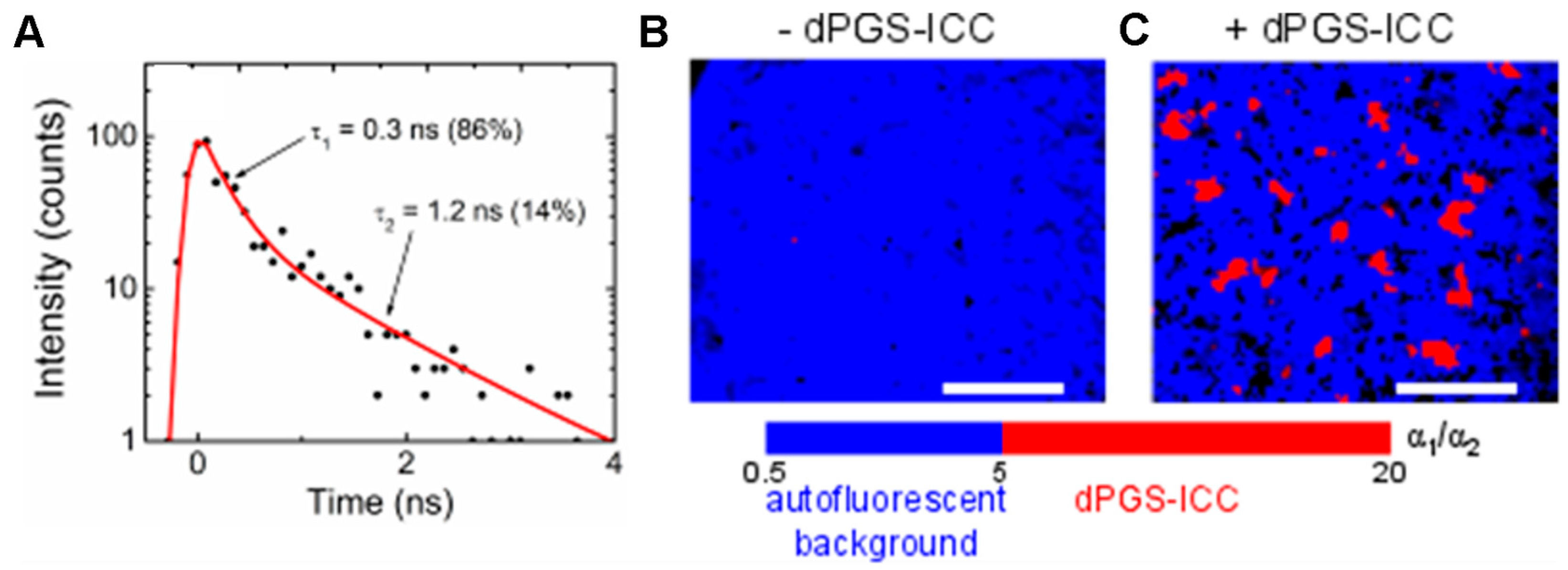
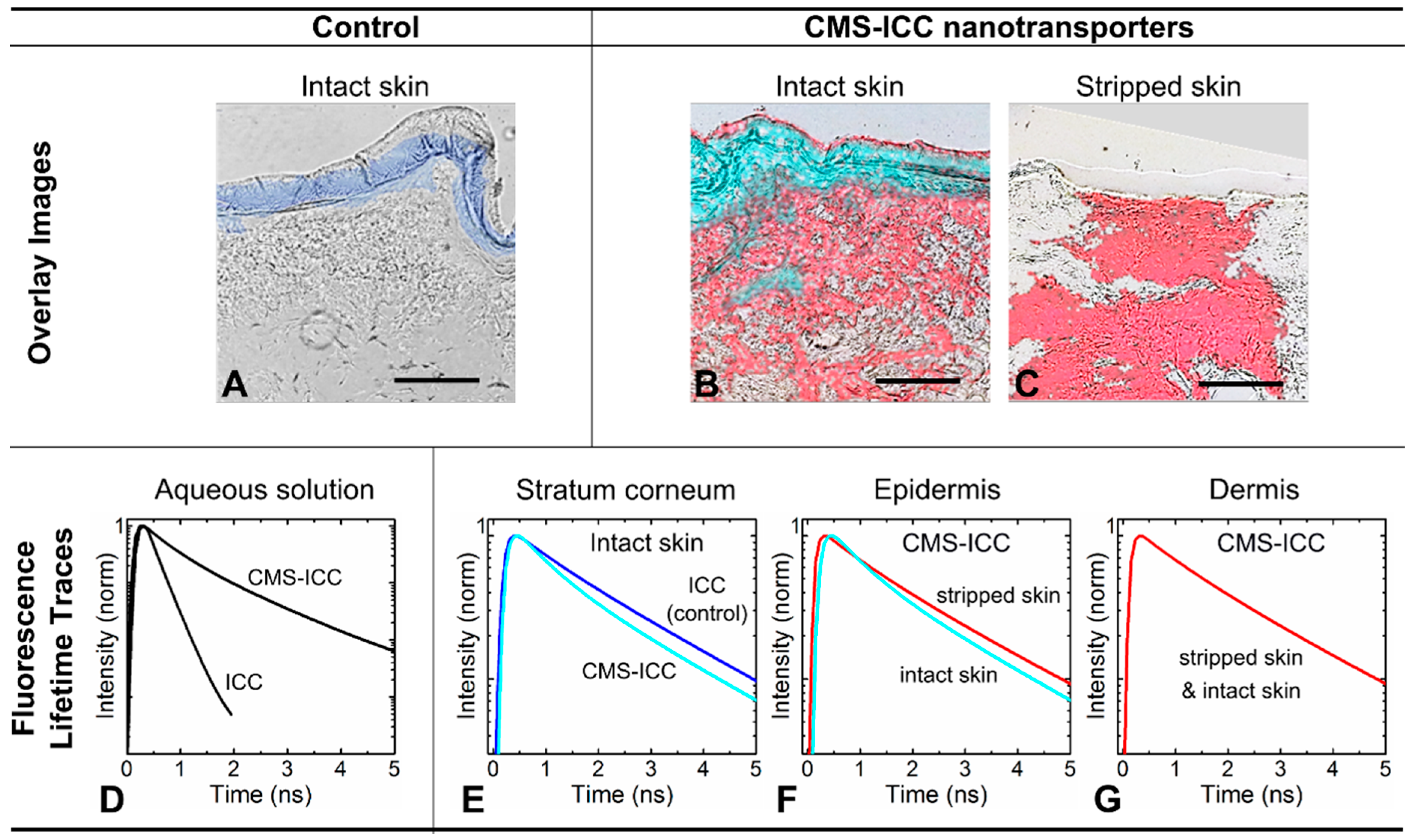
4.1. Distinguishing Between Nanoparticles and Autofluorescence Using the Unique Fluorescence Lifetime Signature
4.2. Unique Fluorescence Lifetime Signature as a Tool to Detect Environmental Effects
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CMS | Core-multi-shell |
| CMS-ICC | Indocarbocyanine bound to core-multi-shell nanocarrier |
| dPGS | Dendritic polyglycerol sulfate |
| dPGS-ICC | Indocarbocyanine bound to dendritic polyglycerol sulfate |
| FLIM | Fluorescence lifetime imaging microscopy |
| FRET | Förster resonance energy tranfer |
| ICC | Indocarbocyanine |
| TCSPC | Time-correlated single photon counting |
References
- Rizzo, L.Y.; Theek, B.; Storm, G.; Kiessling, F.; Lammers, T. Recent progress in nanomedicine: Therapeutic, diagnostic and theranostic applications. Curr. Opin. Biotechnol. 2013, 24, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Thorley, A.J.; Tetley, T.D. New perspectives in nanomedicine. Pharmacol. Ther. 2013, 140, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. Faseb. J. 2005, 19, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Alexiev, U.; Farrens, D.L. Fluorescence spectroscopy of rhodopsins: Insights and approaches. Biochim. Biophys. Acta 2014, 1837, 694–709. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, T.P.; Lim, Y.H.; Flores, J.A.; Heo, G.S.; Zhang, F.; Zhang, S.; Samarajeewa, S.; Raymond, J.E.; Wooley, K.L. Holistic assessment of covalently labeled core-shell polymeric nanoparticles with fluorescent contrast agents for theranostic applications. Langmuir 2014, 30, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.; Wischke, C.; Neffe, A.T.; Ma, N.; Alexiev, U.; Lendlein, A. Nanocarriers for drug delivery into and through the skin—do existing technologies match clinical challenges? J. Control. Release 2016, 242, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Festy, F.; Ameer-Beg, S.M.; Ng, T.; Suhling, K. Imaging proteins in vivo using fluorescence lifetime microscopy. Mol. Biosyst. 2007, 3, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, R.I.; Papkovsky, D.B. Optical probes and techniques for O2 measurement in live cells and tissue. Cell. Mol. Life Sci. 2012, 69, 2025–2039. [Google Scholar] [CrossRef] [PubMed]
- Hille, C.; Berg, M.; Bressel, L.; Munzke, D.; Primus, P.; Lohmannsroben, H.G.; Dosche, C. Time-domain fluorescence lifetime imaging for intracellular pH sensing in living tissues. Anal. Bioanal. Chem. 2008, 391, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef] [PubMed]
