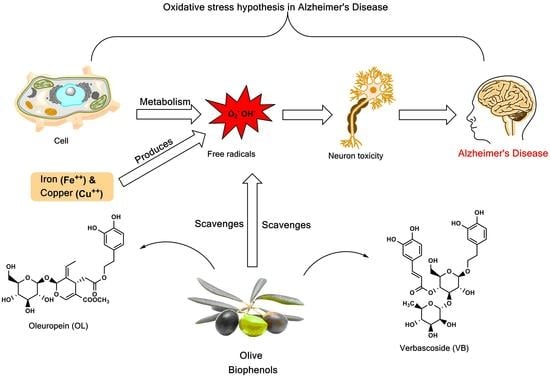
Olive (Olea europaea L.) Biophenols: A Nutriceutical against Oxidative Stress in SH-SY5Y Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Plant Extracts
2.2. Sample Preparation
2.3. Determination of Total Phenol Content (TPC) in Commercial Extracts
2.4. Determination of Total Flavonoid Content (TFC) in Commercial Extracts
2.5. HPLC-DAD-Online ABTS Radical Scavenging Analysis
2.6. Liquid Chromatography-Mass Spectrometry (LC-MS)
2.7. Superoxide Radical (SOR) Scavenging Assay
2.8. In Vitro H2O2 Scavenging Assay
2.9. Ferric Reducing/Antioxidant Power (FRAP) Assay
2.10. Cell Culture
2.11. H2O2-Induced Toxicity in SH-SY5Y Cells and Neuroprotective Potential of Olive Biophenols
2.12. Copper (Cu)-Induced SH-SY5Y Cells Toxicity and Olive Biophenols Treatment
2.13. Cell Viability Assay
2.14. Statistical Analysis
3. Results and Discussion
3.1. Total Phenol and Total Flavonoid Contents
3.2. Identification and Antioxidant Profile of Commercial Olive Extracts
3.3. Superoxide Radical (SOR) Scavenging Activities
3.4. H2O2 Radical Scavenging Activities by Olive Biophenols
3.5. Ferric (Fe3+) Reducing Antioxidant Power Assay (FRAP)
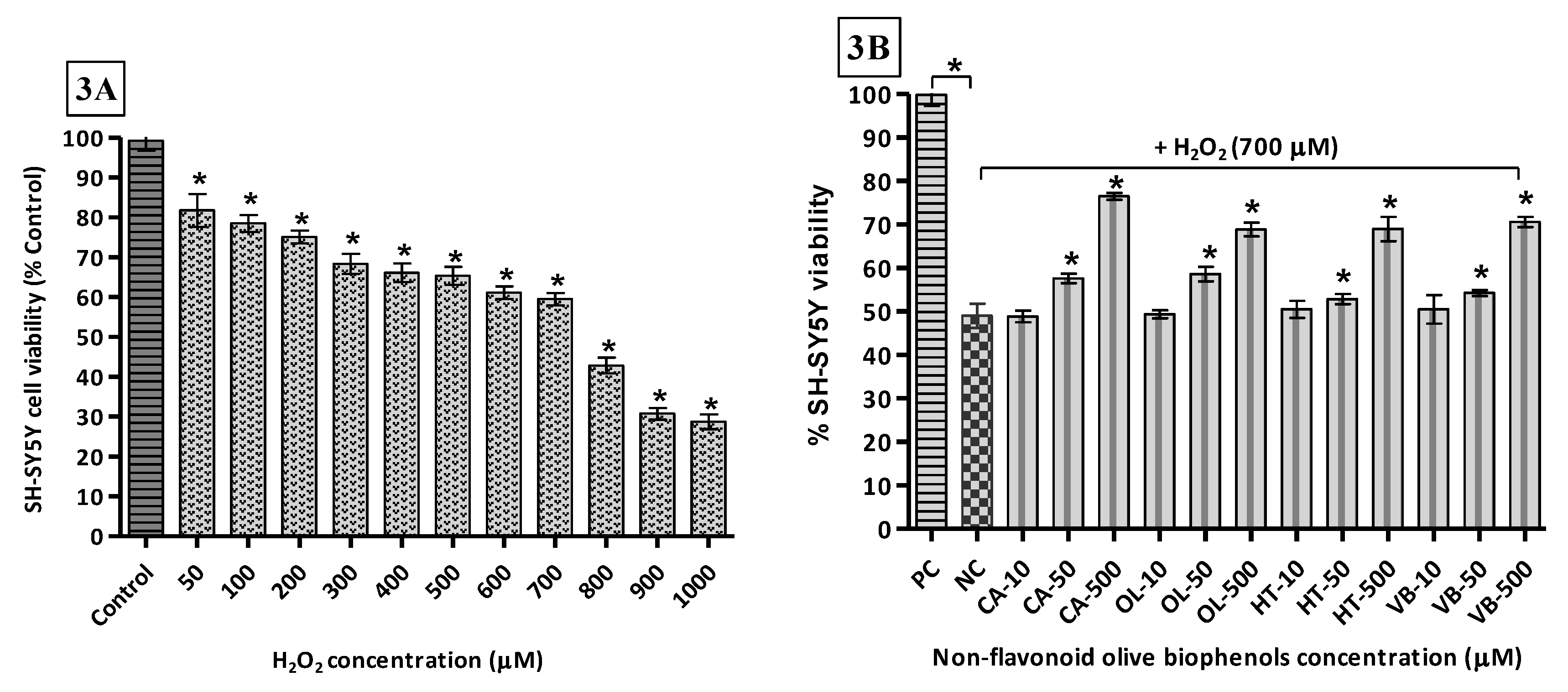
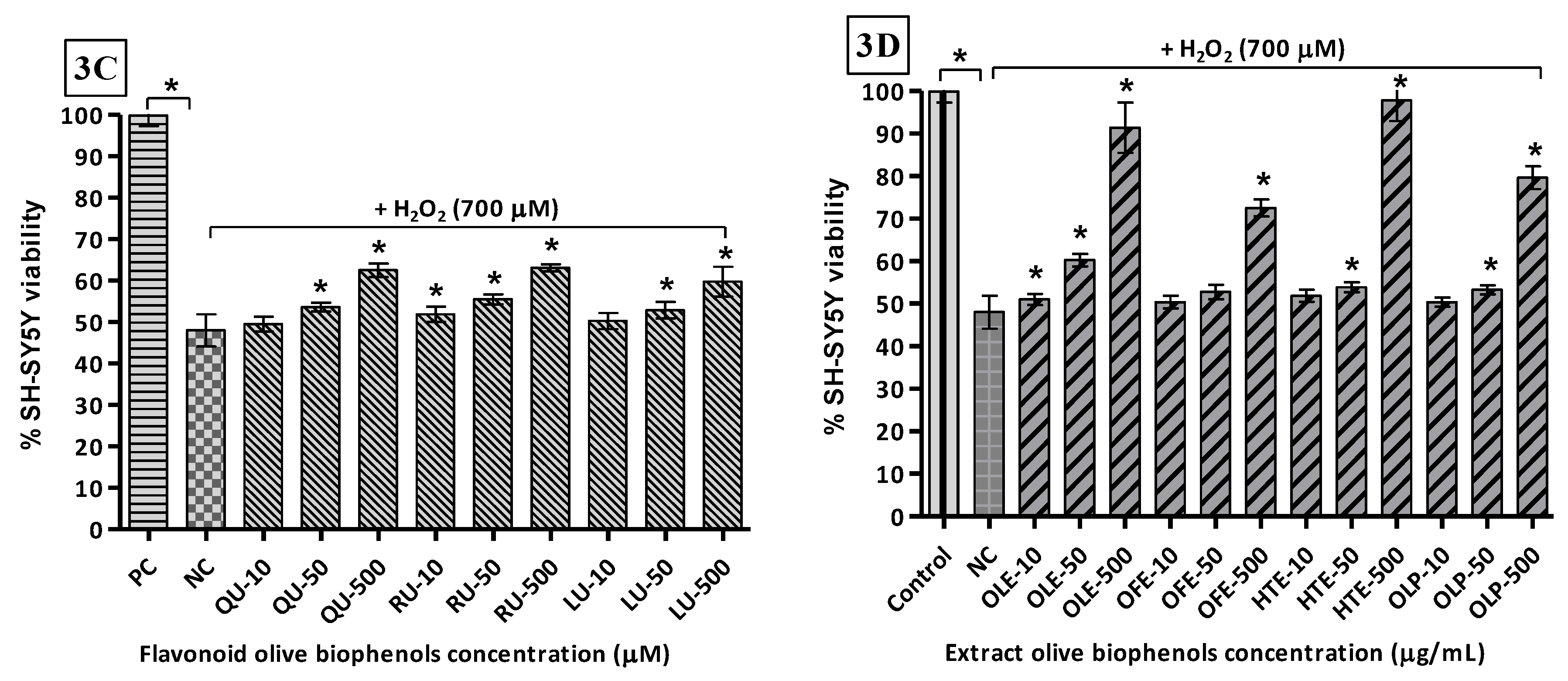
3.6. Neuroprotective Effect of Olive Biophenols against H2O2-Induced Cytotoxicity in SH-SY5Y Cells
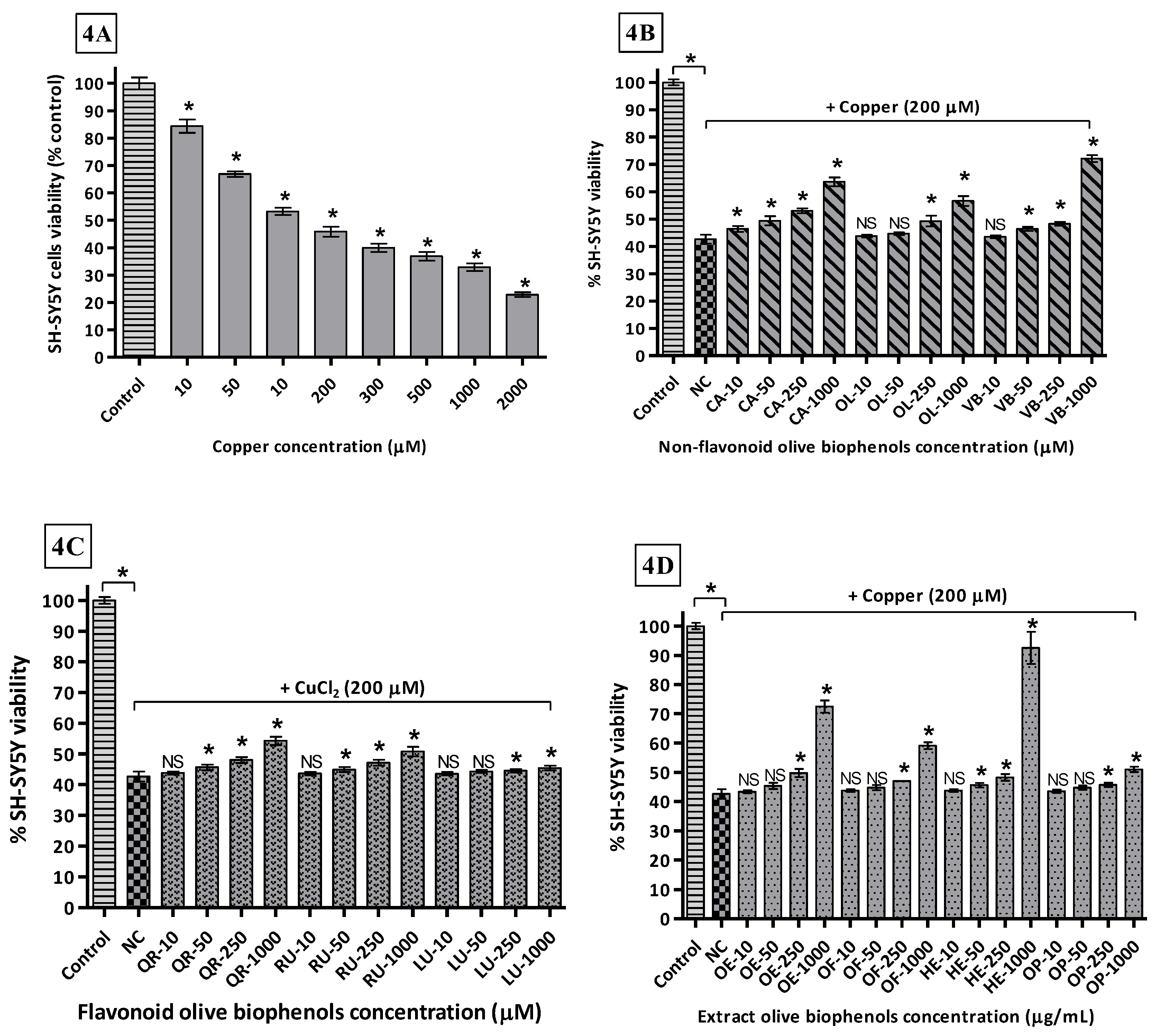
3.7. Neuroprotective Effect of Olive Biophenols against Copper-Induced Cytotoxicity in SH-SY5Y Cells
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Rottkamp, C.A.; Nunomura, A.; Raina, A.K.; Perry, G. Oxidative stress in Alzheimer’s disease. BBA Mol. Basis Dis. 2000, 1502, 139–144. [Google Scholar] [CrossRef]
- Pratico, D. Oxidative stress hypothesis in Alzheimer’s disease: A reappraisal. Trends Pharmacol. Sci. 2008, 29, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Harris, P.L.; Sayre, L.M.; Perry, G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl. Acad. Sci. USA 1997, 94, 9866–9868. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Vondrakova, D.; Lawson, M.; Valko, M. Metals, oxidative stress and neurodegenerative disorders. Mol. Cell. Biochem. 2010, 345, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H. Biophenols pharmacology against the amyloidogenic activity in Alzheimer’s disease. Biomed. Pharmacother. 2017, 89, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Borenstein, A.R.; Wu, Y.; Jackson, J.C.; Larson, E.B. Fruit and vegetable juices and Alzheimer’s disease: The kame project. Am. J. Med. 2006, 119, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Ovaskainen, M.L.; Torronen, R.; Koponen, J.M.; Sinkko, H.; Hellstrom, J.; Reinivuo, H.; Mattila, P. Dietary intake and major food sources of polyphenols in finnish adults. J. Nutr. 2008, 138, 562–566. [Google Scholar] [PubMed]
