Tryptophan-Containing Cyclic Decapeptides with Activity against Plant Pathogenic Bacteria
Abstract
:1. Introduction
2. Results
2.1. Design and Synthesis
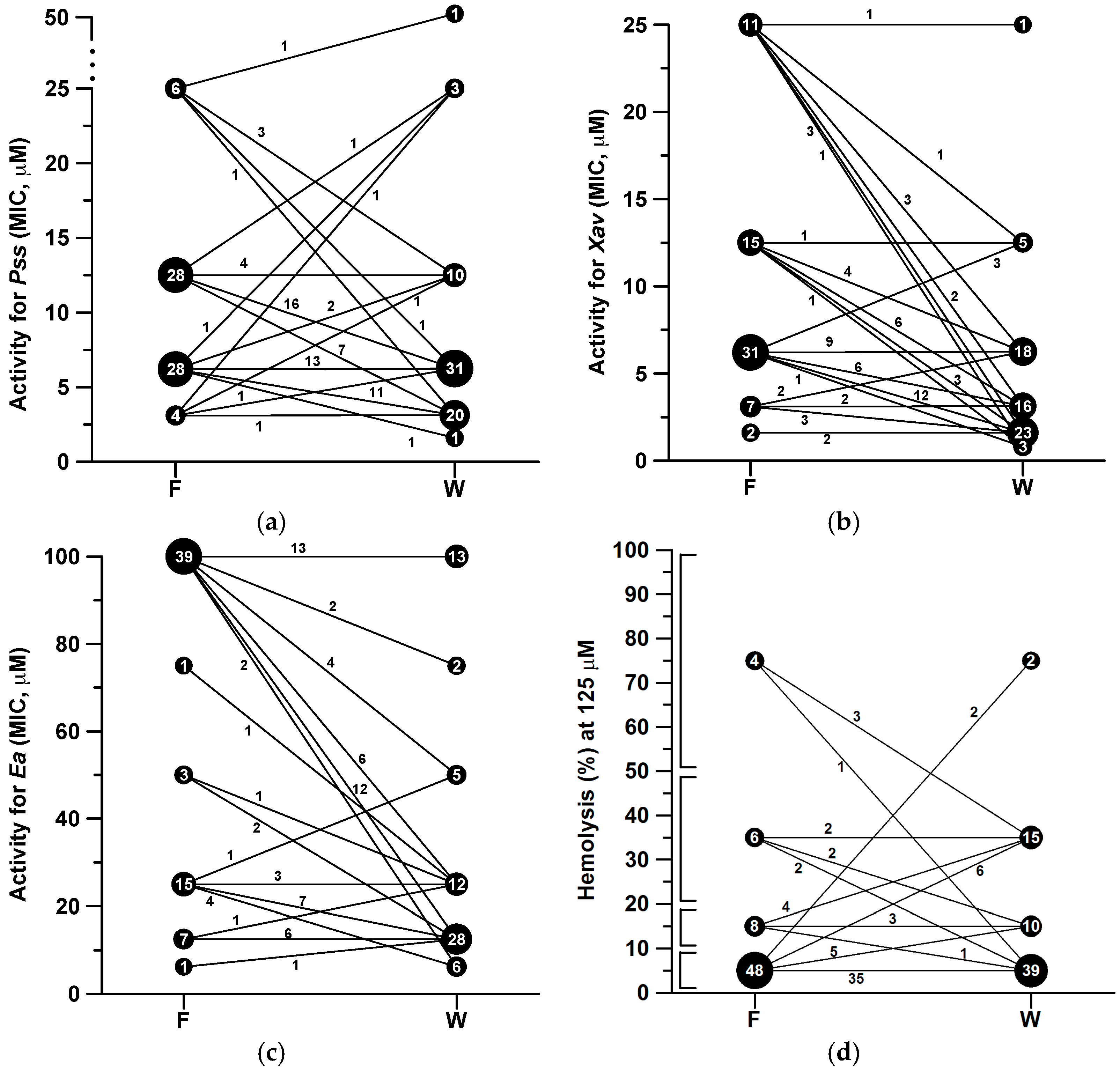
2.2. Antibacterial and Hemolytic Activities
3. Discussion
4. Materials and Methods
4.1. Chemicals and Instruments
4.2. Synthesis of the Cyclic Decapeptide Library
4.3. Bacterial Strains and Growth Conditions
4.4. Antibacterial Activity
4.5. Hemolytic Activity
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Agrios, G.N. Plant Pathology, 5th ed.; Academic Press: San Diego, CA, USA, 2005. [Google Scholar]
- Vidaver, A.K. Uses of antimicrobials in plant agriculture. Clin. Infect. Dis. 2002, 34, S107–S110. [Google Scholar] [CrossRef] [PubMed]
- McManus, P.S.; Stockwell, V.O.; Sundin, V.O.; Jones, A.L. Antibiotic use in plant agriculture. Annu. Rev. Phytopathol. 2002, 46, 443–465. [Google Scholar] [CrossRef] [PubMed]
- Sundin, G.W.; Bender, C.L. Ecological and genetic analysis of copper and streptomycin resistance in Pseudomonas syringae pv. syringae. Appl. Environ. Microbiol. 1993, 59, 1018–1024. [Google Scholar] [PubMed]
- Hancock, R.E.W.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, E. Antimicrobial peptides and plant disease control. FEMS Microbiol. Lett. 2007, 270, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Marcos, J.F.; Muñoz, A.; Pérez-Payá, E.; Misra, S.; López-García, B. Identification and rational design of novel antimicrobial peptides for plant protection. Annu. Rev. Phytopathol. 2008, 46, 271–301. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, E.; Bardají, E. Synthetic antimicrobial peptides as agricultural pesticides for plant-disease control. Chem. Biodivers. 2008, 5, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Keymanesh, K.; Soltani, S.; Sardari, S. Application of antimicrobial peptides in agriculture and food industry. World J. Microbiol. Biotechnol. 2009, 25, 933–944. [Google Scholar] [CrossRef]
- Kang, S.-J.; Park, S.J.; Mishig-Ochir, T.; Lee, B.-J. Antimicrobial peptides: Therapeutic potentials. Expert Rev. Anti-Infect. Ther. 2014, 12, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.C.; Pena, O.M.; Hancock, R.E.W. Host defense peptides: Front-line immunomodulators. Trends Immunol. 2014, 35, 443–450. [Google Scholar] [CrossRef] [PubMed]
- da Costa, J.P.; Cova, M.; Ferreira, R. Antimicrobial peptides: An alternative for innovative medicines? Appl. Microbiol. Biotechnol. 2015, 99, 2023–2040. [Google Scholar] [CrossRef] [PubMed]