- Alnasif, N.; Zoschke, C.; Fleige, E.; Brodwolf, R.; Boreham, A.; Ruhl, E.; Eckl, K.M.; Merk, H.F.; Hennies, H.C.; Alexiev, U.; et al. Penetration of normal, damaged and diseased skin—An in vitro study on dendritic core-multishell nanotransporters. J. Control. Release 2014, 185, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Boreham, A.; Kim, T.Y.; Spahn, V.; Stein, C.; Mundhenk, L.; Gruber, A.D.; Haag, R.; Welker, P.; Licha, K.; Alexiev, U. Exploiting Fluorescence Lifetime Plasticity in FLIM: Target Molecule Localization in Cells and Tissues. ACS Med. Chem. Lett. 2011, 2, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, A.; Nordmeyer, D.; Boreham, A.; Brodwolf, R.; Mundhenk, L.; Fluhr, J.W.; Lademann, J.; Graf, C.; Ruhl, E.; Alexiev, U.; Gruber, A.D. Skin barrier disruptions in tape stripped and allergic dermatitis models have no effect on dermal penetration and systemic distribution of AHAPS-functionalized silica nanoparticles. Nanomedicine 2014, 10, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, A.; Nordmeyer, D.; Boreham, A.; Holzhausen, C.; Mundhenk, L.; Graf, C.; Meinke, M.C.; Vogt, A.; Hadam, S.; Lademann, J.; Ruhl, E.; Alexiev, U.; Gruber, A.D. Overview about the localization of nanoparticles in tissue and cellular context by different imaging techniques. Beilstein J. Nanotech. 2015, 6, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Boreham, A.; Brodwolf, R.; Pfaff, M.; Kim, T.-Y.; Schlieter, T.; Mundhenk, L.; Gruber, A.D.; Gröger, D.; Licha, K.; Haag, R.; Alexiev, U. Temperature and environment dependent dynamic properties of a dendritic polyglycerol sulfate. Polym Adv. Technol. 2014, 25, 1329–1336. [Google Scholar] [CrossRef]
- Witting, M.; Boreham, A.; Brodwolf, R.; Vavrova, K.; Alexiev, U.; Friess, W.; Hedtrich, S. Interactions of hyaluronic acid with the skin and implications for the dermal delivery of biomacromolecules. Mol. Pharmaceutics 2015, 12, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Gaspar, R. Nanomedicine(s) under the Microscope. Mol. Pharmaceut 2011, 8, 2101–2141. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A. Special Issue: “Functional Dendrimers”. Molecules 2016, 21, 1035. [Google Scholar] [CrossRef] [PubMed]
- Lukowiak, M.C.; Thota, B.N.; Haag, R. Dendritic core-shell systems as soft drug delivery nanocarriers. Biotechnol. Adv. 2015, 33, 1327–1341. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, A.Y.; Cheprakov, A.V.; Sakadzic, S.; Boas, D.A.; Wilson, D.F.; Vinogradov, S.A. Dendritic Phosphorescent Probes for Oxygen Imaging in Biological Systems. Acs. Appl. Mater. Inter. 2009, 1, 1292–1304. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.S.; Li, Z.B.A.; Loh, X.J. Recent development of unimolecular micelles as functional materials and applications. Polym. Chem. 2016, 7, 5898–5919. [Google Scholar] [CrossRef]
