Adsorptive Desulfurization of Model Gasoline by Using Different Zn Sources Exchanged NaY Zeolites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization Results
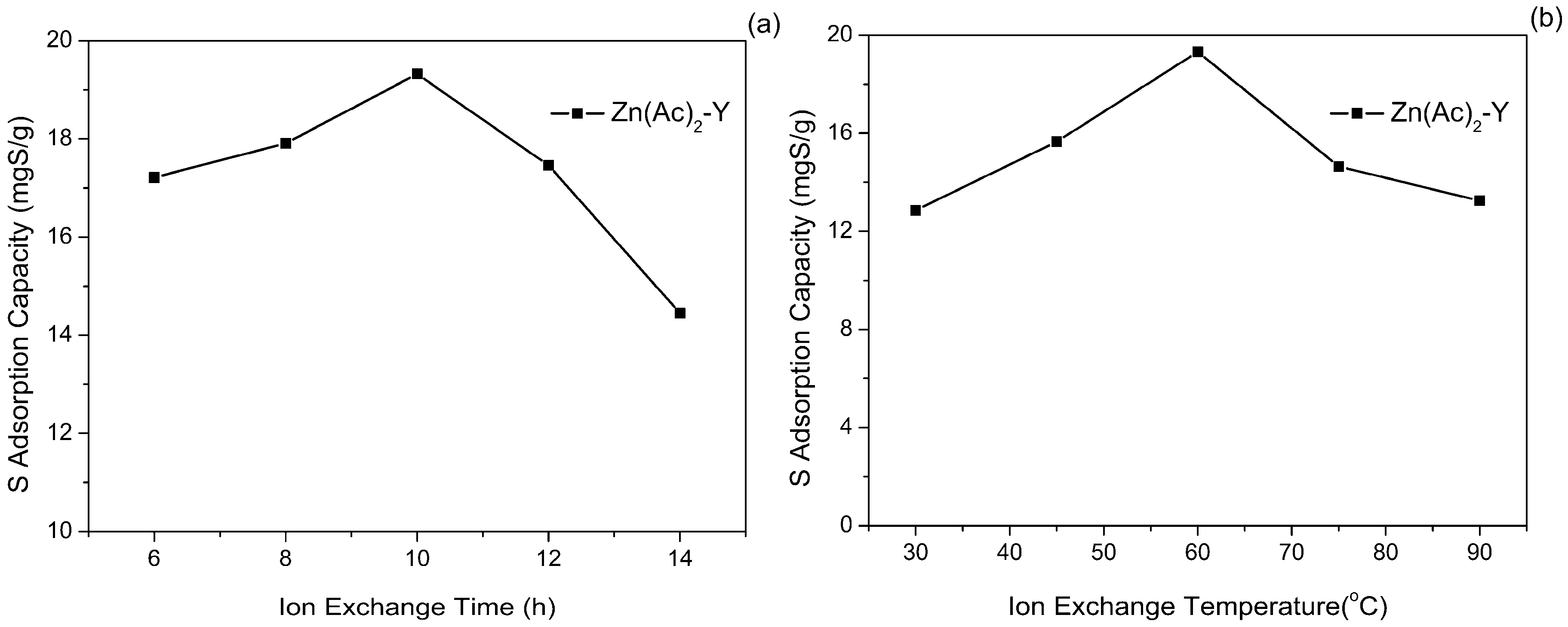
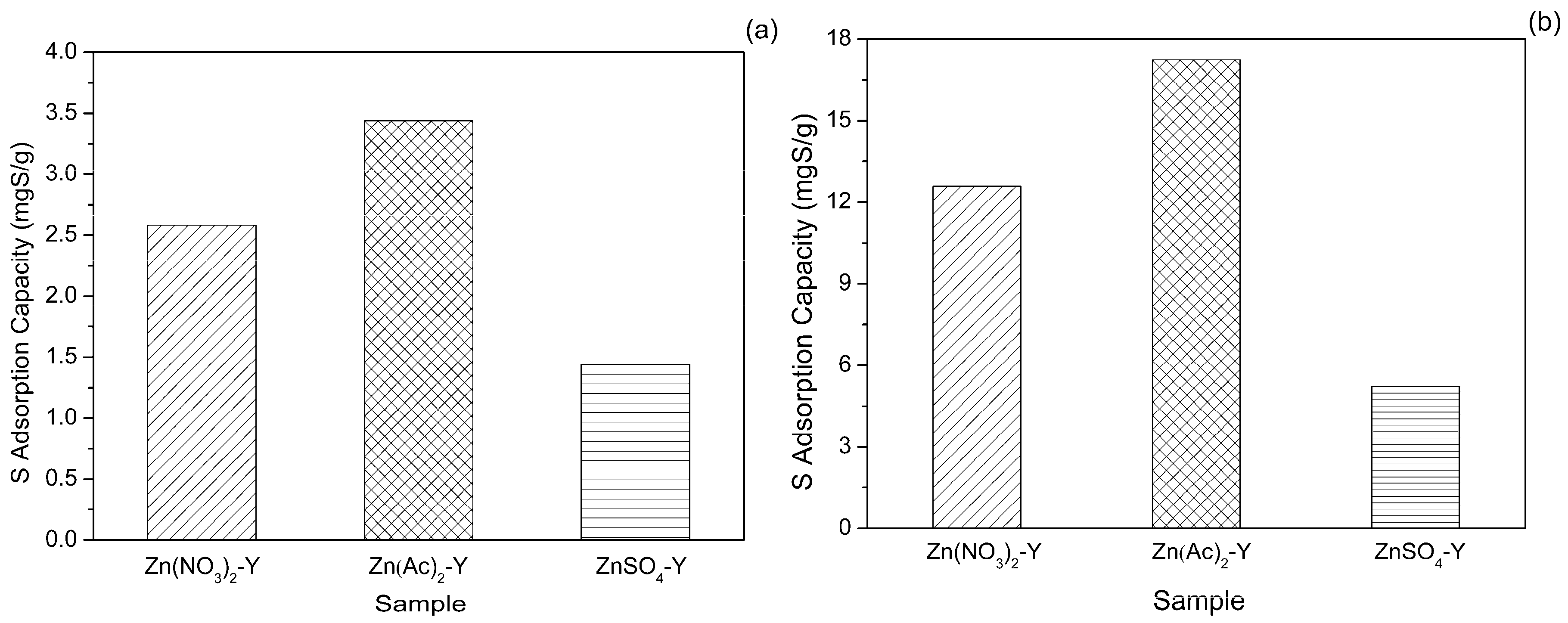
2.2. Effect of Adsorbent Preparation Conditions
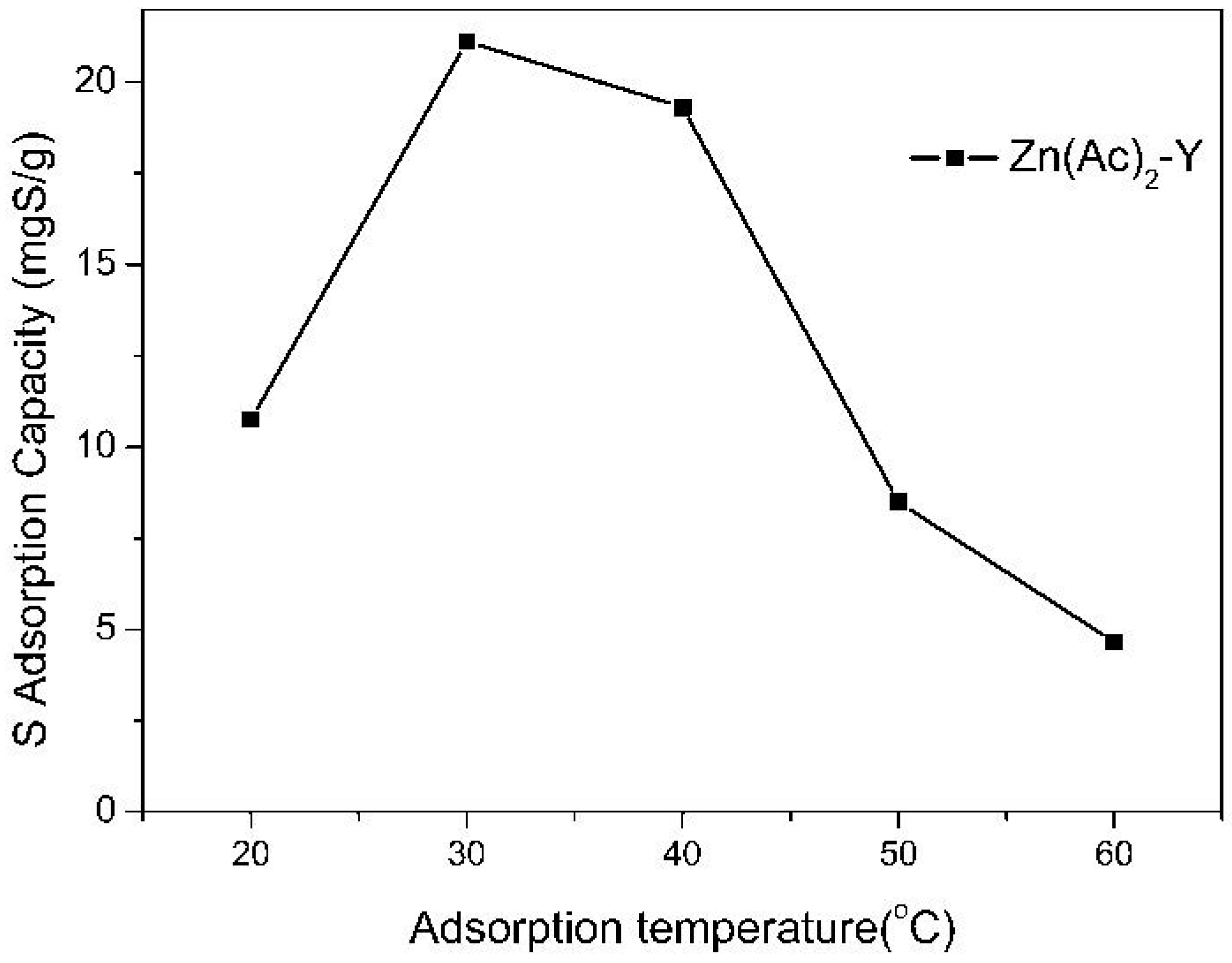
2.3. Effect of Adsorption Conditions
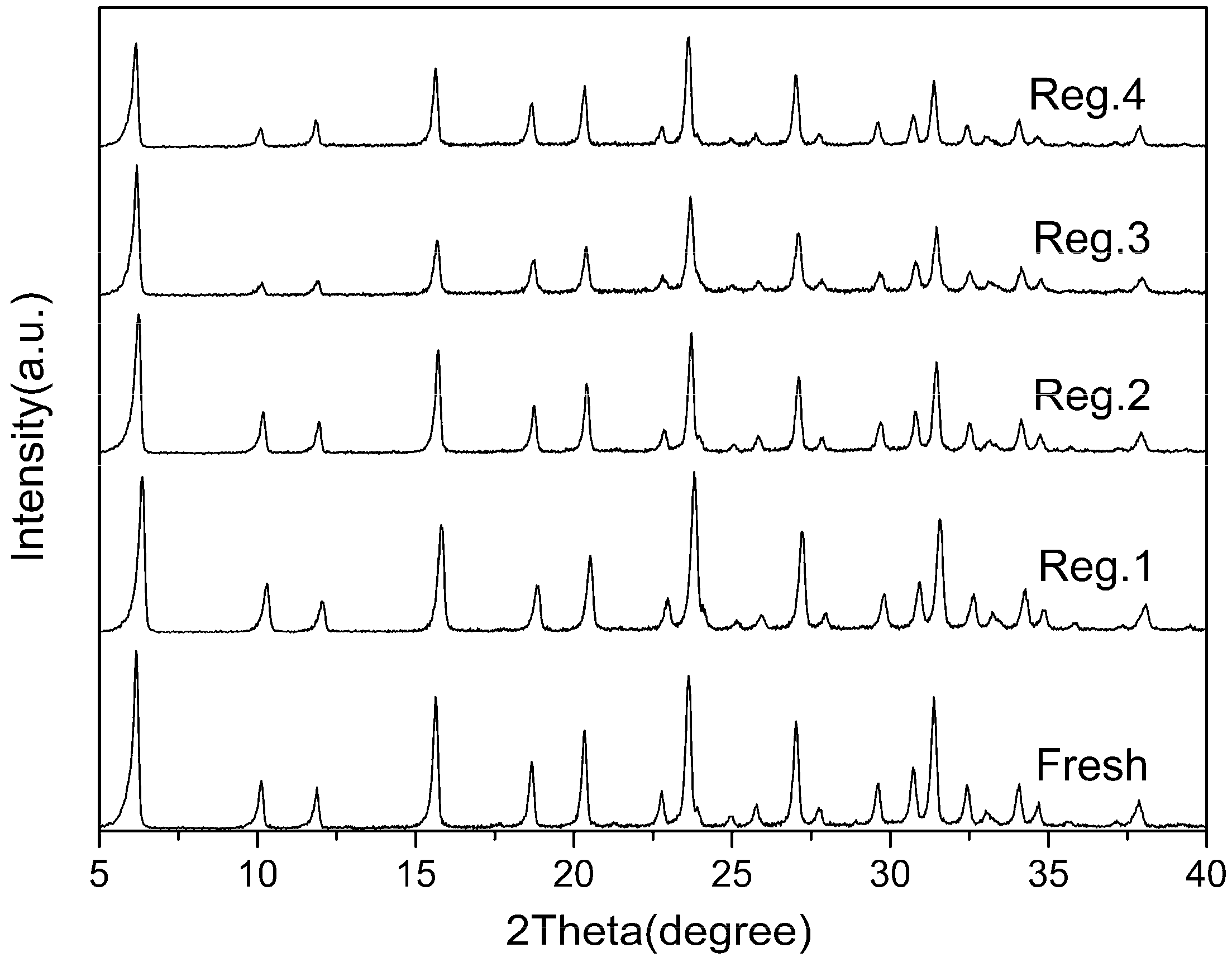
