Design, Synthesis and Bioactivity Evaluation of Novel β-carboline 1,3,4-oxadiazole Derivatives
Abstract
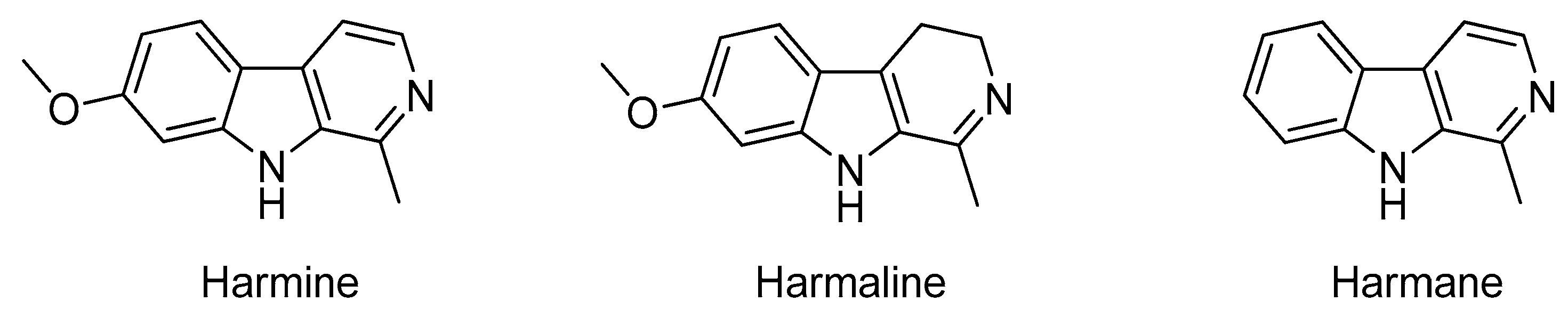
:1. Introduction
2. Results and Discussion
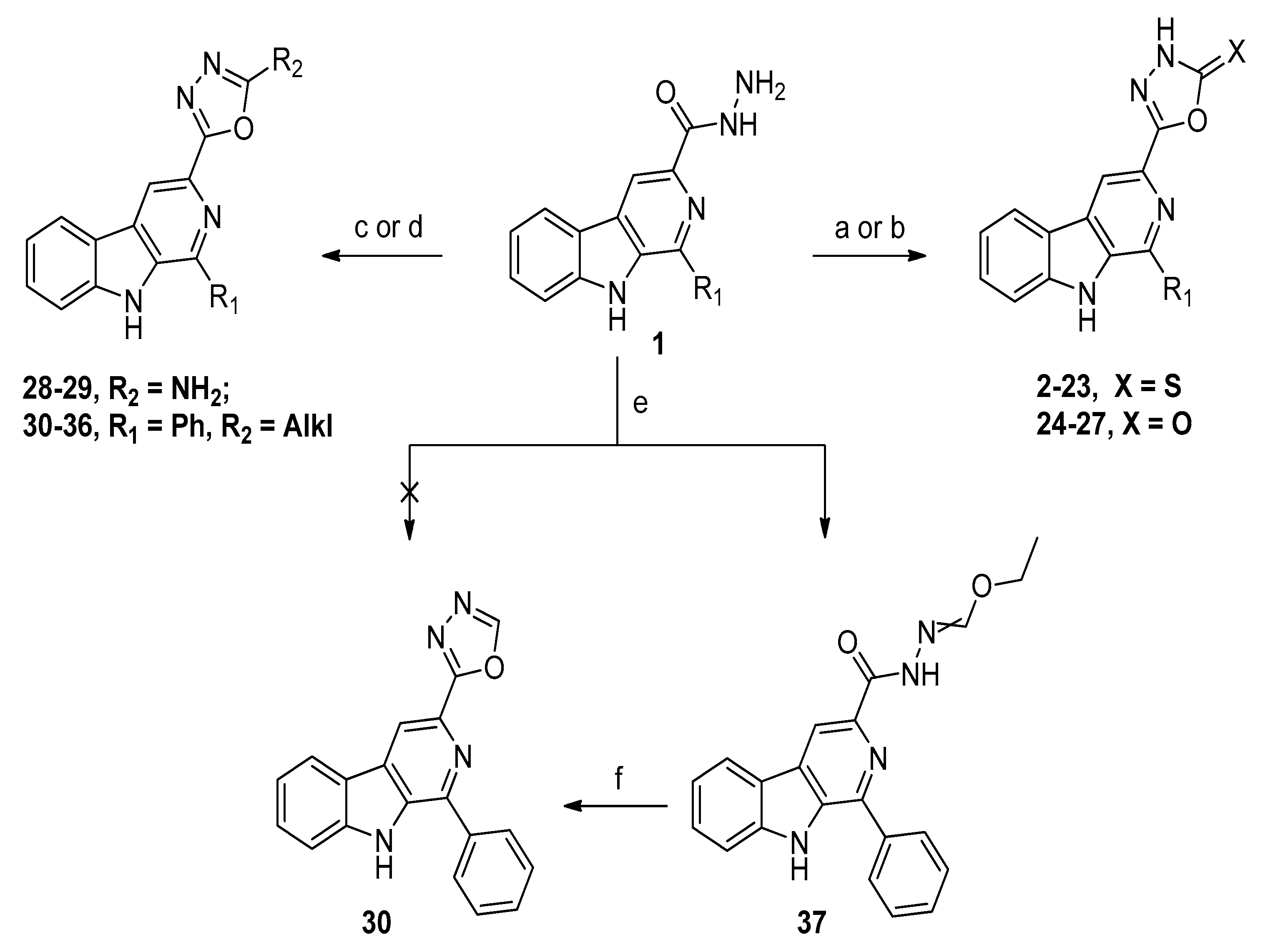
2.1. Chemistry
2.2. Biological Activity
2.2.1. Anti-Proliferative Activity against Sf9 Cells
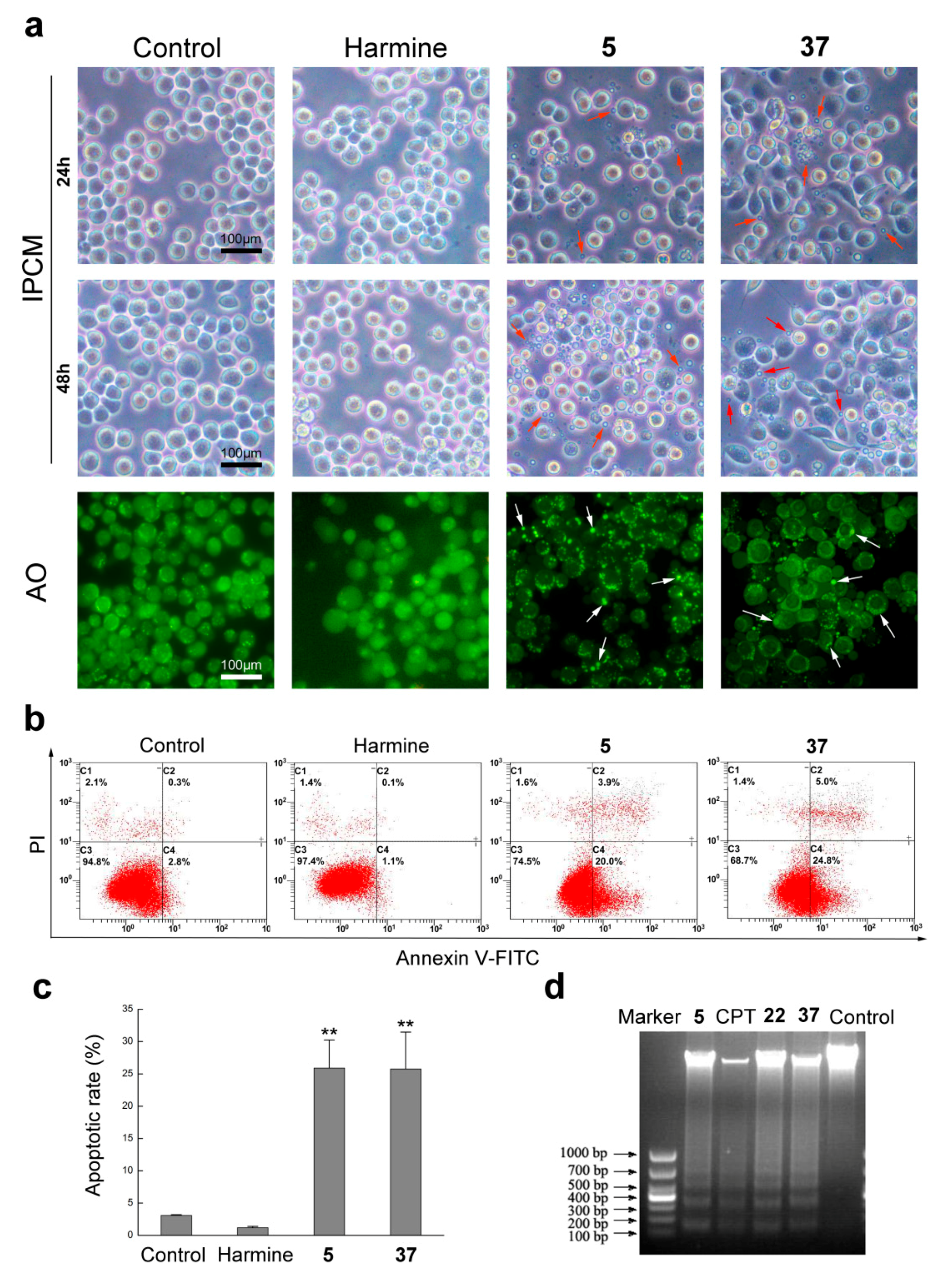
2.2.2. Compounds 5 and 37 Could Induce Cell Apoptosis in Sf9 Cells
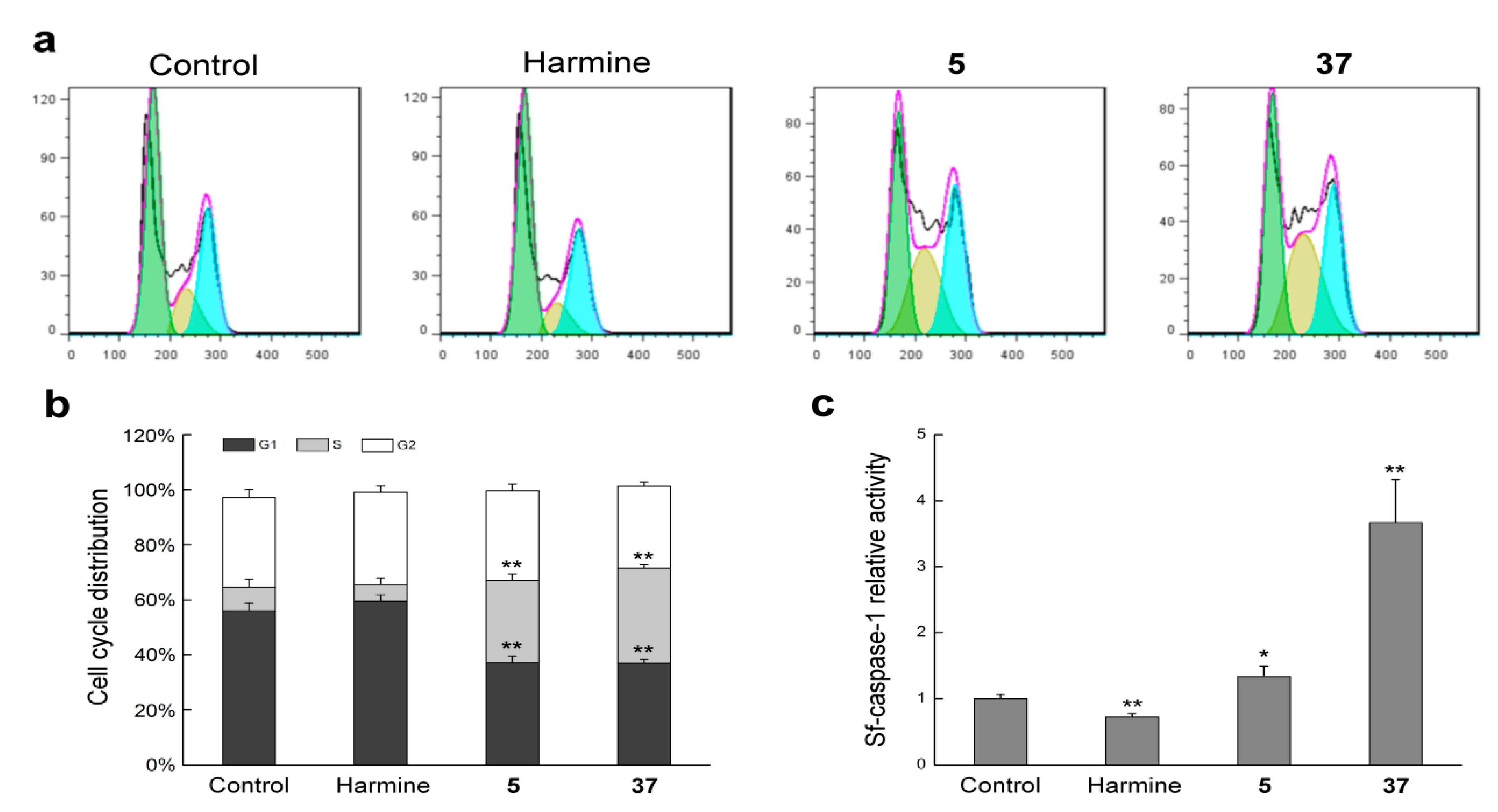
2.2.3. Compounds 5 and 37 Could Induce Cell Cycle Arrest in Sf9 Cells
2.2.4. Compounds 5 and 37 Could Simulate Sf-Caspase-1 Activation
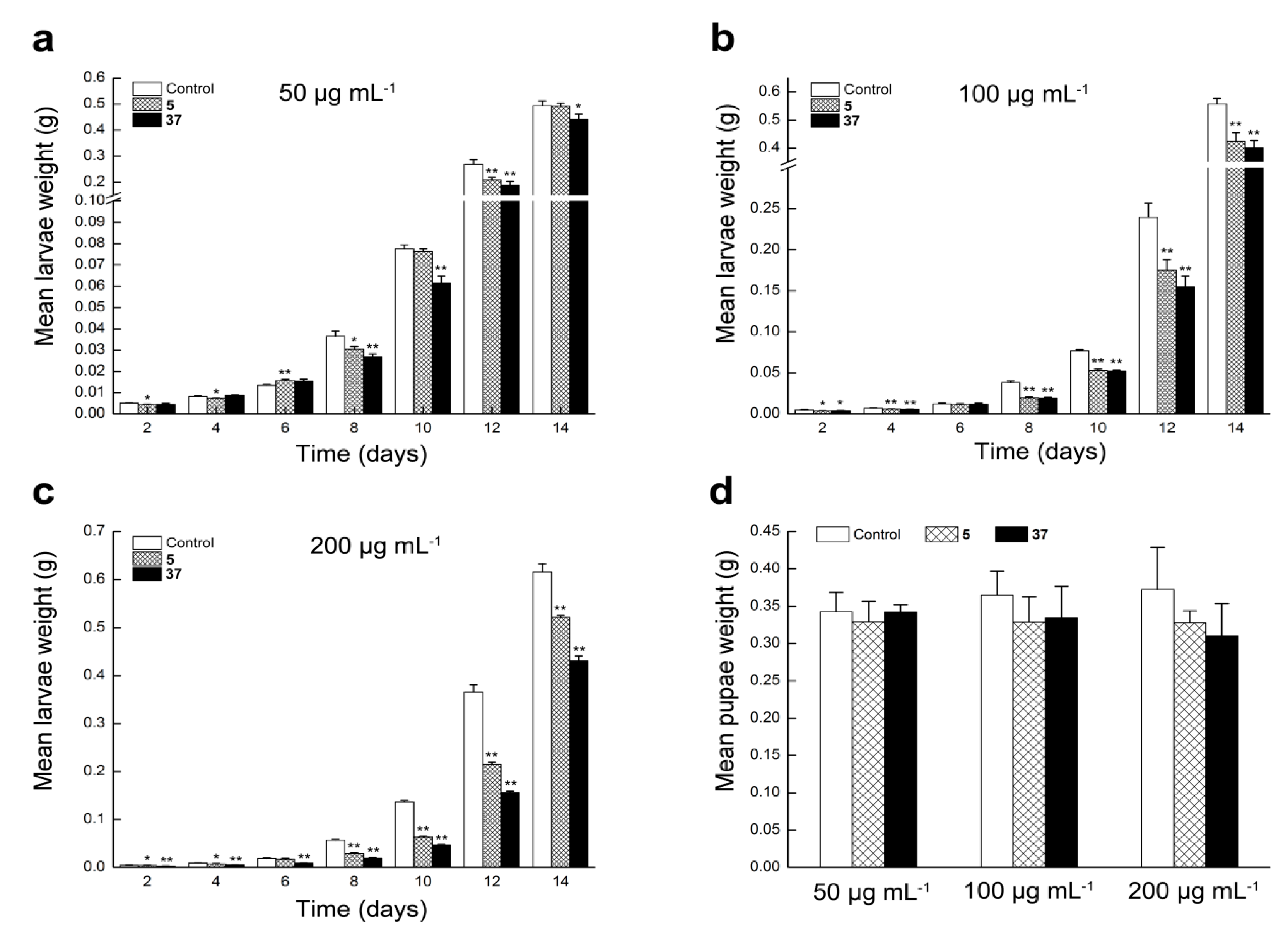
2.2.5. Compounds 5 and 37 Could Inhibit Larvae Growth of S. litura
3. Materials and Methods
3.1. Chemistry
3.1.1. General Synthetic Procedure for Target Compounds 2–23
3.1.2. General Synthetic Procedure for Target Compounds 24–27
3.1.3. General Synthetic Procedure for Target Compounds 28–29
3.1.4. General Synthetic Procedure for Target Compounds 30–36
3.1.5. General Synthetic Procedure for Ethyl Hydrazonoformate 37
3.2. Biology
3.2.1. Cell Culture
3.2.2. Anti-Proliferation Assay
3.2.3. Cell Morphological Observation
3.2.4. Acridine Orange (AO) Staining Analysis
3.2.5. Apoptosis Rate Analysis
3.2.6. DNA Ladder Assay
3.2.7. Sf-Caspase-1 Activity Assay
3.2.8. Cell Cycle Analysis
3.2.9. Insect Growth Inhibition against Tobacco Cutworm (Spodoptera litura)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef] [PubMed]
- Rosell, G.; Quero, C.; Coll, J.; Guerrero, A. Biorational insecticides in pest management. J. Pestic. Sci. 2008, 33, 103–121. [Google Scholar] [CrossRef]