- Wang, D.L.; Zhao, T.Y.; Zhu, X.Y.; Yan, D.Y.; Wang, W.X. Bioapplications of hyperbranched polymers. Chem Soc. Rev. 2015, 44, 4023–4071. [Google Scholar] [CrossRef] [PubMed]
- Calderón, M.; Strumia, M. Hyperbranched and hiperfunctionalized materials from dendritic chemistry. Rev. LatinAm Metal. Mater. 2013, 33, 2–14. [Google Scholar]
- Khandare, J.; Calderon, M.; Dagia, N.M.; Haag, R. Multifunctional dendritic polymers in nanomedicine: Opportunities and challenges. Chem. Soc. Rev. 2012, 41, 2824–2848. [Google Scholar] [CrossRef] [PubMed]
- Dernedde, J.; Rausch, A.; Weinhart, M.; Enders, S.; Tauber, R.; Licha, K.; Schirner, M.; Zugel, U.; von Bonin, A.; Haag, R. Dendritic polyglycerol sulfates as multivalent inhibitors of inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 19679–19684. [Google Scholar] [CrossRef] [PubMed]
- Quadir, M.A.; Radowski, M.R.; Kratz, F.; Licha, K.; Hauff, P.; Haag, R. Dendritic multishell architectures for drug and dye transport. J. Control. Release 2008, 132, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Calderón, M.; Quadir, M.A.; Sharma, S.K.; Haag, R. Dendritic polyglycerols for biomedical applications. Adv. Mater. 2010, 22, 190–218. [Google Scholar] [CrossRef] [PubMed]
- Licha, K.; Welker, P.; Weinhart, M.; Wegner, N.; Kern, S.; Reichert, S.; Gemeinhardt, I.; Weissbach, C.; Ebert, B.; Haag, R.; Schirner, M. Fluorescence imaging with multifunctional polyglycerol sulfates: novel polymeric near-IR probes targeting inflammation. Bioconjug Chem. 2011, 22, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Papp, I.; Dernedde, J.; Enders, S.; Haag, R. Modular synthesis of multivalent glycoarchitectures and their unique selectin binding behavior. Chem. Commun. 2008, 5851–5853. [Google Scholar] [CrossRef] [PubMed]
- Weinhart, M.; Groger, D.; Enders, S.; Dernedde, J.; Haag, R. Synthesis of dendritic polyglycerol anions and their efficiency toward l-selectin inhibition. Biomacromolecules 2011, 12, 2502–2511. [Google Scholar] [CrossRef] [PubMed]
- Paulus, F.; Schulze, R.; Steinhilber, D.; Zieringer, M.; Steinke, I.; Welker, P.; Licha, K.; Wedepohl, S.; Dernedde, J.; Haag, R. The effect of polyglycerol sulfate branching on inflammatory processes. Macromol. Biosci. 2014, 14, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Khanna, S.N. A Systematic Framework and Nanoperiodic Concept for Unifying Nanoscience: Hard/Soft Nanoelements, Superatoms, Meta-Atoms, New Emerging Properties, Periodic Property Patterns, and Predictive Mendeleev-like Nanoperiodic Tables. Chem. Rev. 2016, 116, 2705–2774. [Google Scholar] [CrossRef] [PubMed]
