2.2. Analysis of UPLC Fingerprints and Similarities
Relative retention time, relative peak area and similarities were used to evaluate the quality of the fingerprints. The precision was determined via six consecutive injections of the same sample solution. The relative standard deviations (RSDs) of the relative retention time and relative peak area of the common peaks were all below 0.87% and 1.47%, respectively, and the similarities of different chromatograms were all above 0.95. The repeatability was evaluated by analysing six reduplicative samples. For these samples, the RSDs of relative retention time and relative peak area of the common peaks were all below 1.59% and 1.97%, respectively, and the similarities of different chromatograms were all above 0.95. Stability was assessed by testing one sample solution at different times (0, 2, 4, 6, 8, 12 and 24 h) over 24 h. The RSDs of the relative retention time and relative peak area of the common peaks were all below 0.96% and 1.98%, respectively, and the similarities of different chromatograms were all above 0.95. All these results indicated that the samples remained stable during the testing period and that the fingerprint analysis conditions were satisfactory.
Similarity analysis (SA) of the extracts of the six different parts of cultivated
C. deserticola was conducted under optimal conditions and matched automatically using a Similarity Evaluation System (SES) for Chromatographic Fingerprint of Traditional Chinese Medicine software (Version 2004 A; Manufacturer, Beijing, China), which was recommended by the China Food and Drug Administration (CFDA). Then, a reference chromatogram (R) was generated by this software by comparing the six different extract chromatograms using the median method. The fingerprints revealed the presence of nine common peaks, which were assigned as “characteristic peaks”, in the chromatograms of the six extracts from cultivated
C. deserticola.
Figure 2A shows the overlay chromatograms of the six different part extracts at 330 nm, and
Figure 2B shows the reference chromatogram of the sample with the nine marker compounds.
All samples differed in chromatographic patterns and peak abundances. Furthermore, there were still some differences between the chemical fingerprints of the different parts, including peak numbers and peak abundances. The similarities between the entire chromatographic profiles of the six different part extracts and the reference chromatogram were evaluated by SES software and their correlation coefficients of their chemical fingerprints are shown in
Table 1. The results showed that the correlation coefficients of these samples ranged from 0.07 (R6) to 0.993 (R2), which indicated that obvious chemical composition differences existed between the different parts of cultivated
C. deserticola. The differences in the correlation coefficients indicated variation in the fingerprint and internal qualities of these samples.
2.3. Quantitative Analysis of Six Phenylethanoid Glycosides (PhGs) by UPLC-PDA
An UPLC-PDA method for the simultaneous determination of six PhGs in the different parts of cultivated
C. deserticola was established. A 2 μL aliquot of the working solutions was injected into UPLC for analysis. The UPLC-PDA chromatograms of the mixed standards and samples under the optimized conditions described in
Section 3.4 are shown in
Figure 3. The proposed UPLC-PDA quantitative analysis method was validated by determining the linearity, limit of detection (LOD), limit of quantification (LOQ), intra- and inter-day precisions, repeatability, stability and accuracy.
The calibration curve for each compound was established by plotting the peak area (
y) versus the concentration (
x) of each analyte at seven concentrations run in triplicate (
n = 3). The limit of detection (LOD) and the limit of quantitation (LOQ) for the six PhGs were determined at signal-to-noise ratios (S/N) of 3 and 10, respectively, by injecting a series of dilute solutions of known concentrations. All of the compounds showed good linearity (r
2 > 0.9995) within the linear range. The calibration curves, linear range, r
2 value, LOD and LOQ are listed in
Table 2. The intra- and inter-day precisions were investigated by analysing a mixed PhG standard solution injected six times (
n = 6) during a single day and by duplicating the experiment on three successive days. To further confirm the repeatability of the developed assay, six independent working solutions, all of which were prepared from Sample R1, were analysed using the above method. The stability was analysed by injecting Sample R1 at room temperature at 0, 6, 12, 24, 48 and 72 h. The RSD was calculated as a measurement of the precision, repeatability and stability. Accuracy was determined using the recovery percentage. The recovery test was determined by spiking a known amount of the six PhGs into a sample with a known concentration and then extracting and analysing the resulting solution using the same procedures. Six replicates were performed for this test. The ratio of the determined amount and the added amount was used to calculate the recovery. As shown in
Table 3, the RSDs of the intra-and inter-day variations, repeatability and stability of the six PhGs were less than 2%, and the average recoveries were between 97.6% and 102.2% with an RSD of less than 3%. The abovementioned method validation results demonstrated that our method was precise, accurate and sensitive enough for simultaneous quantitative analysis of the six PhGs in different parts of cultivated
