Dibasic Ammonium Phosphate Application Enhances Aromatic Compound Concentration in Bog Bilberry Syrup Wine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Indexes
2.2. Total Phenols, Total Flavonoids, Total Anthocyanins, and Color Attributes
2.3. Volatile Compounds
2.3.1. Esters
2.3.2. Higher Alcohols
2.3.3. Acids
2.3.4. Aldehydes and Ketones
2.3.5. Terpene Derivatives
2.3.6. Other Volatile Compounds
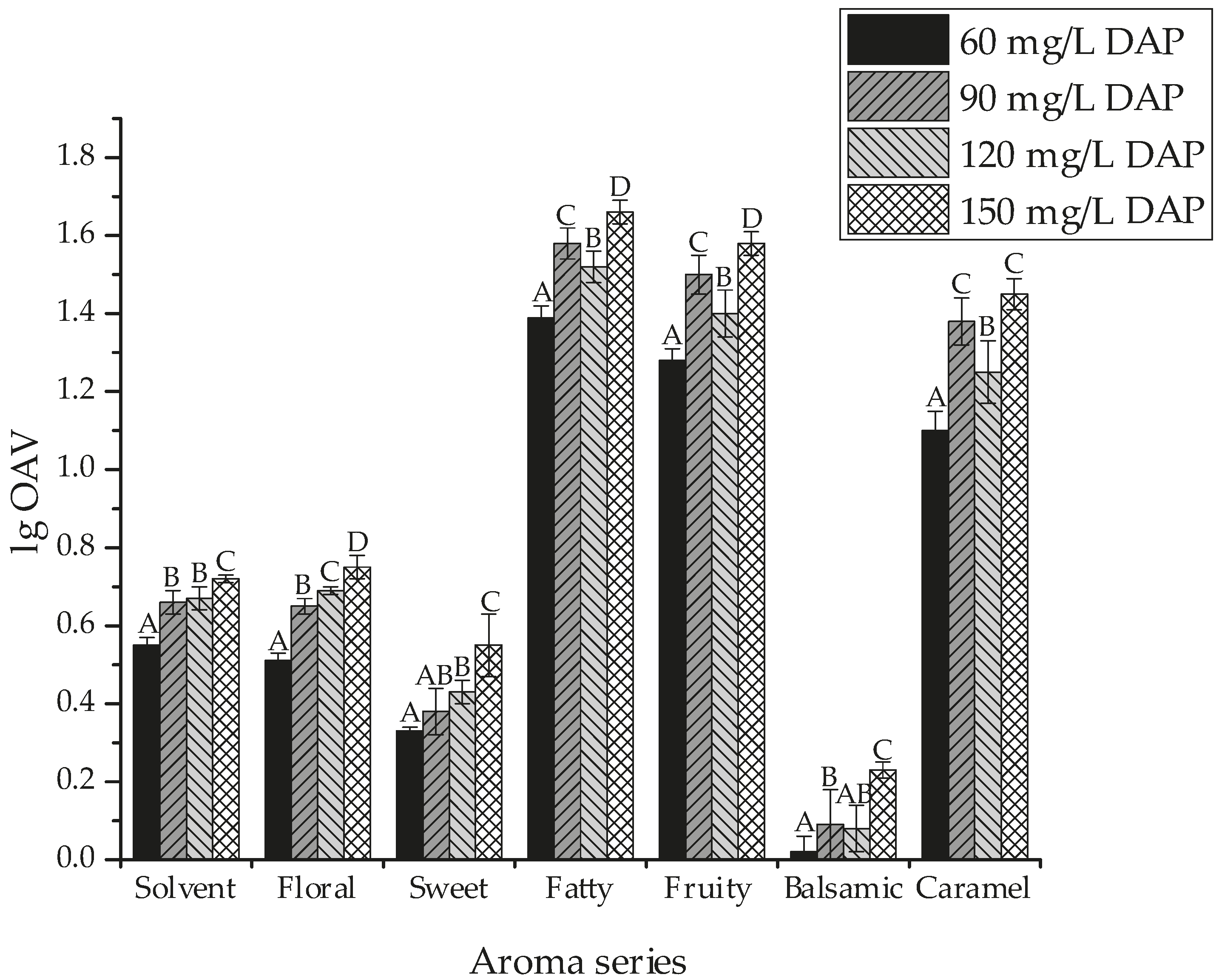
2.4. Global Aroma Attributes
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Bog Bilberry Syrup Wine Fermentation
3.3. Physicochemical Indexes
3.4. Total Phenols, Total Flavonoids, Total Anthocyanins, and Color Attributes
3.5. Volatile Compounds
3.6. Sensory Attributes and Aromatic Series
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Colak, N.; Torun, H.; Gruz, J.; Strnad, M.; Hermosín-Gutiérrez, I.; Hayirlioglu-Ayaz, S.; Ayaz, F.A. Bog bilberry phenolics, antioxidant capacity and nutrient profile. Food Chem. 2016, 201, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, P.; Guo, Q.; Wang, Z. Anthocyanin composition and content of the Vaccinium uliginosum berry. Food Chem. 2011, 125, 116–120. [Google Scholar] [CrossRef]
- Mattila, P.; Hellström, J.; Törrönen, R. Phenolic acids in berries, fruits, and beverages. J. Agric. Food Chem. 2006, 54, 7193–7199. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, H.; Li, S.; Zhang, J.; Li, T.; Zhu, B.; Zhang, B. Polyphenolic compositions and chromatic characteristics of bog bilberry syrup wines. Molecules 2015, 20, 19865–19877. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, S.; Yuan, G.; Ouyang, X.; Liu, Y.; Zhu, B.; Zhang, B. Effect of oak chips on evolution of phenolic compounds and color attributes of bog bilberry syrup wine during bottle-aging. J. Food Sci. 2016, 81, C2697–C2707. [Google Scholar] [CrossRef] [PubMed]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking; Springer: New York, NY, USA, 1999. [Google Scholar]
- Srisamatthakarn, P. Improvement of Varietal Aroma in Grape and Tropical Fruit Wines by Optimal Choice of Yeasts and Nutrient Supplements. Ph.D. Thesis, Justus-Liebig-University Giessen, Giessen, Germany, January 2011. [Google Scholar]
- Lee, P.; Toh, M.; Yu, B.; Curran, P.; Liu, S. Manipulation of volatile compound transformation in durian wine by nitrogen supplementation. Int. J. Food Sci. Technol. 2013, 48, 650–662. [Google Scholar] [CrossRef]
- Torrea, D.; Varela, C.; Ugliano, M.; Ancin-Azpilicueta, C.; Leigh Francis, I.; Henschke, P.A. Comparison of inorganic and organic nitrogen supplementation of grape juice—Effect on volatile composition and aroma profile of a Chardonnay wine fermented with Saccharomyces cerevisiae yeast. Food Chem. 2011, 127, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Ugliano, M.; Travis, B.; Francis, I.L.; Henschke, P.A. Volatile composition and sensory properties of shiraz wines as affected by nitrogen supplementation and yeast species: Rationalizing nitrogen modulation of wine aroma. J. Agric. Food Chem. 2010, 58, 12417–12425. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, U.B.; Bapat, V.A. Wines from fruits other than grapes: Current status and future prospectus. Food Biosci. 2015, 9, 80–96. [Google Scholar] [CrossRef]