- Cavallarin, L.; Andreu, D.; San Segundo, B. Cecropin A-derived peptides are potent inhibitors of fungal plant pathogens. Mol. Plant-Microbe Interact. 1998, 11, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Güell, I.; Cabrefiga, J.; Badosa, E.; Ferre, R.; Talleda, M.; Bardají, E.; Planas, M.; Feliu, L.; Montesinos, E. Improvement of the efficacy of linear undecapeptides against plant-pathogenic bacteria by incorporation of d-amino acids. Appl. Environ. Microbiol. 2011, 77, 2667–2675. [Google Scholar] [CrossRef] [PubMed]
- Ng-Choi, I.; Soler, M.; Cerezo, V.; Badosa, E.; Montesinos, E.; Planas, M.; Feliu, L. Solid-phase synthesis of 5-arylhistidine-containing peptides with antimicrobial activity through a microwave-assisted Suzuki–Miyaura cross-coupling. Eur. J. Org. Chem. 2012, 4321–4332. [Google Scholar] [CrossRef]
- Montesinos, E.; Badosa, E.; Cabrefiga, J.; Planas, M.; Feliu, L.; Bardají, E. Antimicrobial peptides for plant disease control. From discovery to application. In Small Wonders: Peptides for Disease Control; Rajasekaran, K., Cary, J.W., Jaynes, J.M., Montesinos, E., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2012; pp. 235–261. [Google Scholar]
- Ng-Choi, I.; Soler, M.; Güell, I.; Badosa, E.; Cabrefiga, J.; Bardají, E.; Montesinos, E.; Planas, M.; Feliu, L. Antimicrobial peptides incorporating non-natural amino acids as agents for plant protection. Protein Pept. Lett. 2014, 21, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Puig, M.; Moragrega, C.; Ruz, L.; Montesinos, E.; Llorente, I. Postinfection activity of synthetic antimicrobial peptides against Stemphylium vesicarium in pear. Phytopathology 2014, 104, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.T.; Evans, K.O.; Dowd, P.F. Antifungal activity of a synthetic cationic peptide against the plant pathogens Colletotrichum graminicola and three Fusarium species. Plant Pathol. J. 2015, 31, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Monroc, S.; Badosa, E.; Besalú, E.; Planas, M.; Bardají, E.; Montesinos, E.; Feliu, L. Improvement of cyclic decapeptides against plant pathogenic bacteria using a combinatorial chemistry approach. Peptides 2006, 27, 2575–2584. [Google Scholar] [CrossRef] [PubMed]
- Monroc, S.; Badosa, E.; Feliu, L.; Planas, M.; Montesinos, E.; Bardají, E. De novo designed cyclic cationic peptides as inhibitors of plant pathogenic bacteria. Peptides 2006, 27, 2567–2574. [Google Scholar] [CrossRef] [PubMed]
- Vilà, S.; Badosa, E.; Montesinos, E.; Planas, M.; Feliu, L. Synthetic cyclolipopeptides selective agaisnt microbial, plant and animal cell targets by incorporation of d-amino acids or histidine. PLoS ONE 2016, 11, e0151639. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Kim, V.S. Antimicrobial cyclic peptides for plant disease control. Plant Pathol. J. 2015, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Aiello, C.; Grieco, P.; Marasco, D. Targetting “undruggable” proteins: Design of synthetic cyclopeptides. Curr. Med. Chem. 2016, 23, 748–762. [Google Scholar] [CrossRef] [PubMed]