C. deserticola.
The contents of the six PhGs in the different parts of cultivated
C. deserticola are summarized in
Table 4 as the average values of three replicate injections. As shown in
Table 4, remarkable differences between the contents of the six PhGs in the different parts were observed, suggesting that each part has its own chemical characteristics. The total content of the six PhGs ranged considerably from 4.91 to 72.56 mg/g and decreased significantly from the bottom to the top of cultivated
C. deserticola. Between the different parts, the total contents of the six PhGs approximately 3–20-fold. The highest value was found in the stems (72.56 ± 0.96 mg/g), whereas the lowest value was observed in the inflorescence stalks (4.91 ± 0.22 mg/g). In addition, the content of each determined PhG was found to differ strongly between these different parts. As shown in the comparison between the different parts in
Figure 4, echinacoside (10.51–42.80 mg/g, the main compound), cistanoside A (4.21–27.28 mg/g) and acteoside (1.37–2.96 mg/g) were the three most abundant ingredients, whereas relative minor distributions were observed for isoacteoside (0.32–0.51 mg/g), 2′-acetylacteoside (0.37–2.83 mg/g) and tubuloside B (0.23–0.89 mg/g) in the stems, axes and inflorescences. However, in the flowers (without seeds), inflorescence stalks and corollas, 2′-acetylacteoside (3.16–17.89 mg/g, the main compound), tubuloside B (0.6–1.36 mg/g) and acteoside (0.67–0.93 mg/g) were found in relatively higher amounts, whereas echinacoside (0.09–0.31 mg/g), cistanoside A (0.03–0.07 mg/g) and isoacteoside (0.17–0.23 mg/g) were scarcer. Thus, our data are helpful for obtaining a better understanding of the differences in PhG distribution between these different parts. Interestingly, from the bottom to the top of cultivated
C. deserticola, the echinacoside and cistanoside A content decreased significantly; by contrast, the 2′-acetylacteoside content increased. These results highlight the need for proper quality control of cultivated
C. deserticola.
2.4. Antioxidant Capacity of Different Parts of Cultivated C. deserticola Evaluated by Off-Line DPPH Assay
Previous studies [
8] have shown that PhG aglycone is a powerful antioxidant. The free radical scavenging activity of DPPH is extensively used to evaluate the radical scavenging ability of natural antioxidants. In the present study, the total antioxidant activities of the different parts of
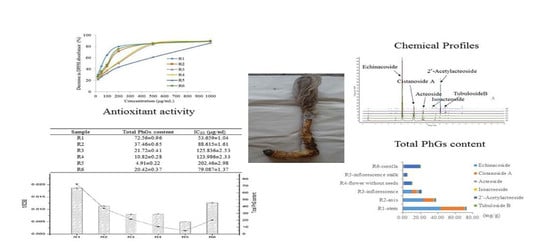
C. deserticola were assayed using an offline DPPH method. The percentage decrease in DPPH absorbance of the samples at various concentrations is shown in
Figure 5, which reveals that the abilities to scavenge the DPPH radical of the six different parts of cultivated
C. deserticola as well as those of the standards (Trolox and
l-ascorbic acid), behaved in a concentration-dependent manner and were varied. Accordingly, the IC
50 values, i.e., the sample concentrations required to scavenge 50% of DPPH radicals, were determined to measure the total antioxidant capacity of the different parts of the cultivated plant. Lower IC
50 values indicate stronger antioxidant activities. The results, as shown in
Table 5, indicated that all extracts had good dose-dependent inhibitory activity against the DPPH radical, although all of them were less potent than the reference antioxidants Trolox and
l-ascorbic acid (IC
50 values of 2.18 ± 0.03 and 1.78 ± 0.01 mg/mL, respectively). Among the extracts, the R1 (stem) and R5 (inflorescence stalk) extracts showed the highest (IC
50 of 53.659 ± 1.04) and lowest antioxidant activity (IC
50 of 202.46 ± 2.98 μg/mL), respectively. The results also revealed that the order of antioxidant potency (IC