- Petroski, R.J.; Stanley, D.W. Natural compounds for pest and weed control. J. Agric. Food Chem. 2009, 57, 8171–8179. [Google Scholar] [CrossRef] [PubMed]
- Crombie, L. Natural product chemistry and its part in the defence against insects and fungi in agriculture. Pestic. Sci. 1999, 55, 761–774. [Google Scholar] [CrossRef]
- Kartal, M.; Altun, M.L.; Kurucu, S. HPLC method for the analysis of harmol, harmalol, harmine and harmaline in the seeds of Peganum harmala L. J. Pharm. Biomed. 2003, 31, 263–269. [Google Scholar] [CrossRef]
- Li, S.P.; Cheng, X.M.; Wang, C.H. A review on traditional uses, phytochemistry, pharmacology, pharmacokinetics and toxicology of the genus Peganum. J. Ethnopharmacol. 2017, 203, 127–162. [Google Scholar] [CrossRef] [PubMed]
- Bournine, L.; Bensalem, S.; Fatmi, S.; Bedjou, F.; Mathieu, V.; Iguer-Ouada, M.; Kiss, R.; Duez, P. Evaluation of the cytotoxic and cytostatic activities of alkaloid extracts from different parts of Peganum harmala L. (Zygophyllaceae). Eur. J. Integr. Med. 2017, 9, 91–96. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Ramezanloo, F. Chemistry, pharmacology and medicinal properties of Peganum harmala L. Afr. J. Pharm. Pharmacol. 2012, 6, 1573–1580. [Google Scholar] [CrossRef]
- Formagio, A.S.N.; Santos, P.R.; Zanoli, K.; Ueda-Nakamura, T.; Tonin, L.T.D.; Nakamura, C.V.; Sarragiotto, M.H. Synthesis and antiviral activity of beta-carboline derivatives bearing a substituted carbohydrazide at C-3 against poliovirus and herpes simplex virus (HSV-1). Eur. J. Med. Chem. 2009, 44, 4695–4701. [Google Scholar] [CrossRef] [PubMed]