- de Veer, S.J.; Weidmann, J.; Craik, D.J. Cyclotides as tools in chemical biology. Acc. Chem. Res. 2017, 50, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- López-García, B.; Pérez-Payá, E.; Marcos, J.F. Identification of novel hexapeptides bioactive against phytopathogenic fungi through screening of a synthetic peptide combinatorial library. Appl. Environ. Microbiol. 2002, 68, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.D.; Edwards, D.L.; Gonzalez, C.F. Synthetic peptide combinatorial libraries: A method for the identification of bioactive peptides against phytopathogenic fungi. Mol. Plant Microbe Interact. 1997, 10, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Bishop-Hurley, S.L.; Mounter, S.A.; Laskey, J.; Morris, R.O.; Elder, J.; Roop, P.; Rouse, C.; Schmidt, F.J.; English, J.T. Phage-displayed peptides as developmental agonists for Phytophthora capsici zoospores. Appl. Environ. Microbiol. 2002, 68, 3315–3320. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Park, H.C.; Yoon, M.Y. Screening for peptides binding on Phytophthora capsici extracts by phage display. J. Microbiol. Methods 2009, 78, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.; López-García, B.; Pérez-Payá, E.; Marcos, J.F. Antimicrobial properties of derivatives of the cationic tryptophan-rich hexapeptide PAF26. Biochem. Biophys. Res. Commun. 2007, 354, 172–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebollar, A.; Marcos, J.F.; López-García, B. Screening of a synthetic peptide combinatorial library to identify inhibitors of the appressorium formation in Magnaporthe oryzae. Biochem. Biophys. Res. Commun. 2014, 454, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Moon, E. Identification of novel bioactive hexapeptides against phytopathogenic bacteria through rapid screening of a synthetic combinatorial library. J. Microbiol. Biotechnol. 2009, 19, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Badosa, E.; Ferre, R.; Planas, M.; Feliu, L.; Besalú, E.; Cabrefiga, J.; Bardají, E.; Montesinos, E. A library of linear undecapeptides with bactericidal activity against phytopathogenic bacteria. Peptides 2007, 28, 2276–2285. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Lee, K.-C.; Cross, T.A. Tryptophans in membrane proteins: Indole ring orientations and functional implications in the gramicidin channel. Biochemistry 1993, 32, 7035–7047. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.I.; Prenner, E.J.; Vogel, H.J. Tryptophan- and arginine-rich antimicrobial peptides: Structures and mechanisms of action. Biochim. Biophys. Acta 2006, 1758, 1184–1202. [Google Scholar] [CrossRef] [PubMed]