- Perez-Jimenez, J.; Fezeu, L.; Touvier, M.; Arnault, N.; Manach, C.; Hercberg, S.; Galan, P.; Scalbert, A. Dietary intake of 337 polyphenols in french adults. Am. J. Clin. Nutr. 2011, 93, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Medina-Remon, A.; Perez-Jimenez, J.; Martinez-Gonzalez, M.A.; Covas, M.I.; Corella, D.; Salas-Salvado, J.; Gomez-Gracia, E.; Lapetra, J.; Aros, F.; et al. Dietary intake and major food sources of polyphenols in a spanish population at high cardiovascular risk: The predimed study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Stepaniak, U.; Topor-Madry, R.; Szafraniec, K.; Pajak, A. Estimated dietary intake and major food sources of polyphenols in the polish arm of the hapiee study. Nutrition 2014, 30, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, C.; Fukushima, Y.; Kishimoto, Y.; Suzuki-Sugihara, N.; Saita, E.; Takahashi, Y.; Kondo, K. Estimated dietary polyphenol intake and major food and beverage sources among elderly Japanese. Nutrients 2015, 7, 10269–10281. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remon, A.; M’Hiri, N.; Garcia-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-explorer 3.0: A major update of the phenol-explorer database to incorporate data on the effects of food processing on polyphenol content. Database J. Biol. Databases Curation 2013, 2013, bat070. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J. Agric. Food Chem. 2003, 51, 7513–7521. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. The protective role of plant biophenols in mechanisms of Alzheimer’s disease. J. Nutr. Biochem. 2017, 47, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Obied, H.K.; Prenzler, P.D.; Omar, S.H.; Ismael, R.; Servili, M.; Esposto, S.; Taticchi, A.; Selvaggini, R.; Urbani, S. Chapter six—Pharmacology of olive biophenols. In Advances in Molecular Toxicology; James, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 6, pp. 195–242. [Google Scholar]
- Knoops, K.T.; de Groot, L.C.; Kromhout, D.; Perrin, A.E.; Moreiras-Varela, O.; Menotti, A.; van Staveren, W.A. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly european men and women: The hale project. JAMA 2004, 292, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health. Biofactors 2013, 39, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lapiscina, E.H.; Clavero, P.; Toledo, E.; San Julian, B.; Sanchez-Tainta, A.; Corella, D.; Lamuela-Raventos, R.M.; Martinez, J.A.; Martinez-Gonzalez, M.A. Virgin olive oil supplementation and long-term cognition: The predimed-navarra randomized, trial. J. Nutr. Health Aging 2013, 17, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Kountouri, A.M.; Mylona, A.; Kaliora, A.C.; Andrikopoulos, N.K. Bioavailability of the phenolic compounds of the fruits (drupes) of olea europaea (olives): Impact on plasma antioxidant status in humans. Phytomedicine 2007, 14, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Uccella, N. Biophenolic components of olives. Food Res. Int. 2000, 33, 475–485. [Google Scholar] [CrossRef]
- Le Tutour, B.; Guedon, D. Antioxidative activities of olea europaea leaves and related phenolic compounds. Phytochemistry 1992, 31, 1173–1178. [Google Scholar] [CrossRef]
- Benavente-Garcia, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. Leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Casaburi, I.; Puoci, F.; Chimento, A.; Sirianni, R.; Ruggiero, C.; Avena, P.; Pezzi, V. Potential of olive oil phenols as chemopreventive and therapeutic agents against cancer: A review of in vitro studies. Mol. Nutr. Food Res. 2013, 57, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Obied, H.K.; Prenzler, P.D.; Konczak, I.; Rehman, A.U.; Robards, K. Chemistry and bioactivity of olive biophenols in some antioxidant and antiproliferative in vitro bioassays. Chem. Res. Toxicol. 2009, 22, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Spiegelhalder, B.; Bartsch, H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer 2000, 36, 1235–1247. [Google Scholar] [CrossRef]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R., Jr.; Prenzler, P.D.; Robards, K. Investigation of australian olive mill waste for recovery of biophenols. J. Agric. Food Chem. 2005, 53, 9911–9920. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Kamran, M.; Hamlin, A.S.; Scott, C.J.; Obied, H.K. Drying at high temperature for a short time maximizes the recovery of olive leaf biophenols. Ind. Crops Prod. 2015, 78, 29–38. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (frap) as a measure of “antioxidant power”: The frap assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Huang, C.; Wu, J.; Sun, L.; Hao, W.; Leung, L.K.; Huang, J. The neuroprotective effects of ipriflavone against H2O2 and amyloid beta induced toxicity in human neuroblastoma SH-SY5Y cells. Eur. J. Pharmacol. 2013, 721, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Arciello, M.; Rotilio, G.; Rossi, L. Copper-dependent toxicity in SH-SY5Y neuroblastoma cells involves mitochondrial damage. Biochem. Biophys. Res. Commun. 