- Vilanova, M.; Ugliano, M.; Varela, C.; Siebert, T.; Pretorius, I.S.; Henschke, P.A. Assimilable nitrogen utilisation and production of volatile and non-volatile compounds in chemically defined medium by Saccharomyces cerevisiae wine yeasts. Adv. Appl. Microbiol. 2007, 77, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Orte, P.; Bely, M.; Cacho, J.; Ferreira, V. Impact of ammonium additions on volatile acidity, ethanol, and aromatic compound production by different Saccharomyces cerevisiae strains during fermentation in controlled synthetic media. Aust. J. Grape Wine Res. 2006, 12, 150–160. [Google Scholar] [CrossRef]
- Ugliano, M.; Fedrizzi, B.; Siebert, T.; Travis, B.; Magno, F.; Versini, G.; Henschke, P.A. Effect of nitrogen supplementation and saccharomyces species on hydrogen sulfide and other volatile sulfur compounds in shiraz fermentation and wine. J. Agric. Food Chem. 2009, 57, 4948–4955. [Google Scholar] [CrossRef] [PubMed]
- Lattey, K.A.; Bramley, B.R.; Francis, I.L. Consumer acceptability, sensory properties and expert quality judgements of Australian Cabernet Sauvignon and Shiraz wines. Aust. J. Grape Wine Res. 2010, 16, 189–202. [Google Scholar] [CrossRef]
- Wright, C.A.; Bruhn, C.M.; Heymann, H.; Bamforth, C.W. Beer and wine consumers’ perceptions of the nutritional value of alcoholic and nonalcoholic beverages. J. Food Sci. 2008, 73, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Charters, S.; Pettigrew, S. The dimensions of wine quality. Food Qual. Preference 2007, 18, 997–1007. [Google Scholar] [CrossRef]
- Li, Z.; Pan, Q.; Jin, Z.; Mu, L.; Duan, C. Comparison on phenolic compounds in Vitis vinifera cv. Cabernet Sauvignon wines from five wine-growing regions in China. Food Chem. 2011, 125, 77–83. [Google Scholar] [CrossRef]
- Bakker, J.; Clarke, R.J. Wine Flavour Chemistry, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2011. [Google Scholar]
- Gobbi, M.; Comitini, F.; D’Ignazi, G.; Ciani, M. Effects of nutrient supplementation on fermentation kinetics, H2S evolution, and aroma profile in Verdicchio DOC wine production. Eur. Food Res. Technol. 2013, 236, 145–154. [Google Scholar] [CrossRef]
- Ugliano, M.; Siebert, T.; Mercurio, M.; Capone, D.; Henschke, P.A. Volatile and color composition of young and model-aged shiraz wines as affected by diammonium phosphate supplementation before alcoholic fermentation. J. Agric. Food Chem. 2008, 56, 9175–9182. [Google Scholar] [CrossRef] [PubMed]
- Carrau, F.M.; Karina, M.; Laura, F.; Eduardo, B.; Henschke, P.A.; Eduardo, D. Production of fermentation aroma compounds by Saccharomyces cerevisiae wine yeasts: Effects of yeast assimilable nitrogen on two model strains. FEMS Yeast Res. 2008, 8, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Orte, P.; Ibarz, M.J.; Cacho, J.; Ferreira, V. Effect of the addition of ammonium and amino acids to musts of Airen variety on aromatic composition and sensory properties of the obtained wine. Food Chem. 2005, 74, 242–255. [Google Scholar] [CrossRef]
- Bell, S.-J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P.; Edelenbos, M.; Kreutzmann, S. Flavours and Fragrances; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Gao, Y.; Tian, Y.; Liu, D.; Li, Z.; Zhang, X.; Li, J.; Huang, J.; Wang, J.; Pan, Q. Evolution of phenolic compounds and sensory in bottled red wines and their co-development. Food Chem. 2015, 172, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arribas, M.V.; Polo, M.C. Wine Chemistry and Biochemistry; Springer: New York, NY, USA, 2009. [Google Scholar]
- Huang, X.; Chai, Z.; Yang, Z.; Liu, Y. Research of the change rules of antioxidant substances of blueberry wines during fermentation. Sci. Technol. Food Ind. 2013, 34, 103–105. [Google Scholar]