- Domalaon, R.; Zhanel, G.G.; Schweizer, F. Short antimicrobial peptides and peptide scaffolds as promising antibacterial agents. Curr. Top. Med. Chem. 2016, 16, 1217–1230. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, G.; Kamalakannan, R.; Shin, S.Y. Enhancement of the anti-inflammatory activity of temporin-1Tl-derived antimicrobial peptides by tryptophan, arginine and lysine substitutions. J. Pept. Sci. 2015, 21, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, R.; Li, X.; Lim, K.; Mohanram, H.; Peng, L.; Mishra, B.; Basu, A.; Lee, J.-M.; Bhattacharjya, S.; Leong, S.S. Design of short membrane selective antimicrobial peptides containing tryptophan and arginine residues for improve activity, salt-resistance, and biocompatibility. Biotechnol. Bioeng. 2014, 111, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Wessolowski, A.; Bienert, M.; Dathe, M. Antimicrobial activity of arginine- and tryptophan-rich hexapeptides: The effects of aromatic clusters, the amino acid substitution and cyclization. J. Pept. Res. 2004, 64, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Baek, K.-H.; Moon, E. Antimicrobial effects of a hexapeptide KCM21 against Pseudomonas syringae pv. tomato DC3000 and Clavibacter michiganensis subsp. michiganensis. Plant Pathol. J. 2014, 30, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Grau-Campistany, A.; Manresa, A.; Pujol, M.; Rabanal, F.; Cajal, Y. Tryptophan-containing lipopeptide antibiotics derived from polymyxin B with activity against Gram positive and Gram negative bacteria. Biochim. Biophys. Acta 2016, 1858, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhao, U.; Li, S.; Yan, Z.; Li, J.; Li, Y.; Mou, L.; Zhang, B.; Yang, W.; Miao, X.; et al. Novel antimicrobial peptide CPF-C1 analogs with superior stabilities and activities against multidrug-resistant bacteria. Chem. Biol. Drug Des. 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Strömsted, A.A.; Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Evaluation of strategies for improving proteolytic resistance of antimicrobial peptides by using variants of EFK17, an internal segment of LL-37. Antimicrob. Agents Chemother. 