50) of the six parts was, in decreasing order: stem > corolla ~axis > flower (without seeds) ~inflorescence > inflorescence stalk. According to previous reports [
20,
21], the stem extracts of
C. deserticola possess good antioxidant activities. Furthermore, PhGs were claimed to be responsible for the antioxidant activity because of their hydroxyl groups. Similarly, our results reveal that there are abundant antioxidative phytochemicals in the extracts of cultivated
C. deserticola, especially in the stem extract. In addition, our results indicated that there may be a positive relationship between composition, PhG content and antioxidant activity.
2.5. UPLC-PDA Coupled with Pre-Column DPPH Assay for Screening Antioxidant Profiles
The UPLC coupled with a pre-column DPPH assay has successfully combined the determination of active compounds with the free radical scavenging capability to evaluate the quality of herbal medicines, and could reflect the bioactivity of the herbal medicines directly [
22,
23]. Therefore, this method would be superior to multicomponent quantification for evaluating herbal medicine quality.
The extract with the best antioxidative activity (Sample 1, R1) was further screened using UPLC-PDA coupled with the pre-column DPPH assay. First, 1 mL of the R1 methanol solution (12 mg/mL) and 2 mL of 3 mM DPPH in methanol were mixed and reacted for 60 min. Both the reaction mixture and R1 methanol solution were then analysed using UPLC-PDA under the same chromatographic conditions. Subsequently, comparing to the chromatogram of the reaction mixture to that of R1 revealed that some peaks had disappeared or decreased in height and therefore could be considered as antioxidant peaks. Radical scavenging profiles revealed the six PhG peaks in chromatographic fingerprint possessing obvious free radical inhibition effects.
Figure 6A,B display the chromatographic fingerprint of Sample 1 (R1) and the antioxidant activity fingerprint detected at 330 nm, respectively. The disappeared or reduced peaks in the activity fingerprint indicate that these components have free radical scavenging activity. Clearly, antioxidant activity was observed for the marker compounds found in the chromatographic fingerprint, indicating that the antioxidant activity of cultivated
C. deserticola is attributable to the presence of PhGs. The other five parts showed similar antioxidant activity fingerprints (not shown). UPLC-PDA coupled with the pre-column DPPH assay has a clear advantage over the offline assay method because the individual contribution to the total antioxidant activity of each chemical component can be determined, and the main radical scavengers can be further identified by HPLC-MS.
2.6. Identification of Six Phenylethanoid Glycosides (PhGs) by UPLC-ESI-QTOF/MS
According to previous studies, the structures of the PhGs in cultivated
C. deserticola have several similarities [
7]. The empirical structural features of these PhGs are as follows: for the disaccharide glycosides, the sugar moiety comprises glucose (Glc) and rhamnose (Rha) connected by a Glc (3→1) Rha linkage, the Glc unit typically links directly to the aglycone, and a coumaroyl or caffeoyl moiety is usually located at the C4 or C6 position. For the trisaccharide glycosides, there is another Glc or Rha unit at the C6 position of the inner Glc unit. Under the optimized MS conditions, negative-ion mode was used to identify the six antioxidant peaks, which were further validated by comparing their retention time, UV/Vis data and MS data with those of standards. Peaks 3, 4, 5, 6, 8 and 9 detected in the fingerprint chromatograms were tentatively identified as echinacoside, cistanoside A, acteoside, isoacteoside, 2′-Acetylacteoside and tubuloside B, respectively, all of which are PhGs. Their retention time (RT) and MS and MS/MS data are illustrated in
Table 6.