- Villamor, R.R.; Evans, M.A.; Mattinson, D.S.; Ross, C.F. Effects of ethanol, tannin and fructose on the headspace concentration and potential sensory significance of odorants in a model wine. Food Res. Int. 2013, 50, 38–45. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Regueiro, J.; Cancho-Grande, B.; Simal-Gándara, J. Garnacha Tintorera-based sweet wines: Detailed phenolic composition by HPLC/DAD-ESI/MS analysis. Food Chem. 2014, 143, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Magariño, S.; González-Sanjosé, M.L. Application of absorbance values used in wineries for estimating CIELAB parameters in red wines. Food Chem. 2003, 81, 301–306. [Google Scholar] [CrossRef]
- Schreier, P.; Jennings, W.G. Flavor composition of wines: A review. CRC Crit. Rev. Food Sci. Nutr. 1979, 12, 59–111. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Yuan, G.; Ren, J.; Wang, L.; Wang, M.; Li, Y.; Zhang, B.; Zhu, B. Aromatic compounds and organoleptic features of fermented wolfberry wine: Effects of maceration time. Int. J. Food. Prop. 2016, in press. [Google Scholar] [CrossRef]
- Cai, J.; Zhu, B.; Wang, Y.; Lu, L.; Lan, Y.; Reeves, M.J.; Duan, C. Influence of pre-fermentation cold maceration treatment on aroma compounds of Cabernet Sauvignon wines fermented in different industrial scale fermenters. Food Chem. 2014, 154, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Esters DETECTION THRESHOLDS & Molecular Structures. Available online: http://www.leffingwell.com/esters1.htm (accessed on 28 December 2016).
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Agric. Food Chem. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- The Good Scents Company Information System. Available online: http://www.thegoodscentscompany.com/ (accessed on 28 December 2016).
- Jiang, B.; Zhang, Z. Volatile compounds of young wines from cabernet sauvignon, cabernet gernischet and chardonnay varieties grown in the loess plateau region of China. Molecules 2010, 15, 9184–9196. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Palomo, E.; Gómez García-Carpintero, E.; Alonso-Villegas, R.; González-Viñas, M.A. Characterization of aroma compounds of Verdejo white wines from the La Mancha region by odour activity values. Flavour Fragr. J. 2010, 25, 456–462. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Compilations of Odour Threshold Values in Air, Water and Other Media, 2nd ed.; Oliemans Punter & Partners BV: Vollenhove, The Netherlands, 2011. [Google Scholar]
- Juan, F.S.; Cacho, J.; Ferreira, V.; Escudero, A. Aroma chemical composition of red wines from different price categories and its relationship to quality. J. Agric. Food Chem. 2012, 60, 5045–5056. [Google Scholar] [CrossRef] [PubMed]
- Crandles, M.; Reynolds, A.G.; Khairallah, R.; Bowen, A. The effect of yeast strain on odor active compounds in Riesling and Vidal blanc icewines. LWT Food Sci. Technol. 2015, 64, 243–258. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, S.; Zhao, L.; Gao, Z.; Luo, M.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, S. Aroma characterization based on aromatic series analysis in table grapes. Sci. Rep. (UK) 2016, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Torrens, J.; Urpí, P.; Riu-Aumatell, M.; Vichi, S.; López-Tamames, E.; Buxaderas, S. Different commercial yeast strains affecting the volatile and sensory profile of cava base wine. Int. J. Food Microbiol. 2008, 124, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Schneiderbanger, H.; Koob, J.; Poltinger, S.; Jacob, F.; Hutzler, M. Gene expression in wheat beer yeast strains and the synthesis of acetate esters. J. Inst. Brew. 2016, 122, 403–411. [Google Scholar] [CrossRef]