2005, 327, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhao, L.; Zhu, B.; Li, Q.; Yew, D.T.; Yao, Z.; Xu, J. Protective effects of ginkgo biloba extract (egb761) and its constituents quercetin and ginkgolide B against β-amyloid peptide-induced toxicity in SH-SY5Y cells. Chem. Biol. Interact. 2009, 181, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Chetsawang, J.; Govitrapong, P.; Chetsawang, B. Hydrogen peroxide toxicity induces ras signaling in human neuroblastoma SH-SY5Y cultured cells. J. Biomed. Biotechnol. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Mylonaki, S.; Kiassos, E.; Makris, D.P.; Kefalas, P. Optimisation of the extraction of olive (Olea europaea) leaf phenolics using water/ethanol-based solvent systems and response surface methodology. Anal. Bioanal. Chem. 2008, 392, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, S.M.; Guyot, S.; Marnet, N.; Lopes-da-Silva, J.A.; Renard, C.M.G.C.; Coimbra, M.A. Characterisation of phenolic extracts from olive pulp and olive pomace by electrospray mass spectrometry. J. Sci. Food Agric. 2005, 85, 21–32. [Google Scholar] [CrossRef]
- Hayes, J.E.; Allen, P.; Brunton, N.; O’Grady, M.N.; Kerry, J.P. Phenolic composition and in vitro antioxidant capacity of four commercial phytochemical products: Olive leaf extract (Olea europaea L.), lutein, sesamol and ellagic acid. Food Chem. 2011, 126, 948–955. [Google Scholar] [CrossRef]
- Niaounakis, M.; Halvadakis, C.P. Olive Processing Waste Management: Literature Review and Patent Survey; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Ryan, D.; Robards, K.; Lavee, S. Determination of phenolic compounds in olives by reversed-phase chromatography and mass spectrometry. J. Chromatogr. A 1999, 832, 87–96. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Charisiadis, P.; Margianni, E.; Lamari, F.N.; Gerothanassis, I.P.; Tzakos, A.G. Olive leaf extracts are a natural source of advanced glycation end product inhibitors. J. Med. Food 2013, 16, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Inbaraj, B.S.; Chen, B.H. Determination of phenolic acids and flavonoids in Taraxacum formosanum Kitam by liquid chromatography-tandem mass spectrometry coupled with a post-column derivatization technique. Int. J. Mol. Sci. 2012, 13, 260–285. [Google Scholar] [CrossRef] [PubMed]
- Savarese, M.; De Marco, E.; Sacchi, R. Characterization of phenolic extracts from olives (Olea europaea cv. Pisciottana) by electrospray ionization mass spectrometry. Food Chem. 2007, 105, 761–770. [Google Scholar] [CrossRef]