- Fleige, E.; Ziem, B.; Grabolle, M.; Haag, R.; Resch-Genger, U. Aggregation Phenomena of Host and Guest upon the Loading of Dendritic Core-Multishell Nanoparticles with Solvatochromic Dyes. Macromolecules 2012, 45, 9452–9459. [Google Scholar] [CrossRef]
- Kuchler, S.; Radowski, M.R.; Blaschke, T.; Dathe, M.; Plendl, J.; Haag, R.; Schafer-Korting, M.; Kramer, K.D. Nanoparticles for skin penetration enhancement—A comparison of a dendritic core-multishell-nanotransporter and solid lipid nanoparticles. Eur. J. Pharm Biopharm. 2009, 71, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Radowski, M.R.; Shukla, A.; von Berlepsch, H.; Bottcher, C.; Pickaert, G.; Rehage, H.; Haag, R. Supramolecular aggregates of dendritic multishell architectures as universal nanocarriers. Angew Chem. Int. Ed. Engl. 2007, 46, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Turk, H.; Shukla, A.; Alves Rodrigues, P.C.; Rehage, H.; Haag, R. Water-soluble dendritic core-shell-type architectures based on polyglycerol for solubilization of hydrophobic drugs. Chemistry 2007, 13, 4187–4196. [Google Scholar] [CrossRef] [PubMed]
- Wolf, N.B.; Kuchler, S.; Radowski, M.R.; Blaschke, T.; Kramer, K.D.; Weindl, G.; Kleuser, B.; Haag, R.; Schafer-Korting, M. Influences of opioids and nanoparticles on in vitro wound healing models. Eur. J. Pharm. Biopharm. 2009, 73, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Pischon, H.; Radbruch, M.; Ostrowski, A.; Volz, P.; Gerecke, C.; Unbehauen, M.; Hönzke, S.; Hedtrich, S.; Fluhr, J.; Haag, R.; Kleuser, B.; Alexiev, U.; Gruber, A.; Mundhenk, L. Stratum corneum targeting by dendritic core-multishell-nanocarriers in a mouse model of psoriasis. Nanomedicine 2016, 13, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Hönzke, S.; Gerecke, C.; Elpelt, A.; Zhang, N.; Unbehauen, M.; Kral, V.; Fleige, E.; Paulus, F.; Haag, R.; Schäfer-Korting, M.; Kleuser, B.; Hedtrich, S. Tailored dendritic core-multishell nanocarriers for efficient dermal drug delivery: A systematic top-down approach from synthesis to preclinical testing. J. Control. Release 2016, 242, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Kurniasih, I.N.; Keilitz, J.; Haag, R. Dendritic nanocarriers based on hyperbranched polymers. Chem. Soc. Rev. 2015, 44, 4145–4164. [Google Scholar] [CrossRef] [PubMed]
- Hertzberg, R.P.; Pope, A.J. High-throughput screening: New technology for the 21st century. Curr. Opin. Chem. Biol. 2000, 4, 445–451. [Google Scholar] [CrossRef]