- Bartowsky, E.; Bellon, J.; Borneman, A.; Chambers, P.; Cordente, A.; Costello, P.; Curtin, C.; Forgan, A.; Henschke, P.; Kutyna, D.; et al. Not all wine yeast are equal. Microbiol. Aust. 2007, 28, 55–58. [Google Scholar]
- Mason, A.B.; Dufour, J.-P. Alcohol acetyltransferases and the significance of ester synthesis in yeast. Yeast 2000, 16, 1287–1298. [Google Scholar] [CrossRef]
- Saerens, S.M.; Delvaux, F.; Verstrepen, K.J.; van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters affecting ethyl ester production by Saccharomyces cerevisiae during fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Swiegers, J.H.; Pretorius, I.S. Yeast modulation of wine flavor. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2005; Volume 57, pp. 131–175. [Google Scholar]
- Romano, P.; Brandolini, V.; Ansaloni, C.; Menziani, E. The production of 2,3-Butanediol as a differentiating character in wine yeasts. World J. Microbiol. Biotechnol. 1998, 14, 649–653. [Google Scholar] [CrossRef]
- Bely, M.; Rinaldi, A.; Dubourdieu, D. Influence of assimilable nitrogen on volatile acidity production by Saccharomyces cerevisiae during high sugar fermentation. J. Biosci. Bioeng. 2003, 96, 507–512. [Google Scholar] [CrossRef]
- Blackmore, S.J. Composition of Volatiles of Pinot Noir Wine as Affected by Differing Levels of DAP Supplementation. Master’s Thesis, Lincoln University, Lincoln, New Zealand, 2014. [Google Scholar]
- Fugelsang, K.C.; Edwards, C.G. Wine Microbiology: Practical Applications and Procedures, 2nd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Culleré, L.; Cacho, J.; Ferreira, V. An assessment of the role played by some oxidation-related aldehydes in wine aroma. J. Agric. Food Chem. 2007, 55, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Tufariello, M.; Capone, S.; Siciliano, P. Volatile components of Negroamaro red wines produced in Apulian Salento area. Food Chem. 2012, 132, 2155–2164. [Google Scholar] [CrossRef]
- Palomo, E.S.; Dı́az-Maroto Hidalgo, M.C.; González-Viñas, M.Á.; Pérez-Coello, M.S. Aroma enhancement in wines from different grape varieties using exogenous glycosidases. Food Chem. 2005, 92, 627–635. [Google Scholar] [CrossRef]
- Ugliano, M.; Bartowsky, E.J.; McCarthy, J.; Moio, L.; Henschke, P.A. Hydrolysis and transformation of grape glycosidically bound volatile compounds during fermentation with three saccharomyces yeast strains. J. Agric. Food Chem. 2006, 54, 6322–6331. [Google Scholar] [CrossRef] [PubMed]
- General Administration of Quality Supervision, Inspection and Quarantine (AQSIQ); Standardization Administration of China (SAC). Analytical Methods of Wine and Fruit Wine; China Standard Press: Beijing, China, 2006; Volume GBT 15038-2006. [Google Scholar]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [PubMed]
- Zhang, M.; Xu, Q.; Duan, C.; Qu, W.; Wu, Y. Comparative study of aromatic compounds in young red wines from cabernet sauvignon, cabernet franc, and cabernet gernischet varieties in China. J. Food Sci. 2007, 72, C248–C252. [Google Scholar] [CrossRef] [PubMed]
- NIST Chemistry WebBook. Available online: http://webbook.nist.gov/chemistry/ (accessed on 28 December 2016).
- Sample Availability: Samples of the frozen wines are available from the authors.