2009, 53, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Molhoek, E.M.; van Dijk, A.; Veldhuizen, E.J.A.; Dijk-Knijnenburg, H.; Mars-Groenendijk, R.H.; Boele, L.C.L.; Kaman-van Zanten, W.E.; Haagsman, H.P.; Bikker, F.J. Chicken cathelicidin-2-derived peptides with enhanced immunomodulatory and antibacterial activities against biological warfare agents. Int. J. Antimicrob. Agents 2010, 36, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.H.; Bang, J.-K.; Jacob, B.; Park, I.-S.; Shin, S.Y. Prokaryotic selectivity and LPS-neutralizing activity of short antimicrobial peptides designed from the human antimicrobial peptide LL-37. Peptides 2012, 35, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Li, W.; Zhang, L.; Zhang, Y.; Cao, B. Cecropin A-melittin mutant with improved proteolytic stability and enhanced antimicrobial activity against bacteria and fungi associated with gastroenteritis in vitro. Biochem. Biophys. Res. Commun. 2014, 451, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Blondelle, S.E.; Lohner, K. Combinatorial libraries: A tool to design antimicrobial and antifungal peptide analogues having lytic specificities for structure-activity relationship studies. Biopolymers 2000, 55, 74–87. [Google Scholar] [CrossRef]
- Oh, D.; Shin, S.Y.; Lee, S.; Kang, J.H.; Kim, S.D.; Ryu, P.D.; Hahm, K.-S.; Kim, Y. Role of the hinge region and the tryptophan residue in the synthetic antimicrobial peptides, cecropin A(1–8)–magainin 2(1–12) and its analogues, on their antibiotic activities and structures. Biochemistry 2000, 39, 11855–11864. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |


| Peptide | Sequence | tR (min) a | Purity (%) b | HRMS |
|---|---|---|---|---|
| BPC016W | c(KLKLKWKLKQ) | 6.19 | 91 | 647.9420 [M + 2H]2+, 1294.8736 [M + H]+ |
| BPC058W | c(KKKKKWLLLQ) | 6.16 | >99 | 647.9400 [M + 2H]2+, 1294.8709 [M + H]+ |
| BPC060W | c(KKKKLWKLLQ) | 6.34 | >99 | 647.9389 [M + 2H]2+, 1294.8709 [M + H]+ |
| BPC062W | c(KKKLKWKLLQ) | 6.15 | >99 | 647.9371 [M + 2H]2+, 1294.8681 [M + H]+ |
| BPC064W | c(KKLKKWKLLQ) | 6.35 | >99 | 647.9427 [M + 2H]2+, 1294.8752 [M + H]+ |
| BPC066W | c(KLKKKWKLLQ) | 6.48 | 88 | 647.9423 [M + 2H]2+, 1294.8752 [M + H]+ |
| BPC068W | c(LKKKKWKLLQ) | 6.00 | >99 | 647.9424 [M + 2H]2+, 1294.8700 [M + H]+ |
| BPC070W | c(KKKKLWLKLQ) | 6.04 | >99 | 647.9411 [M + 2H]2+, 1294.8716 [M + H]+ |
| BPC072W | c(KKKLKWLKLQ) | 6.04 | >99 | 647.9414 [M + 2H]2+, 1294.8718 [M + H]+ |
| BPC074W | c(KKLKKWLKLQ) | 6.33 | 90 | 647.9422 [M + 2H]2+, 1294.8692 [M + H]+ |
| BPC076W | c(KLKKKWLKLQ) | 6.11 | 96 | 647.9423 [M + 2H]2+, 1294.8737 [M + H]+ |
| BPC078W | c(LKKKKWLKLQ) | 5.94 | >99 | 647.9410 [M + 2H]2+, 1294.8709 [M + H]+ |
| BPC080W | c(KKKLLWKKLQ) | 5.90 | >99 | 647.9396 [M + 2H]2+, 1294.8694 [M + H]+ |
| BPC082W | c(KKLKLWKKLQ) | 6.22 | 88 | 647.9424 [M + 2H]2+, 1294.8727 [M + H]+ |