- Hamden, K.; Allouche, N.; Damak, M.; Elfeki, A. Hypoglycemic and antioxidant effects of phenolic extracts and purified hydroxytyrosol from olive mill waste in vitro and in rats. Chem. Biol. Interact. 2009, 180, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.; Antolovich, M.; Herlt, T.; Prenzler, P.D.; Lavee, S.; Robards, K. Identification of phenolic compounds in tissues of the novel olive cultivar hardy’s mammoth. J. Agric. Food Chem. 2002, 50, 6716–6724. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Temirzoda, T.N.; Segura-Carretero, A.; Quirantes, R.; Plaza, M.; Ibanez, E. New possibilities for the valorization of olive oil by-products. J. Chromatogr. A 2011, 1218, 7511–7520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, D.; Antolovich, M.; Prenzler, P.; Robards, K.; Lavee, S. Biotransformations of phenolic compounds in Olea europaea L. Sci Hort 2002, 92, 147–176. [Google Scholar] [CrossRef]
- Servili, M.; Baldioli, M.; Selvaggini, R.; Macchioni, A.; Montedoro, G. Phenolic compounds of olive fruit: One- and two-dimensional nuclear magnetic resonance characterization of nuzhenide and its distribution in the constitutive parts of fruit. J. Agric. Food Chem. 1999, 47, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S.V.; Steenken, S.; Tosic, M.; Marjanovic, B.; Simic, M.G. Flavonoids as antioxidants. J. Am. Chem. Soc. 1994, 116, 4846–4851. [Google Scholar] [CrossRef]
- D’Imperio, M.; Cardinali, A.; D’Antuono, I.; Linsalata, V.; Minervini, F.; Redan, B.W.; Ferruzzi, M.G. Stability–activity of verbascoside, a known antioxidant compound, at different ph conditions. Food Res. Int. 2014, 66, 373–378. [Google Scholar] [CrossRef]
- Cai, W.; Chen, Y.; Xie, L.; Zhang, H.; Hou, C. Characterization and density functional theory study of the antioxidant activity of quercetin and its sugar-containing analogues. Eur. Food Res. Technol. 2014, 238, 121–128. [Google Scholar] [CrossRef]
- Masuoka, N.; Matsuda, M.; Kubo, I. Characterisation of the antioxidant activity of flavonoids. Food Chem. 2012, 131, 541–545. [Google Scholar] [CrossRef]
- Son, T.G.; Camandola, S.; Mattson, M.P. Hormetic dietary phytochemicals. Neuromol. Med. 2008, 10, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.R.; Snyder, B.S.; Beard, J.L.; Fine, R.E.; Mufson, E.J. Regional distribution of iron and iron-regulatory proteins in the brain in aging and Alzheimer’s disease. J. Neurosci. Res. 1992, 31, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Zecca, L.; Youdim, M.B.; Riederer, P.; Connor, J.R.; Crichton, R.R. Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 2004, 5, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Shin, R.W.; Hasegawa, K.; Naiki, H.; Sato, H.; Yoshimasu, F.; Kitamoto, T. Iron (iii) induces aggregation of hyperphosphorylated tau and its reduction to iron (ii) reverses the aggregation: Implications in the formation of neurofibrillary tangles of Alzheimer’s disease. J. Neurochem. 2002, 82, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Dairam, A.; Fogel, R.; Daya, S.; Limson, J.L. Antioxidant and iron-binding properties of curcumin, capsaicin, and S-allylcysteine reduce oxidative stress in rat brain homogenate. J. Agric. Food Chem. 2008, 56, 3350–3356. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef] [PubMed]
- Bindoli, A.; Fukuto, J.M.; Forman, H.J. Thiol chemistry in peroxidase catalysis and redox signaling. Antioxid. Redox Signal. 2008, 10, 1549–1564. [Google Scholar] [CrossRef] [PubMed]
- Milton, N.G. Role of hydrogen peroxide in the aetiology of Alzheimer’s disease: Implications for treatment. Drugs Aging 2004, 21, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Chen, Y.; Liu, H.; Zhang, K.; Zhang, T.; Lin, A.; Jing, N. Hydrogen peroxide promotes abeta production through jnk-dependent activation of gamma-secretase. J. Biol. Chem. 2008, 283, 17721–17730. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.G.; Arumugam, T.V.; Woo, H.N.; Park, J.S.; Tang, S.C.; Mughal, M.; Hyun, D.H.; Park, J.H.; Choi, Y.H.; Gwon, A.R.; et al. Evidence that γ-secretase mediates oxidative stress-induced β-secretase expression in Alzheimer’s disease. Neurobiol. Aging 2010, 31, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Arnal, N.; de Alaniz, M.J.; Marra, C.A. Cytotoxic effects of copper overload on human-derived lung and liver cells in culture. Biochim. Biophys. Acta 2012, 1820, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Watt, N.T.; Hooper, N.M. The response of neurones and glial cells to elevated copper. Brain Res. Bull. 2001, 55, 219–224. [Google Scholar] [CrossRef]
- Deane, R.; Bell, R.D.; Sagare, A.; Zlokovic, B.V. Clearance of amyloid-β peptide across the blood-brain barrier: Implication for therapies in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2009, 8, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Bandaruk, Y.; Mukai, R.; Terao, J. Cellular uptake of quercetin and luteolin and their effects on monoamine oxidase-α in human neuroblastoma SH-SY5Y cells. Toxicol. Rep. 2014, 1, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Mariani, C.; Braca, A.; Vitalini, S.; De Tommasi, N.; Visioli, F.; Fico, G. Flavonoid characterization and in vitro antioxidant activity of Aconitum anthora L. (ranunculaceae). Phytochemistry 