| Physicochemical Index | Wine with Dibasic Ammonium Phosphate (mg/L) a | |||
|---|---|---|---|---|
| 60 | 90 | 120 | 150 | |
| Fermentation Period (days) | 19 | 17 | 14 | 14 |
| Alcohol (%, vol) | 9.1 ± 0.2 a | 9.8 ± 0.1 b | 10.6 ± 0.2 c | 11.0 ± 0.1 d |
| Total Sugar (g/L) | 18.84 ± 0.11 c | 10.11 ± 0.43 b | 10.10 ± 0.17 b | 8.32 ± 0.31 a |
| Reducing Sugar (g/L) | 16.07 ± 0.39 c | 7.25 ± 0.54 b | 7.22 ± 0.03 b | 5.84 ± 0.32 a |
| pH | 3.14 ± 0.01 a | 3.14 ± 0.01 a | 3.14 ± 0.01 a | 3.14 ± 0.01 a |
| Total Acidity (g/L) | 8.47 ± 0.17 a | 8.66 ± 0.21 a | 8.56 ± 0.02 a | 8.71 ± 0.07 a |
| Content | Wine with Dibasic Ammonium Phosphate (mg/L) d | |||
|---|---|---|---|---|
| 60 | 90 | 120 | 150 | |
| Phenolic compounds | ||||
| Total anthocyanins (mg/L) a | 36.86 ± 0.30 a | 35.36 ± 0.09 a | 36.24 ± 1.17 a | 38.22 ± 3.65 a |
| Total flavonoids (mg/L) b | 12.88 ± 0.15 a | 14.26 ± 0.01 b | 14.51 ± 0.13 bc | 14.72 ± 0.16 c |
| Total phenols (mg/L) c | 749.72 ± 24.67 a | 757.42 ± 33.51 a | 836.41 ± 22.24 b | 892.27 ± 33.51 b |
| Color attributes | ||||
| L* value | 47.93 ± 1.15 ab | 46.11 ± 0.96 a | 46.39 ± 1.05 ab | 48.68 ± 0.50 b |
| a* value | 66.74 ± 1.54 b | 82.94 ± 1.00 c | 81.00 ± 1.04 c | 61.60 ± 0.73 a |
| b* value | −5.40 ± 1.13 a | −6.72 ± 0.71 a | −6.89 ± 1.07 a | −4.75 ± 0.56 a |
| Volatile Compound a | Concentration of Volatile in Wine with DAP (mg/L) b | Odor Threshold (mg/L) | Aroma Descriptor | |||
|---|---|---|---|---|---|---|
| 60 | 90 | 120 | 150 | |||
| Esters | ||||||
| Acetate esters | ||||||
| Ethyl acetate (mg/L) A | 12.88 ± 1.08 a | 15.55 ± 3.06 a | 14.92 ± 2.05 a | 20.70 ± 1.14 b | 12.3 [34] | Pineapple, fruity, solvent, balsamic [35] |
| Isobutyl acetate A | 16.54 ± 0.71 a | 21.94 ± 4.47 b | 21.42 ± 3.31 ab | 30.94 ± 3.27 c | 1.6 [34] | Banana, fruity, sweet [34] |
| Isoamyl acetate A | 258.32 ± 16.06 a | 251.02 ± 52.85 a | 279.42 ± 34.35 ab | 354.55 ± 73.61 b | 0.16 [36] | Banana, fruity, sweet [35] |
| Octyl acetate B | 0.06 ± 0.01 a | 0.08 ± 0.01 ab | 0.09 ± 0.01 bc | 0.10 ± 0.01 c | ||
| Phenethyl acetate A | 93.98 ± 5.23 a | 163.75 ± 7.57 b | 229.35 ± 15.36 c | 293.72 ± 29.85 d | 1.8 [36] | Flowery [35] |
| Total acetate esters (mg/L) | 13.25 ± 1.01 a | 15.99 ± 3.04 a | 15.45 ± 2.05 a | 21.38 ± 1.18 b | ||
| Ethyl esters | ||||||
| Ethyl butanoate A | 39.34 ± 4.02 a | 49.04 ± 11.56 ab | 40.9 ± 6.85 ab | 52.39 ± 5.04 b | 0.02 [34] | Strawberry, apple, banana [35] |
| Ethyl-2-methylbutyrate B | 13.34 ± 2.65 a | 12.92 ± 2.61 a | 14.11 ± 1.32 a | 15.44 ± 2.57 a | ||
| Ethyl hexanoate A | 157.75 ± 6.77 a | 215.04 ± 31.92 b | 196.54 ± 30.32 ab | 205.95 ± 44.44 ab | 0.08 [36] | Fruity, green apple, banana, brandy, wine-like [35] |
| Ethyl heptanoate B | 1.27 ± 0.06 a | 1.58 ± 0.24 ab | 1.72 ± 0.31 b | 2.30 ± 0.31 c | ||
| Ethyl lactate (mg/L) A | 4.88 ± 0.18 a | 7.16 ± 0.67 b | 9.40 ± 0.42 c | 11.63 ± 0.76 d | 154.636 [35] | Fruity, buttery [35] |
| Ethyl octanoate A | 307.31 ± 24.64 a | 475.80 ± 20.62 b | 556.63 ± 137.48 b | 807.86 ± 121.61 c | 0.58 [36] | Sweet, floral, fruity, banana, pear, brandy [35] |
| Ethyl 7-octenoate B | 6.90 ± 0.47 a | 18.55 ± 2.25 b | 19.85 ± 1.40 b | 22.8 ± 0.38 c | ||
| Ethyl 3-hydroxybutyrate B | 2.98 ± 0.23 a | 3.28 ± 0.28 a | 3.77 ± 0.60 ab | 4.78 ± 1.57 b | ||
| Ethyl nonanoate A | 1.26 ± 0.19 b | 0.88 ± 0.24 a | 1.43 ± 0.25 b | 1.14 ± 0.20 ab | 0.85 [37] | Fatty, oily, cognac, nut-like odor; oily, fatty-fruity taste [37] |