As shown in
Table 6, peak 5 and peak 6 (retention times 10.99 and 12.35 min), which have the same predominant deprotonated ion at
m/
z 623 and fragment ions at
m/
z 461, 315, 179, 161 and 135, were identified as acteoside and isoacteoside by comparing their retention times and mass spectra with those of authentic standards. Both peaks 8 and 9 (retention times 14.44 and 15.09 min) exhibited an ion at
m/
z 665, which was 42 Da (the molecular weight of the acetyl (Ac) group) higher than those of peaks 5 and 6, and displayed a fragment ion at
m/
z 623 in the MS/MS spectrum corresponding to the loss of the Ac moiety (42 Da). Similar characteristic fragment ions, such as
m/
z 461, 315, 179, 161 and 135, were also obtained in the MS/MS spectra of the two peaks; therefore, we finally established peaks 8 and 9 to be 2′-acetylacteoside and tubuloside B, respectively, as they share the same retention times and mass spectra with the authentic reference standards. As peaks 5, 6 and peaks 8, 9 were only substituted positional isomers, the fragment ions were almost the same, preventing the MS from distinguishing between them. Fortunately, peaks 5, 6 and peaks 8, 9 were isolated as reference compounds and could be identified by their distinct UPLC retention times. The ion of peak 3 (retention time 7.22 min) was 162 Da (molecular weight of Glc) higher than those of peaks 5 and 6. The peak also showed characteristic fragment ions at
m/
z 623 in addition to at
m/
z 461, 315, 179, 161 and 135, and the fragment ion at
m/
z 623 was attributed to the loss of a Glc unit from the predominant deprotonated ion at
m/
z 785. It was identical to that of echinacoside, and its identity confirmed by comparing the retention time and mass spectra with those of the authentic reference standard. Peak 4 (retention time 8.88 min) had a predominant deprotonated ion at
m/
z 799, which was 14 Da (CH2) higher than that of peak 3, and fragment ions at
m/
z 637 and 475 (14 Da higher compared with the fragment ions at
m/
z 623 and 461). Additionally, the lower molecular weight characteristic fragment ions (lower than 200 Da), including
m/
z 179, 161 and 135, were the same as those mentioned above. It was determined as cistanoside A, which corresponds to the retention time and mass spectra of the authentic reference standard. Thus, the peak was determined as cistanoside A, as its retention time and mass spectra correspond to those of the authentic reference standard. Finally, the antioxidants were identified by their mass spectra and fragmentation patterns with the aid of standard references and literature reports [
24,
25,
26,
27,
28,
29,
30,
31,
32].