2008, 69, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.E.; Khodr, H.; Hider, R.C.; Rice-Evans, C.A. Structural dependence of flavonoid interactions with Cu2+ ions: Implications for their antioxidant properties. Biochem. J. 1998, 330 Pt 3, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Artajo, L.S.; Romero, M.P.; Morello, J.R.; Motilva, M.J. Enrichment of refined olive oil with phenolic compounds: Evaluation of their antioxidant activity and their effect on the bitter index. J. Agric. Food Chem. 2006, 54, 6079–6088. [Google Scholar] [CrossRef] [PubMed]
- Firuzi, O.; Lacanna, A.; Petrucci, R.; Marrosu, G.; Saso, L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim. Biophys. Acta 2005, 1721, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.N.; Zock, P.L.; Katan, M.B. Bioavailability and antioxidant effects of olive oil phenols in humans: A review. Eur. J. Clin. Nutr. 2004, 58, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Mellon, F.; Barron, D.; Sarrazin, G.; Morgan, M.R.; Williamson, G. Human metabolism of dietary flavonoids: Identification of plasma metabolites of quercetin. Free Radic. Res. 2001, 35, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Pertiñez, M.; Gómez-Cabrera, A.; Garrido, A. Predicting the nutritive value of the olive leaf (Olea europaea): Digestibility and chemical composition and in vitro studies. Anim. Feed Sci. Technol. 2000, 87, 187–201. [Google Scholar] [CrossRef]
- Omar, S.H. Chapter 4—Biophenols: Impacts and prospects in anti-Alzheimer drug discovery a2-brahmachari, goutam. In Discovery and Development of Neuroprotective Agents from Natural Products; Elsevier: Amsterdam, The Netherlands, 2018; pp. 103–148. [Google Scholar]
- Lindenmeier, M.; Burkon, A.; Somoza, V. A novel method to measure both the reductive and the radical scavenging activity in a linoleic acid model system. Mol. Nutr. Food Res. 2007, 51, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Young, I.S.; Woodside, J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.P.; Ferreira, I.C.; Marcelino, F.; Valentao, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrancosa) leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
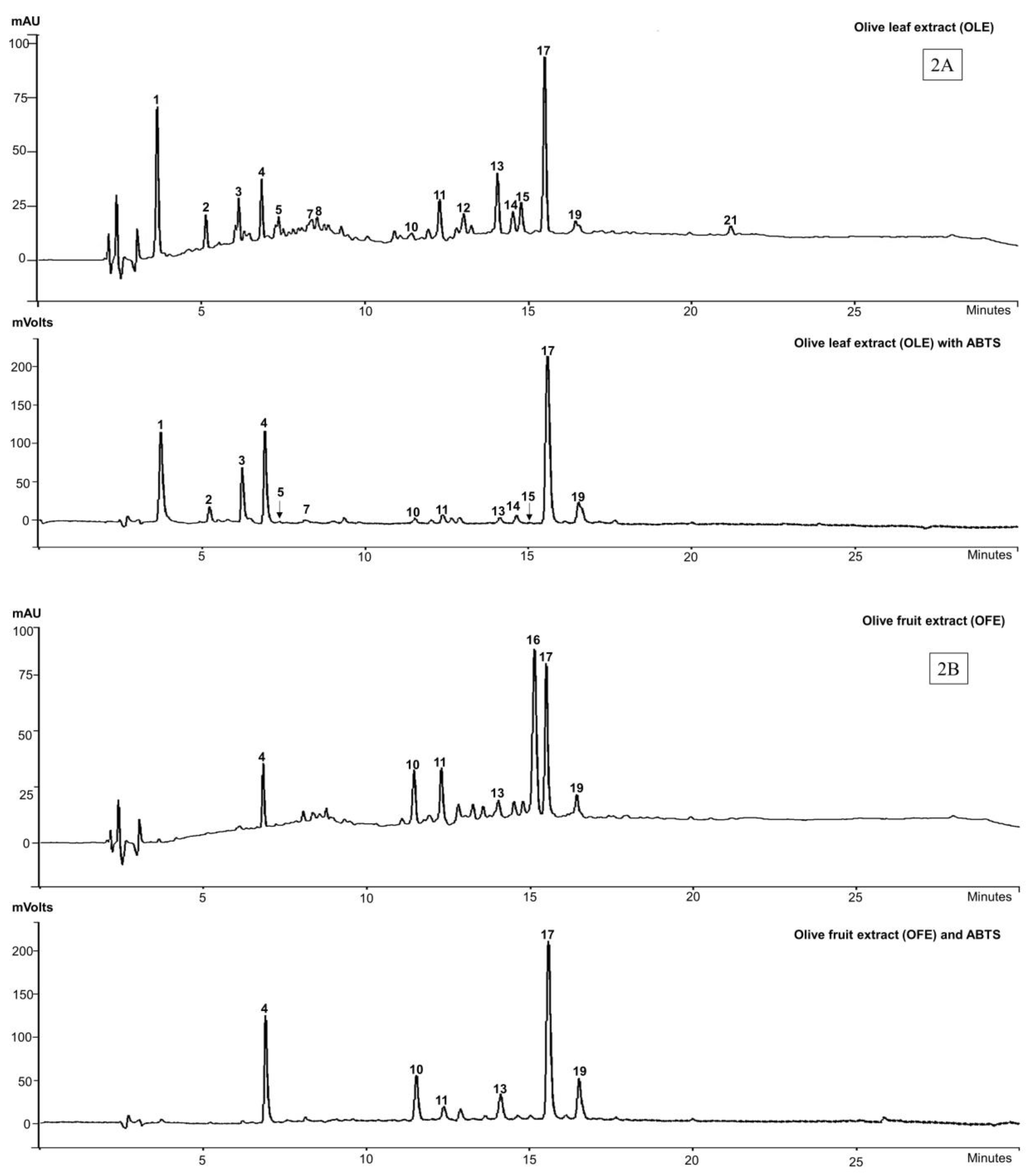

| Peak | TR | λmax (nm) | Identification | ABTS | [M − H]− | Extract | References |
|---|---|---|---|---|---|---|---|
| 1 | 3.6 | 231, 278 | Unknown | Yes | 191 | OLE | |
| 2 | 5.1 | 231, 279 | Unknown | Yes | 487 | OLE | |
| 3 | 6.1 | 283 | Hydroxytyrosol glucoside | Yes | 315 | OLE, HTE | [42] |
| 4 | 6.8 | 231, 275 | Hydroxytyrosol (3) | Yes | 153 | OLE, OFE, HTE, OLP | [42] |