| Ethyl 2-hydroxy-4-methylpentanoate (mg/L) B | 1.49 ± 0.32 a | 2.98 ± 0.06 b | 3.58 ± 0.10 c | 4.33 ± 0.12 d | ||
| Ethyl furoate B | 0.46 ± 0.01 a | 0.77 ± 0.01 b | 0.91 ± 0.03 c | 1.00 ± 0.10 d | ||
| Ethyl decanoate A | 270.21 ± 24.26 a | 259.4 ± 54.90 a | 367.62 ± 97.66 b | 495.84 ± 15.03 c | 0.2 [38] | Brandy, fruity, grape [35] |
| Ethyl benzoate B | 3.45 ± 0.08 a | 5.52 ± 0.31 b | 6.39 ± 0.41 c | 7.42 ± 0.54 d | ||
| Diethyl succinate (mg/L) A | 0.38 ± 0.03 a | 0.68 ± 0.03 b | 0.96 ± 0.03 c | 1.15 ± 0.07 d | 1200 [35] | Fruity, melon [35] |
| Ethyl 9-decenoate B | 100.11 ± 6.33 a | 129.61 ± 13.08 ab | 155.78 ± 34.29 b | 212.55 ± 24.71 c | ||
| Ethyl phenylacetate B | 4.18 ± 0.14 a | 6.01 ± 0.39 b | 6.97 ± 0.34 c | 7.53 ± 0.41 d | ||
| Ethyl dodecanoate A | 82.31 ± 6.78 a | 83.8 ± 10.88 a | 116.89 ± 5.56 b | 145.15 ± 24.82 c | 1.5 [35] | Oily, fatty, fruity [35] |
| Ethyl myristate B | 70.20 ± 2.75 a | 93.73 ± 9.75 b | 118.03 ± 5.58 c | 133.89 ± 15.4 d | ||
| Diethyl malate B | 5.56 ± 1.36 a | 12.76 ± 4.01 b | 11.71 ± 1.94 b | 13.61 ± 2.48 b | ||
| Ethyl 3-hydroxy tridecanoate B | 8.13 ± 0.22 a | 17.57 ± 1.86 b | 23.04 ± 2.78 c | 26.12 ± 1.92 c | ||
| Ethyl pentadecanoate B | 0.39 ± 0.11 a | 0.72 ± 0.10 a | 1.17 ± 0.19 b | 1.33 ± 0.47 b | ||
| Ethyl palmitate B | 26.02 ± 1.41 a | 32.35 ± 2.79 a | 39.07 ± 3.24 b | 44.8 ± 6.86 b | ||
| Ethyl 9-hexadecenoate B | 70.38 ± 4.17 c | 20.42 ± 1.84 a | 22.68 ± 1.60 ab | 26.32 ± 1.74 b | ||
| Ethyl hydrogen succinate B | 16.34 ± 4.59 a | 43.27 ± 12.6 b | 35.55 ± 3.68 b | 43.71 ± 4.93 b | ||
| Total ethyl esters (mg/L) | 6.89 ± 0.31 a | 10.92 ± 0.67 b | 13.81 ± 0.38 c | 17.07 ± 0.88 d | ||
| Other esters | ||||||
| Isobutyl caproate B | 0.11 ± 0.01 a | 0.15 ± 0.03 a | 0.25 ± 0.05 b | 0.29 ± 0.08 b | ||
| Isoamyl hexanoate A | 0.89 ± 0.08 a | 1.30 ± 0.10 ab | 1.70 ± 0.46 b | 2.22 ± 0.36 c | - | Fruity, banana, apple, pineapple, green [39] |
| Isobutyl octanoate B | 1.26 ± 0.15 a | 1.41 ± 0.19 a | 3.07 ± 0.54 b | 3.70 ± 0.84 b | ||
| Isoamyl octanoate B | 2.42 ± 0.08 a | 2.46 ± 0.26 a | 3.22 ± 0.60 b | 3.83 ± 0.34 c | ||
| Isopentyl decanoate B | 20.75 ± 1.17 a | 19.87 ± 1.79 a | 24.12 ± 3.47 a | 30.03 ± 3.46 b | ||
| Total other esters | 25.42 ± 1.34 a | 25.19 ± 2.22 a | 32.35 ± 4.95 b | 40.06 ± 4.80 c | ||
| Total esters (mg/L) | 20.17 ± 1.38 a | 26.93 ± 3.66 b | 29.30 ± 2.24 b | 38.49 ± 1.75 c | ||
| Higher Alcohols | ||||||
| Isobutanol (mg/L) A | 74.69 ± 2.07 a | 91.84 ± 6.81 b | 104.38 ± 4.45 c | 118.51 ± 8.49 d | 75 [36] | Alcohol, nail polish [35] |
| 1-Butanol (mg/L) A | 0.42 ± 0.01 a | 0.58 ± 0.05 ab | 0.71 ± 0.04 b | 1.15 ± 0.29 c | 150 [35] | Medicinal, phenolic [35] |
| Isoamyl alcohol (mg/L) A | 146.81 ± 4.49 a | 197.55 ± 7.01 b | 204.03 ± 7.77 bc | 213.25 ± 9.72 c | 60 [36] | Solvent, sweet, alcohol, nail polish [35] |
| 4-Methyl-1-pentanol A | 12.44 ± 0.80 a | 18.63 ± 0.96 b | 18.64 ± 1.25 b | 18.81 ± 1.17 b | 50 [35] | Almond, toasted [35] |
| 3-Methyl-1-pentanol B | 40.04 ± 1.03 a | 49.13 ± 1.01 b | 46.85 ± 4.20 b | 42.34 ± 2.52 a | ||
| 1-Hexanol A | 8.70 ± 0.30 a | 11.77 ± 0.64 b | 12.87 ± 1.05 b | 12.52 ± 1.04 b | 1.1 [35] | Herbaceous, grass, woody [35] |
| cis-3-Hexenol B | 17.44 ± 3.77 a | 21.38 ± 1.87 ab | 25.61 ± 2.35 bc | 29.17 ± 3.22 c | ||
| 1-Heptanol A | 4.93 ± 0.55 a | 11.24 ± 0.71 b | 15.80 ± 1.67 c | 22.05 ± 2.49 d | 0.2 [35] | Oily [35] |
| 2-Nonanol A | 2.77 ± 0.06 a | 4.22 ± 0.18 b | 4.07 ± 0.14 b | 4.18 ± 0.25 b | 0.058 [40] | Waxy green creamy citrus orange cheese fruity [39] |