- Farouk, L.; Laroubi, A.; Aboufatima, R.; Benharref, A.; Chait, A. Evaluation of the analgesic effect of alkaloid extract of Peganum harmala L.: Possible mechanisms involved. J. Ethnopharmacol. 2008, 115, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Gadewar, M.; Tripathi, R.; Prasad, S.K.; Patel, D.K. A review on medicinal importance, pharmacological activity and bioanalytical aspects of beta-carboline alkaloid “Harmine”. Asian Pac. J. Trop. Biomed. 2012, 2, 660–664. [Google Scholar] [CrossRef]
- Jbilou, R.; Amri, H.; Boulayad, N.; Ghailani, N.; Ennabili, A.; Sayah, F. Insecticidal effects of extracts of seven plant species on larval development, alpha-amylase activity and offspring production of Tribolium castaneum (Herbst) (Insecta: Coleoptera: Tenebrionidae). Bioresour. Technol. 2008, 99, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Nenaah, G. Toxicity and growth inhibitory activities of methanol extract and the beta-carboline alkaloids of Peganum harmala L. against two coleopteran stored-grain pests. J. Stored Prod. Res. 2011, 47, 255–261. [Google Scholar] [CrossRef]
- Abbasipour, H.; Mahmoudvand, M.; Rastegar, F.; Basij, M. Insecticidal activity of Peganum harmala seed extract against the diamondback moth, Plutella xylostella. B Insectol. 2010, 63, 259–263. [Google Scholar]
- ElGengaihi, S.E.; Dimetry, N.Z.; Mohamed, S.M. Chemical and biological investigation of harmal plant. 2. Alkaloidal investigation. J. Appl. Entomol. 1997, 121, 165–167. [Google Scholar] [CrossRef]