| BPC084W | c(KLKKLWKKLQ) | 6.42 | 92 | 647.9411 [M + 2H]2+, 1294.8706 [M + H]+ |
| BPC086W | c(LKKKLWKKLQ) | 5.98 | 91 | 647.9405 [M + 2H]2+, 1294.8696 [M + H]+ |
| BPC088W | c(KKLLKWKKLQ) | 6.09 | 88 | 647.9419 [M + 2H]2+, 1294.8700 [M + H]+ |
| BPC090W | c(KLKLKWKKLQ) | 6.03 | >99 | 647.9426 [M + 2H]2+, 1294.8740 [M + H]+ |
| BPC092W | c(LKKLKWKKLQ) | 5.89 | >99 | 647.9421 [M + 2H]2+, 1294.8706 [M + H]+ |
| BPC094W | c(KLLKKWKKLQ) | 6.39 | 83 | 647.9410 [M + 2H]2+, 1294.8693 [M + H]+ |
| BPC096W | c(LKLKKWKKLQ) | 6.07 | 88 | 647.9418 [M + 2H]2+, 1294.8712 [M + H]+ |
| BPC098W | c(LLKKKWKKLQ) | 6.07 | >99 | 647.9418 [M + 2H]2+, 1294.8714 [M + H]+ |
| BPC100W | c(KKKKLWLLKQ) | 5.84 | >99 | 647.9411 [M + 2H]2+, 1294.8695 [M + H]+ |
| BPC102W | c(KKKLKWLLKQ) | 6.38 | 96 | 647.9405 [M + 2H]2+, 1294.8694 [M + H]+ |
| BPC104W | c(KKLKKWLLKQ) | 6.28 | >99 | 647.9419 [M + 2H]2+, 1294.8700 [M + H]+ |
| BPC106W | c(KLKKKWLLKQ) | 5.89 | >99 | 647.9421 [M + 2H]2+, 1294.8640 [M + H]+ |
| BPC108W | c(LKKKKWLLKQ) | 6.02 | 96 | 647.9420 [M + 2H]2+, 1294.8747 [M + H]+ |
| BPC110W | c(KKKLLWKLKQ) | 6.16 | >99 | 647.9411 [M + 2H]2+, 1294.8743 [M + H]+ |
| BPC112W | c(KKLKLWKLKQ) | 6.09 | >99 | 647.9425 [M + 2H]2+, 1294.8746 [M + H]+ |
| BPC114W | c(KLKKLWKLKQ) | 6.20 | 84 | 647.9423 [M + 2H]2+, 1294.8750 [M + H]+ |
| BPC116W | c(LKKKLWKLKQ) | 6.06 | 89 | 647.9425 [M + 2H]2+, 1294.8730 [M + H]+ |
| BPC118W | c(KKLLKWKLKQ) | 6.10 | >99 | 647.9400 [M + 2H]2+, 1294.8707 [M + H]+ |
| BPC120W | c(LKKLKWKLKQ) | 6.11 | 89 | 647.9415 [M + 2H]2+, 1294.8725 [M + H]+ |
| BPC122W | c(KLLKKWKLKQ) | 6.20 | >99 | 647.9398 [M + 2H]2+, 1294.8708 [M + H]+ |
| BPC124W | c(LKLKKWKLKQ) | 5.92 | 97 | 647.9414 [M + 2H]2+, 1294.8727 [M + H]+ |
| BPC126W | c(LLKKKWKLKQ) | 6.04 | 87 | 647.9417 [M + 2H]2+, 1294.8737 [M + H]+ |
| BPC128W | c(KKKLLWLKKQ) | 5.92 | >99 | 647.9424 [M + 2H]2+, 1294.8764 [M + H]+ |
| BPC130W | c(KKLKLWLKKQ) | 6.01 | >99 | 647.9423 [M + 2H]2+, 1294.8745 [M + H]+ |
| BPC132W | c(KLKKLWLKKQ) | 5.93 | >99 | 647.9418 [M + 2H]2+, 1294.8753 [M + H]+ |
| BPC134W | c(LKKKLWLKKQ) | 5.87 | >99 | 647.9411 [M + 2H]2+, 1294.8731 [M + H]+ |
| BPC136W | c(KKLLKWLKKQ) | 6.87 | 90 | 647.9401 [M + 2H]2+, 1294.8697 [M + H]+ |
| BPC138W | c(KLKLKWLKKQ) | 5.98 | >99 | 647.9418 [M + 2H]2+, 1294.8727 [M + H]+ |
| BPC140W | c(LKKLKWLKKQ) | 6.95 | 81 | 647.9406 [M + 2H]2+, 1294.8683 [M + H]+ |
| BPC142W | c(KLLKKWLKKQ) | 6.76 | 87 | 647.9424 [M + 2H]2+, 1294.8755 [M + H]+ |
| BPC144W | c(LKLKKWLKKQ) | 6.72 | 87 | 647.9381 [M + 2H]2+, 1294.8674 [M + H]+ |
| BPC146W | c(LLKKKWLKKQ) | 6.45 | 90 | 647.9419 [M + 2H]2+, 1294.8703 [M + H]+ |