- Janzen, W.P. Screening Technologies for Small Molecule Discovery: The State of the Art. Chem. Biol. 2014, 21, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Owicki, J.C. Fluorescence polarization and anisotropy in high throughput screening: Perspectives and primer. J. Biomol. Screen 2000, 5, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, S.A. High-throughput and ultra-high-throughput screening: Solution- and cell-based approaches. Curr. Opin. Biotechnol. 2000, 11, 47–53. [Google Scholar] [CrossRef]
- Kellogg, R.E.; Bennett, R.G. Radiationless Intermolecular Energy Transfer. III. Determination of Phosphorescence Efficiencies. J. Chem. Phys. 1964, 41, 3042–3045. [Google Scholar] [CrossRef]
- Waggoner, A.; DeBiasio, R.; Conrad, P.; Bright, G.R.; Ernst, L.; Ryan, K.; Nederlof, M.; Taylor, D. Multiple spectral parameter imaging. Methods Cell. Bio. 1989, 30, 449–478. [Google Scholar]
- Eftink, M.R.; Ghiron, C.A. Fluorescence quenching studies with proteins. Anal. Biochem. 1981, 114, 199–227. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer US: Boston, MA, USA, 2006. [Google Scholar]
- Dexter, D.L. A Theory of Sensitized Luminescence in Solids. J. Chem. Phys. 1953, 21, 836–850. [Google Scholar] [CrossRef]
- de Silva, A.P.; Moody, T.S.; Wright, G.D. Fluorescent PET (Photoinduced Electron Transfer) sensors as potent analytical tools. Analyst 2009, 134, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Förster, T. Zwischenmolekulare Energiewanderung und Fluoreszenz. Ann. Phys. 1948, 437, 55–75. [Google Scholar] [CrossRef]
- Alexiev, U.; Rimke, I.; Pohlmann, T. Elucidation of the nature of the conformational changes of the EF-interhelical loop in bacteriorhodopsin and of the helix VIII on the cytoplasmic surface of bovine rhodopsin: A time-resolved fluorescence depolarization study. J. Mol. Biol. 2003, 328, 705–719. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Winkler, K.; Alexiev, U. Picosecond multidimensional fluorescence spectroscopy: A tool to measure real-time protein dynamics during function. Photochem. Photobiol. 2007, 83, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Munro, I.; McGinty, J.; Galletly, N.; Requejo-Isidro, J.; Lanigan, P.M.P.; Elson, D.S.; Dunsby, C.; Neil, M.A.A.; Lever, M.J.; Stamp, G.W.H.; et al. Toward the clinical application of time-domain fluorescence lifetime imaging. J. Biomed. Optics 2005, 10, 051403-9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- König, K. Hybrid multiphoton multimodal tomography of in vivo human skin. IntraVital 2012, 1, 11–26. [Google Scholar] [CrossRef]