- Shonouda, M.; Osman, S.; Salama, O.; Ayoub, A. Toxical effect of Peganum harmala L. leaves on the cotton leaf worm, Spodoptera littoralis boisd and its parasitoids Microplitis rufiventris Kok. Pak. J. Biol. Sci. 2008, 11, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Abbassi, K.; Atay-Kadiri, Z.; Ghaout, S. Biological effects of alkaloids extracted from three plants of Moroccan arid areas on the desert locust. Physiol. Entomol. 2003, 28, 232–236. [Google Scholar] [CrossRef]
- Rehman, J.U.; Wang, X.G.; Johnson, M.W.; Daane, K.M.; Jilani, G.; Khan, M.A.; Zalom, F.G. Effects of Peganum harmala (Zygophyllaceae) seed extract on the olive fruit fly (Diptera: Tephritidae) and its larval parasitoid Psyttalia concolor (Hymenoptera: Braconidae). J. Econ. Entomol. 2009, 102, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.Q.; Zhong, G.H.; Hu, M.Y.; Wang, Q.; Wang, W.Q.; Su, Z.T. The insecticidal activity of the extract of Peganum harmala and other plant species against pest insects. J. South China Agric. Univ. (Nat. Sci. Ed.) 2003, 24, 38–41. [Google Scholar]
- Su, Z.T.; Ma, A.Q.; Hu, M.Y.; Zhong, G.H. Antifeedant property and physiological effect of total alkaloids of Peganum harmala L. against Spodoptera litura F. J. Hubei Agric. Coll. 2004, 24, 85–89. [Google Scholar]
- Zeng, Y.; Zhang, Y.M.; Weng, Q.F.; Hu, M.Y.; Zhong, G.H. Cytotoxic and insecticidal activities of derivatives of harmine, a natural insecticidal component isolated from Peganum harmala. Molecules 2010, 15, 7775–7791. [Google Scholar] [CrossRef] [PubMed]