Detailed mass spectral data and the proposed fragmentation patterns were given in
Figure 7. The structures of the six PhGs identified above are divided into three moieties: I-caffeic acid (CA), II-aglycone and III-sugar (Glc and Rha). In the MS/MS spectra, the mass defects corresponding to the neutral cleavages of a caffeoyl group and Glc and Rha residues dominate the fragmentation pathways of PhGs and generate the diagnostic fragment ions (
Figure 8). Additional MS/MS characteristic fragment ions at
m/
z 179, 161 and 135 further suggested that a caffeoyl substituent exists in their structures. Using these fragmentation features, we could confidently propose fragmentation pathways as follows. Acteoside (
Figure 7c) produced a fragment at
m/
z 461 via the loss of a CA moiety and then produced an ion at
m/
z 315 via a further loss of Rha. The CA moiety was found at
m/
z 179, and its fragment ions found at
m/
z 161 and
m/
z 135 were produced by the loss of H
2O and CO
2, respectively. Isoacteoside (
Figure 7d) produced the same ions as acteoside at
m/
z 461, 315, 179, 161, and 135. 2′-Acetylacteoside (
Figure 7e) produced a fragment at
m/
z 623 via the loss of acetyl and then generated ions at
m/
z 461, 315, 179, 161 and 135, which was identical to the fragmentation pattern of acteoside. Meanwhile, 2′-acetylacteoside also produced a fragment at
m/
z 503 by losing its CA moiety and subsequently lost its Ac group to produce the ion at
m/
z 461. Tubuloside B (
Figure 6f) displayed a similar fragmentation pattern to 2′-acetylacteoside. Echinacoside (
Figure 7a) produced a fragment ion at
m/
z 623 via loss of its CA moiety, and then sequential losses of Rha and Glc moieties yielded the fragment ions at
m/
z 477 and 315. The fragment ion at
m/
z 461 was generated by losing the CA and Glc moieties from the precursor ion. The characteristic CA ions at
m/
z 179, 161 and 135 were also found. Cistanoside A (
Figure 7b) produced a fragment ion at
m/
z 637 via loss of its CA moiety and then generated ions at
m/
z 491 and 475 by the further loss of Rha and Glc moieties, respectively. Therefore, the use of UPLC-PDA-QTOF/MS in combination could make a major contribution towards precisely elucidating the chemical structures of compounds.
2.7. Correlation between Antioxidants and A8ntioxidant Properties
Correlation analysis between the total contents of antioxidants and total antioxidant activities has been described in many studies [
33,
34,
35,
36,
37,
38]. To the best of our knowledge, there is a lack of studies on comparing the antioxidant properties and PhG contents of cultivated
C. deserticola parts using such a concise characterization method. The preliminary results of the six PhG contents described in the “Quantitative analysis” section are summarized in
Table 4, and antioxidant activity (IC
50) determined using the DPPH assay as described in the “Antioxidant capacity” section are listed in
Table 5. First, the total antioxidant contents and activities of each parts are presented in
Figure 9. Clearly, there are conspicuous differences between the different parts. In fact, the obtained results indicated that the antioxidant activity of cultivated
C. deserticola stems was comparable with that of the stems available on the market. These results suggested that the antioxidant activity of the stem extract may be correlated to its PhG content. Therefore, the phytochemicals in the stem extract of cultivated
C. deserticola are proposed to play an important role in its DPPH radical scavenging activity. Overall, our results indicated a strong correlation between total PhG content and antioxidant activity: r = 0.92, **
p < 0.01.
In addition, certain samples with higher total antioxidant contents did not show high antioxidant activities, such as the axis and inflorescence. Meanwhile, the corolla, which had the highest activity of the six parts, had a lower total antioxidant content than the axis and inflorescence.
Figure 4, as described in the “Quantitative analysis” section, shows the relative amounts of the PhG compounds to give a preliminary understanding of their individual contributions. In this study, Partial Least Squares (PLS) regression analysis [
39,
40,
41] was also carried out to compare the correlation between the abundance of each antioxidant and the antioxidant activity in vitro. The PLS model was constructed using the contents of each antioxidant (as the descriptor matrix X) in the fingerprint chromatograms at 330 nm and the antioxidant activities (as the response matrix Y) of all the parts. The coefficient plots and variable importance in projection (VIP) values obtained using SIMCA-P+ software (Version 13.0, Umetrics, Umea, Sweden) are shown in
Figure 10. Notably, the inverse of the IC
50 values (1/IC
50) was selected as the Y variable to establish the models because lower IC
50 values represent stronger antioxidant activity. According to the obtained regression coefficient plot in
Figure 10A, the linear regression models show that all the antioxidants except acteoside were positively correlated with 1/IC
50. The obtained calibration model of PLS is expressed by the regression equation: Y = 0.50X1 + 0.57X2 − 0.11X3 + 0.32X4 + 0.58X5 + 0.24X6. VIP values reflect the importance of variables in the model: the larger the VIP, the more relevant for sample classification. As our results showed, 2′-acetylacteoside was an important contributor to the antioxidant activity (
Figure 10B). This finding was in agreement with a study by Yang et al [
20].