| 5 | 7.4 | 232, 333 | Caffeic acid glucoside | Yes | 339 | OLE | [43] |
| 6 | 8.2 | 277 | Tyrosol (2) | No | ND | OLP | [44] |
| 7 | 8.3 | 229, 278 | Oleuropein aglycone-1 | Yes | 377 | OLE, HTE, OLP | [42] |
| 8 | 8.5 | 233 | Elenolic acid (5) | No | 241 | OLE | [45] |
| 9 | 9.1 | 237 | Elenolic acid glucoside | No | 403 | HTE | [46] |
| 10 | 11.3 | 282, 333 | Verbascoside (6) | Yes | 623 | OLE, OFE OLP, HTE | [47] |
| 11 | 12.3 | 254, 267s, 340 | Luteolin-7-O-glucoside | Yes | 447 | OLE, OFE, HTE | [47] |
| 12 | 13.0 | 278 | Unknown “secoiridoid” | No | 581 | OLE | |
| 13 | 14.0 | 278 | Unknown “secoiridoid” | Yes | 577 | OLE, OFE | |
| 14 | 14.5 | 265, 325, 359 | Flavonoid glycoside | Yes | 447 | OLE | |
| 15 | 14.8 | 266, 341 | Flavonoid glycoside | Yes | 461 | OLE | |
| 16 | 15.1 | 260 | Unknown | No | ND | OFE | |
| 17 | 15.4 | 237, 280 | Oleuropein (4) | Yes | 539 | OFE, OLE | [42] |
| 18 | 16.0 | 232 | Non-phenolic secoiridoid | No | 285 | OLP | |
| 19 | 16.42 | 234 | Oleuroside | Yes | 539 | OFE, OLE | [47] |
| 20 | 19.1 | 287, 290 | Oleuropein aglycone-2 | Yes | 377 | HTE | [42] |
| 21 | 21.2 | 234, 280 | Unknown | No | 415 | OLE |
| Biophenols | SOR EC50 (μM) | H2O2 EC50 (mM) | FRAP mM TE/g | |
|---|---|---|---|---|
| Non-flavonoids | CA (1) | 436.3 | 1.01 | 0.830 ± 0.19 |
| HT (3) | 291.4 | 1.02 | 0.775 ± 0.16 | |
| OL (4) | 258 | 1.02 | 0.713 ± 0.18 | |
| VB (6) | 119.4 | 0.66 | 1.173 ± 0.27 | |
| FRAP mM TE/g | ||||
| Flavonoids | QU (9) | 93.97 | NS | 1.272 ± 0.29 |
| RU (10) | 143.2 | NS | 0.957 ± 0.22 | |
| LU (8) | 234 | NS | 1.011 ± 0.23 | |
| SOR EC50 (μg/mL) | H2O2 EC50 (μg/mL) | FRAP mg TE/g | ||
| Extracts | OLE | 1.89 | 120.6 | 2.261 ± 0.51 |
| OFE | 6.71 | 217 | 1.708 ± 0.39 | |
| HTE | 1.98 | 115.8 | 2.824 ± 0.64 | |
| OLP | 2.46 | 280.3 | 1.421 ± 0.32 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, S.H.; Kerr, P.G.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Olive (Olea europaea L.) Biophenols: A Nutriceutical against Oxidative Stress in SH-SY5Y Cells. Molecules 2017, 22, 1858. https://doi.org/10.3390/molecules22111858
Omar SH, Kerr PG, Scott CJ, Hamlin AS, Obied HK. Olive (Olea europaea L.) Biophenols: A Nutriceutical against Oxidative Stress in SH-SY5Y Cells. Molecules. 2017; 22(11):1858. https://doi.org/10.3390/molecules22111858
Chicago/Turabian StyleOmar, Syed Haris, Philip G. Kerr, Christopher J. Scott, Adam S. Hamlin, and Hassan K. Obied. 2017. "Olive (Olea europaea L.) Biophenols: A Nutriceutical against Oxidative Stress in SH-SY5Y Cells" Molecules 22, no. 11: 1858. https://doi.org/10.3390/molecules22111858