- Tabanca, N.; Ali, A.; Bernier, U.R.; Khan, I.A.; Kocyigit-Kaymakcioglu, B.; Oruc-Emre, E.E.; Unsalan, S.; Rollas, S. Biting deterrence and insecticidal activity of hydrazide-hydrazones and their corresponding 3-acetyl-2,5-disubstituted-2,3-dihydro-1,3,4-oxadiazoles against Aedes aegypti. Pest Manag. Sci. 2013, 69, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M.; Kunitomo, J.; Kimura, E.; Iwashita, H.; Uno, Y.; Onishi, T.; Uchiyama, N.; Kawamoto, T.; Tanaka, T.; Mol, C.D.; et al. 2-{3-[4-(Alkylsulpfinyl)phenyl]-1-benzofuran-5-yl}-5-methyl-1,3,4-oxadiazole derivatives as novel inhibitors of glycogen synthase kinase-3 beta with good brain permeability. J. Med. Chem. 2009, 52, 6270–6286. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.J.; Lai, L.H.; Jin, G.Y.; Zhang, Z.X. Synthesis, fungicidal activity, and 3D-QSAR of pyridazinone-substituted 1,3,4-oxadiazoles and 1,3,4-thiadiazoles. J. Agric. Food Chem. 2002, 50, 3757–3760. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, H.; Huang, Y.; Li, J.; Zhao, S.; Song, Y.; Yang, P.; Xiao, Z.; Liu, Y.; Li, Y.; et al. Design, synthesis, and antiviral, fungicidal, and insecticidal activities of tetrahydro-beta-carboline-3-carbohydrazide derivatives. J. Agric. Food Chem. 2014, 62, 9987–9999. [Google Scholar] [CrossRef] [PubMed]
- Formagio, A.S.N.; Tonin, L.T.D.; Foglio, M.A.; Madjarof, C.; de Carvalho, J.E.; da Costa, W.F.; Cardoso, F.P.; Sarragiotto, M.H. Synthesis and antitumoral activity of novel 3-(2-substituted-1,3,4-oxadiazol-5-yl) and 3-(5-substituted-1,2,4-triazol-3-yl) beta-carboline derivatives. Bioorg. Med. Chem. 2008, 16, 9660–9667. [Google Scholar] [CrossRef] [PubMed]