| BPC148W | c(KKLLLWKKKQ) | 5.81 | 97 | 647.9461 [M + 2H]2+, 1294.8842 [M + H]+ |
| BPC150W | c(KLKLLWKKKQ) | 5.92 | 91 | 647.9405 [M + 2H]2+, 1294.8741 [M + H]+ |
| BPC152W | c(LKKLLWKKKQ) | 5.90 | 85 | 647.9411 [M + 2H]2+, 1294.8706 [M + H]+ |
| BPC154W | c(KLLKLWKKKQ) | 6.03 | 87 | 647.9435 [M + 2H]2+, 1294.8759 [M + H]+ |
| BPC156W | c(LKLKLWKKKQ) | 6.37 | >99 | 647.9422 [M + 2H]2+, 1294.8723 [M + H]+ |
| BPC158W | c(LLKKLWKKKQ) | 6.12 | 86 | 647.9425 [M + 2H]2+, 1294.8738 [M + H]+ |
| BPC160W | c(KLLLKWKKKQ) | 5.96 | 88 | 647.9425 [M + 2H]2+, 1294.8739 [M + H]+ |
| BPC162W | c(LKLLKWKKKQ) | 6.00 | 93 | 647.9388 [M + 2H]2+, 1294.8701 [M + H]+ |
| BPC164W | c(LLKLKWKKKQ) | 6.38 | >99 | 647.9417 [M + 2H]2+, 1294.8723 [M + H]+ |
| BPC166W | c(LLLKKWKKKQ) | 6.38 | 90 | 647.9423 [M + 2H]2+, 1294.8742 [M + H]+ |
| BPC184W | c(KLLLKWKKLQ) | 6.97 | 93 | 640.4371 [M + 2H]2+, 1279.8604 [M + H]+ |
| BPC186W | c(KKKLKWKKLQ) | 5.76 | >99 | 655.4473 [M + 2H]2+, 1309.8832 [M + H]+ |
| BPC188W | c(LLLKKWKKLQ) | 6.97 | 93 | 640.4352 [M + 2H]2+, 1279.8585 [M + H]+ |
| BPC190W | c(LKKKKWKKLQ) | 5.70 | >99 | 655.4471 [M + 2H]2+, 1309.8811 [M + H]+ |
| BPC192W | c(LKLLKWKKLQ) | 6.30 | 90 | 640.4380 [M + 2H]2+, 1279.8633 [M + H]+ |
| BPC194W | c(KKLKKWKKLQ) | 6.21 | 91 | 655.4456 [M + 2H]2+, 1309.8802 [M + H]+ |
| BPC196W | c(LLKLKWKKLQ) | 6.60 | 99 | 640.4362 [M + 2H]2+, 1279.8610 [M + H]+ |
| BPC198W | c(KLKKKWKKLQ) | 6.26 | 93 | 655.4468 [M + 2H]2+, 1309.8813 [M + H]+ |
| BPC200W | c(LLLLKWKKLQ) | 7.40 | 96 | 632.9259 [M + 2H]2+, 1264.8505 [M + H]+ |
| BPC202W | c(KKKKKWKKLQ) | 5.51 | >99 | 662.9523 [M + 2H]2+, 1324.8950 [M + H]+ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camó, C.; Torné, M.; Besalú, E.; Rosés, C.; Cirac, A.D.; Moiset, G.; Badosa, E.; Bardají, E.; Montesinos, E.; Planas, M.; et al. Tryptophan-Containing Cyclic Decapeptides with Activity against Plant Pathogenic Bacteria. Molecules 2017, 22, 1817. https://doi.org/10.3390/molecules22111817
Camó C, Torné M, Besalú E, Rosés C, Cirac AD, Moiset G, Badosa E, Bardají E, Montesinos E, Planas M, et al. Tryptophan-Containing Cyclic Decapeptides with Activity against Plant Pathogenic Bacteria. Molecules. 2017; 22(11):1817. https://doi.org/10.3390/molecules22111817
Chicago/Turabian StyleCamó, Cristina, Maria Torné, Emili Besalú, Cristina Rosés, Anna D. Cirac, Gemma Moiset, Esther Badosa, Eduard Bardají, Emilio Montesinos, Marta Planas, and et al. 2017. "Tryptophan-Containing Cyclic Decapeptides with Activity against Plant Pathogenic Bacteria" Molecules 22, no. 11: 1817. https://doi.org/10.3390/molecules22111817