- Yew, E.; Rowlands, C.; So, P.T.C. Application of Multiphoton Microscopy in Dermatological Studies: A Mini-Review. J. Innovative Opt. Health Sci. 2014, 7, 1330010. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.M.; Behne, M.J.; Barry, N.P.; Mauro, T.M.; Gratton, E.; Clegg, R.M. Two-photon fluorescence lifetime imaging of the skin stratum corneum pH gradient. Biophys. J. 2002, 83, 1682–1690. [Google Scholar] [CrossRef]
- Kinosita, K., Jr.; Kawato, S.; Ikegami, A. A theory of fluorescence polarization decay in membranes. Biophys. J. 1977, 20, 289–305. [Google Scholar] [CrossRef]
- Schröder, G.F.; Alexiev, U.; Grubmüller, H. Simulation of Fluorescence Anisotropy Experiments: Probing Protein Dynamics. Biophys. J. 2005, 89, 3757–3770. [Google Scholar] [CrossRef] [PubMed]
- Boreham, A.; Pfaff, M.; Fleige, E.; Haag, R.; Alexiev, U. Nanodynamics of dendritic core-multishell nanocarriers. Langmuir 2014, 30, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Boreham, A.; Pikkemaat, J.; Volz, P.; Brodwolf, R.; Kuehne, C.; Licha, K.; Haag, R.; Dernedde, J.; Alexiev, U. Detecting and Quantifying Biomolecular Interactions of a Dendritic Polyglycerol Sulfate Nanoparticle Using Fluorescence Lifetime Measurements. Molecules 2016, 21, 22. [Google Scholar] [CrossRef] [PubMed]
- Eftink, M.R. The use of fluorescence methods to monitor unfolding transitions in proteins. Biophys. J. 1994, 66, 482–501. [Google Scholar] [CrossRef]
- Winkler, K.; Winter, A.; Rueckert, C.; Uchanska-Ziegler, B.; Alexiev, U. Natural MHC class I polymorphism controls the pathway of peptide dissociation from HLA-B27 complexes. Biophys. J. 2007, 93, 2743–2755. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boreham, A.; Brodwolf, R.; Walker, K.; Haag, R.; Alexiev, U. Time-Resolved Fluorescence Spectroscopy and Fluorescence Lifetime Imaging Microscopy for Characterization of Dendritic Polymer Nanoparticles and Applications in Nanomedicine. Molecules 2017, 22, 17. https://doi.org/10.3390/molecules22010017
Boreham A, Brodwolf R, Walker K, Haag R, Alexiev U. Time-Resolved Fluorescence Spectroscopy and Fluorescence Lifetime Imaging Microscopy for Characterization of Dendritic Polymer Nanoparticles and Applications in Nanomedicine. Molecules. 2017; 22(1):17. https://doi.org/10.3390/molecules22010017
Chicago/Turabian StyleBoreham, Alexander, Robert Brodwolf, Karolina Walker, Rainer Haag, and Ulrike Alexiev. 2017. "Time-Resolved Fluorescence Spectroscopy and Fluorescence Lifetime Imaging Microscopy for Characterization of Dendritic Polymer Nanoparticles and Applications in Nanomedicine" Molecules 22, no. 1: 17. https://doi.org/10.3390/molecules22010017