- Levins, C.G.; Wan, Z.K. Efficient phosphonium-mediated synthesis of 2-amino-1,3,4-oxadiazoles. Org. Lett. 2008, 10, 1755–1758. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, T.; Tabatabai, S.A.; Khoshnoud, M.J.; Shafaghi, B.; Shafiee, A. Design and synthesis of 4H-3-(2-phenoxy)phenyl-1,2,4-triazole derivatives as benzodiazepine receptor agonists. Bioorg. Med. Chem. 2003, 11, 769–773. [Google Scholar] [CrossRef]
- Sridhara, A.M.; Reddy, K.R.; Keshavayya, J.; Goud, P.S.; Somashekar, B.C.; Bose, P.; Peethambar, S.K.; Gaddam, S.K. Synthesis and antimicrobial activity of 2-substituted [4-(1,3,4-oxadiazol-2-yl methyl)] phthalazin-1(2H)-one derivatives. Eur. J. Med. Chem. 2010, 45, 4983–4989. [Google Scholar] [CrossRef] [PubMed]
- Boyapati, S.; Kulandaivelu, U.; Sangu, S.; Vanga, M.R. Synthesis, antimicrobial evaluation, and docking studies of novel 4-substituted quinazoline derivatives as DNA-gyrase inhibitors. Arch. Pharm. (Weinheim) 2010, 343, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Savariz, F.C.; Foglio, M.A.; Ruiz, A.L.; da Costa, W.F.; Silva Mde, M.; Santos, J.C.; Figueiredo, I.M.; Meyer, E.; de Carvalho, J.E.; Sarragiotto, M.H. Synthesis and antitumor activity of novel 1-substituted phenyl 3-(2-oxo-1,3,4-oxadiazol-5-yl) beta-carbolines and their Mannich bases. Bioorg. Med. Chem. 2014, 22, 6867–6875. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chejanovsky, N. Activation pathways and signal-mediated upregulation of the insect Spodoptera frugiperda caspase-1. Apoptosis 2006, 11, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.H.; Hu, M.Y.; Weng, Q.F.; Ma, A.Q.; Xu, W.S. Laboratory and field evaluations of extracts from Rhododendron molle flowers as insect growth regulator to imported cabbage worm, Pieris rapae L. (Lepidoptera: Pieridae). J. Appl. Entomol. 2001, 125, 563–569. [Google Scholar] [CrossRef]
- Zhong, G.H.; Liu, J.X.; Weng, Q.F.; Hu, M.Y.; Luo, J.J. Laboratory and field evaluations of rhodojaponin-III against the imported cabbage worm Pieris rapae (L. (Lepidoptera: Pieridae). Pest. Manag. Sci. 2006, 62, 976–981. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–37 are available from the authors. |
| Compound | R1 | X | R2 | IC50 (μmol L−1) | 95% Confidence Interval |
|---|---|---|---|---|---|
| 2 | CH3 | =S | 35.49 | 23.87–49.90 | |
| 3 | Ph | =S | 45.44 | 28.69–70.64 | |
| 4 | 4-CH3-Ph | =S | 47.35 | 29.99–87.66 | |
| 5 | 4-F-Ph | =S | 16.25 | 0.11–61.37 | |
| 6 | 4-Cl-Ph | =S | 12.80 | 6.36–20.96 | |
| 7 | 4-Br-Ph | =S | 34.23 | 20.84–55.59 | |
| 8 | 4-CF3-Ph | =S | 17.58 | 9.67–31.89 | |
| 9 | 4-OCH3-Ph | =S | 10.39 | 8.09–12.95 | |
| 10 | 2-Cl-Ph | =S | 27.69 | 7.99–57.07 | |
| 11 | 3-Cl-Ph | =S | 13.83 | 1.08–42.37 | |
| 12 | 3-F-Ph | =S | 22.37 | 16.71–29.03 | |
| 13 | 3-NO2-Ph | =S | 63.97 | 32.36–134.99 | |
| 14 | 3,4-di-OCH3-Ph | =S | 64.86 | 22.05–135.19 | |
| 15 | 3,4,5-tri-OCH3-Ph | =S | 14.22 | 8.01–22.03 | |
| 16 | 3,4-diF-Ph | =S | 213.34 | 171.16–293.30 | |
| 17 | 3,4-diCl-Ph | =S | 98.74 | 48.67–316.92 | |
| 18 | 3-F-4-OCH3-Ph | =S | 25.79 | 10.89–42.80 | |
| 19 |  | =S | 56.61 | 34.02–90.47 | |
| 20 |  | =S | 19.11 | 14.48–25.90 | |