| levo-2,3-Butanediol (g/L) A | 1.12 ± 0.16 a | 2.46 ± 0.45 b | 1.60 ± 0.38 a | 2.70 ± 0.30 b | 150 [41] | Fruity, sweet, butter [35] |
| 1-Octanol A | 0.38 ± 0.03 a | 0.66 ± 0.05 b | 0.90 ± 0.02 c | 1.12 ± 0.05 d | 0.8 [35] | Jasmine, lemon [35] |
| 1-Nonanol B | 0.59 ± 0.05 a | 0.89 ± 0.09 b | 1.09 ± 0.07 c | 1.27 ± 0.07 d | ||
| 2-Undecanol B | 2.67 ± 0.22 a | 4.08 ± 0.30 b | 3.87 ± 0.53 b | 4.42 ± 0.57 b | ||
| 1-Decanol A | 0.18 ± 0.01 a | 0.90 ± 0.09 b | 1.24 ± 0.19 c | 1.96 ± 0.11 d | 0.4 [35] | Fatty, waxy, floral, orange, sweet, clean ,watery [39] |
| Benzyl alcohol A | 21.34 ± 3.06 a | 30.14 ± 2.28 b | 33.04 ± 3.14 b | 41.99 ± 2.65 c | 200 [35] | Roasted, sweet, fruity [35] |
| 2-Phenylethanol (mg/L) A | 36.79 ± 2.52 a | 49.63 ± 2.84 b | 52.74 ± 2.63 bc | 56.37 ± 4.55 c | 14 [38] | Roses, honey [35] |
| meso-2,3-Butanediol (mg/L) A | 416.52 ± 72.13 a | 708.72 ± 184.22 b | 592.29 ± 98.02 ab | 988.36 ± 108.14 c | 150 [41] | Fruity, sweet, butter [35] |
| 1-Dodecanol A | tr | tr | 0.03 ± 0.05 a | 0.17 ± 0.12 b | 1 [40] | Flowery [40] |
| Total alcohols (g/L) | 1.35 ± 0.15 a | 2.75 ± 0.44 c | 1.91 ± 0.37 b | 3.03 ± 0.29 c | ||
| Acids | ||||||
| Acetic acid A | 538.66 ± 26.36 a | 639.49 ± 58.90 ab | 747.59 ± 67.60 bc | 873.73 ± 144.99 c | 200 [40] | Acid, fatty [40] |
| Isobutyric acid (mg/L) A | 13.39 ± 0.21 a | 14.44 ± 0.95 a | 16.78 ± 0.72 b | 17.76 ± 0.96 b | 2.3 [38] | Rancid, butter, cheese [42] |
| 3-Methylbutanoic acid B | 14.47 ± 2.05 a | 14.68 ± 4.29 a | 13.31 ± 3.60 a | 17.74 ± 4.31 a | ||
| 2-Methylbutanoic acid (mg/L) B | 2.57 ± 0.19 a | 3.00 ± 0.13 b | 3.03 ± 0.16 b | 3.01 ± 0.24 b | ||
| Butyric acid A | 306.88 ± 13.70 a | 392.08 ± 33.81 b | 451.87 ± 28.89 bc | 520.62 ± 71.77 c | 2.3 [35] | Rancid, butter, cheese [35] |
| Hexanoic acid (mg/L) A | 1.04 ± 0.02 a | 1.14 ± 0.02 b | 1.21 ± 0.02 c | 1.26 ± 0.04 d | 0.42 [38] | Cheese, fatty [35] |
| Octanoic acid (mg/L) A | 1.46 ± 0.09 a | 1.87 ± 0.09 b | 2.20 ± 0.18 c | 2.52 ± 0.28 d | 0.5 [38] | Rancid, cheese, fatty acid [35] |
| 7-Octenoic acid B | 38.50 ± 7.64 a | 50.84 ± 4.46 b | 63.27 ± 4.73 c | 74.96 ± 10.95 c | ||
| n-Decanoic acid A | 715.48 ± 25.30 a | 698.68 ± 32.51 a | 757.30 ± 53.56 ab | 816.75 ± 65.02 b | 1 [35] | Fatty, rancid [35] |
| 9-Decenoic acid B | 258.77 ± 11.74 a | 285.13 ± 11.52 ab | 321.52 ± 21.32 bc | 356.93 ± 36.48 c | ||
| Total acids (mg/L) | 20.33 ± 0.26 a | 22.52 ± 0.96 b | 25.57 ± 1.01 c | 27.20 ± 1.38 d | ||
| Aldehydes | ||||||
| Nonanal A | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 c | 0.02 ± 0.00 b | 0.015 [35] | Green, slightly pungent [35] |
| Furfural B | 0.04 ± 0.01 a | 0.08 ± 0.01 b | 0.10 ± 0.01 c | 0.12 ± 0.02 d | ||
| Decanal A | 2.12 ± 0.43 a | 3.01 ± 0.64 ab | 3.81 ± 0.53 bc | 4.20 ± 0.82 c | 0.01 [35] | Grassy, orange skin-like [40] |
| 5-Methylfurfural B | 9.57 ± 0.76 a | 12.41 ± 1.43 b | 17.09 ± 1.69 c | 23.25 ± 2.05 d | ||
| Dodecanal B | tr | tr | 0.14 ± 0.11 a | 0.38 ± 0.19 b | ||
| Total aldehydes | 11.73 ± 1.00 a | 15.51 ± 1.36 b | 21.15 ± 2.20 c | 27.97 ± 2.68 d | ||
| Ketones | ||||||
| 2-Nonanone B | tr | 0.66 ± 0.62 | tr | tr | ||
| 2-Undecanone B | 3.71 ± 0.53 a | 4.12 ± 0.66 ab | 3.81 ± 0.59 ab | 4.84 ± 0.86 b | ||
| Total ketones | 3.71 ± 0.53 a | 4.78 ± 1.22 a | 3.81 ± 0.59 a | 4.84 ± 0.86 a | ||
| Terpene derivatives | ||||||
| Linalool A | 4.92 ± 0.15 a | 7.08 ± 0.43 b | 6.72 ± 0.38 b | 6.62 ± 0.42 b | 0.025 [35] | Flowery, muscat [35] |