| 21 | 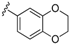 | =S | 30.84 | 3.78–70.23 | |
| 22 | 2-naphthyl | =S | 11.10 | 3.50–21.70 | |
| 23 | 6-quinolyl | =S | 197.81 | 130.28–380.25 | |
| 24 | CH3 | =O | 725.93 | 355.57–2562.85 | |
| 25 | Ph | =O | 18.09 | 5.76–37.16 | |
| 26 | 4-CF3-Ph | =O | 27.35 | 10.22–88.94 | |
| 27 | 3,4,5-tri-OCH3-Ph | =O | 54.92 | 35.49–104.66 | |
| 28 | Ph | NH2 | 28.32 | 18.42–41.27 | |
| 29 | 4-CF3-Ph | NH2 | 44.29 | 18.74–86.43 | |
| 30 | H | 122.52 | 105.73–144.47 | ||
| 31 | CH3 | 129.92 | 110.74–165.14 | ||
| 32 | CH2CH3 | 66.63 | 45.36–97.30 | ||
| 33 | cyclopropyl | 127.76 | 102.16–167.65 | ||
| 34 | Ph | 144.66 | 87.87–463.36 | ||
| 35 | 4-CH3-Ph | >1000 | - | ||
| 36 | 4-Cl-Ph | >1000 | - | ||
| 37 | - | 3.93 | 1.03–8.01 | ||
| Harmine | 140.68 | 75.05–319.53 | |||
| Camptothecin | 18.95 | 12.43–25.68 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-J.; Zhang, J.-J.; Jiang, Z.-Y.; Zhong, G.-H. Design, Synthesis and Bioactivity Evaluation of Novel β-carboline 1,3,4-oxadiazole Derivatives. Molecules 2017, 22, 1811. https://doi.org/10.3390/molecules22111811
Zhang Z-J, Zhang J-J, Jiang Z-Y, Zhong G-H. Design, Synthesis and Bioactivity Evaluation of Novel β-carboline 1,3,4-oxadiazole Derivatives. Molecules. 2017; 22(11):1811. https://doi.org/10.3390/molecules22111811
Chicago/Turabian StyleZhang, Zhi-Jun, Jing-Jing Zhang, Zhi-Yan Jiang, and Guo-Hua Zhong. 2017. "Design, Synthesis and Bioactivity Evaluation of Novel β-carboline 1,3,4-oxadiazole Derivatives" Molecules 22, no. 11: 1811. https://doi.org/10.3390/molecules22111811