| 4-Terpineol B | 6.92 ± 0.17 a | 8.15 ± 0.34 b | 8.56 ± 0.28 b | 9.39 ± 0.50 c | ||
| β-cis-Farnesene B | 9.13 ± 0.55 a | 13.84 ± 0.93 b | 15.85 ± 0.71 c | 17.35 ± 1.39 c | ||
| p-Menth-1-en-8-ol A | 15.00 ± 0.67 a | 19.27 ± 1.14 b | 21.54 ± 1.09 c | 23.20 ± 1.16 c | 0.25 [43] | Pine, terpene, lilac, citrus, woody, floral [39] |
| (Z,E)-α-Farnesene B | 2.41 ± 0.07 a | 2.88 ± 0.37 b | 3.07 ± 0.37 bc | 3.53 ± 0.25 c | ||
| Naphthalene A | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 b | 0.01 ± 0.00 b | - | - |
| 1,1,6-Trimethyl-1,2-dihydronaphtalene B | 0.14 ± 0.01 a | 0.14 ± 0.02 a | 0.19 ± 0.02 b | 0.24 ± 0.04 c | ||
| β-Bisabolene B | 3.13 ± 0.17 a | 3.74 ± 0.41 ab | 4.07 ± 0.51 b | 4.94 ± 0.52 c | ||
| α-Farnesene B | 3.80 ± 0.18 a | 5.03 ± 0.63 b | 5.61 ± 0.85 b | 6.85 ± 0.50 c | ||
| Citronellol A | 8.12 ± 0.41 a | 11.85 ± 0.80 b | 12.39 ± 0.79 b | 12.76 ± 0.74 b | 0.1 [35] | Rose [35] |
| α-Caryophyllene B | 2.99 ± 0.22 a | 3.35 ± 0.47 ab | 3.91 ± 0.51 b | 4.84 ± 0.42 c | ||
| β-Damascenone B | 1.63 ± 0.04 a | 2.11 ± 0.14 b | 2.29 ± 0.14 c | 2.39 ± 0.08 c | ||
| Geranylacetone A | 3.66 ± 0.28 a | 5.32 ± 0.59 b | 7.17 ± 0.96 c | 8.84 ± 0.75 d | 0.06 [44] | Fresh, green, fruity, waxy, rose, woody, magnolia, tropical [39] |
| trans-Nerolidol A | 26.89 ± 1.94 a | 38.39 ± 5.00 b | 38.78 ± 3.47 b | 37.05 ± 3.55 b | 0.25 [45] | Rose, apple, green, citrus, waxy, woody [45] |
| 2,3-Dihydrofarnesol B | 7.51 ± 0.41 a | 14.04 ± 2.04 b | 12.47 ± 2.43 b | 13.82 ± 1.19 b | ||
| FarnesolB | 0.17 ± 0.06 a | 0.44 ± 0.09 c | 0.38 ± 0.07 bc | 0.29 ± 0.02 b | ||
| Total terpene derivatives | 41.66 ± 1.06 a | 55.12 ± 2.36 b | 60.00 ± 2.71 c | 65.28 ± 3.40 d | ||
| Others | ||||||
| Acetoin (mg/L) A | 39.52 ± 2.31 ab | 35.53 ± 2.32 a | 42.32 ± 3.66 b | 42.55 ± 4.79 b | 150 [35] | Buttery, fatty [35] |
| p-Cymene A | 0.31 ± 0.04 a | 0.26 ± 0.06 a | 0.44 ± 0.07 b | 0.44 ± 0.04 b | 0.0114 [45] | Citrus, green [45] |
| 2-Acetylfuran B | 0.58 ± 0.05 a | 1.28 ± 0.12 b | 1.59 ± 0.08 c | 1.88 ± 0.17 d | ||
| γ-Butyrolactone B | 42.49 ± 2.78 a | 64.45 ± 7.64 ab | 81.77 ± 18.78 b | 115.05 ± 20.11 c | ||
| Methionol B | 0.04 ± 0.00 a | 0.06 ± 0.00 c | 0.05 ± 0.00 bc | 0.05 ± 0.01 ab | ||
| 4-Hydroxy-2-methylacetophenone B | 6.51 ± 0.37 a | 9.27 ± 0.71 b | 10.32 ± 0.79 bc | 11.05 ± 1.04 c | ||
| Total others (mg/L) | 39.57 ± 2.31 ab | 35.61 ± 2.32 a | 42.41 ± 3.61 b | 42.68 ± 4.80 b | ||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-Y.; Li, Y.-Q.; Li, T.; Yang, H.-Y.; Ren, J.; Zhang, B.-L.; Zhu, B.-Q. Dibasic Ammonium Phosphate Application Enhances Aromatic Compound Concentration in Bog Bilberry Syrup Wine. Molecules 2017, 22, 52. https://doi.org/10.3390/molecules22010052
Wang S-Y, Li Y-Q, Li T, Yang H-Y, Ren J, Zhang B-L, Zhu B-Q. Dibasic Ammonium Phosphate Application Enhances Aromatic Compound Concentration in Bog Bilberry Syrup Wine. Molecules. 2017; 22(1):52. https://doi.org/10.3390/molecules22010052
Chicago/Turabian StyleWang, Shao-Yang, Yi-Qing Li, Teng Li, Hang-Yu Yang, Jie Ren, Bo-Lin Zhang, and Bao-Qing Zhu. 2017. "Dibasic Ammonium Phosphate Application Enhances Aromatic Compound Concentration in Bog Bilberry Syrup Wine" Molecules 22, no. 1: 52. https://doi.org/10.3390/molecules22010052
APA StyleWang, S.-Y., Li, Y.-Q., Li, T., Yang, H.-Y., Ren, J., Zhang, B.-L., & Zhu, B.-Q. (2017). Dibasic Ammonium Phosphate Application Enhances Aromatic Compound Concentration in Bog Bilberry Syrup Wine. Molecules, 22(1), 52. https://doi.org/10.3390/molecules22010052