2.4. Adsorbent Regeneration
3. Experimental Section
3.1. Materials and Adsorbent Spreparation
3.2. Characterizations
3.3. Adsorption Experiments
3.4. Regeneration Experiments
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Song, C. An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel. Catal. Today 2003, 86, 211–263. [Google Scholar] [CrossRef]
- Srivastava, V.C. An evaluation of desulfurization technologies for sulfur removal from liquid fuels. RSC Adv. 2012, 2, 759–783. [Google Scholar] [CrossRef]
- Song, C.; Ma, X. New design approaches to ultra-clean diesel fuels by deep desulfurization and deep dearomatization. Appl. Catal. B Environ. 2003, 41, 207–238. [Google Scholar] [CrossRef]
- Vasudevan, P.T.; Fierro, J.L.G. A Review of Deep Hydrodesulfurization Catalysis. Catal. Rev. Sci. Eng. 1996, 38, 161–188. [Google Scholar] [CrossRef]
- Ma, X.; Sun, L.; Song, C. A new approach to deep desulfurization of gasoline, diesel fuel and jet fuel by selective adsorption for ultra-clean fuels and for fuel cell applications. Catal. Today 2002, 77, 107–116. [Google Scholar] [CrossRef]
- Ma, X.; Velu, S.; Kim, J.H.; Song, C. Deep desulfurization of gasoline by selective adsorption over solid adsorbents and impact of analytical methods on ppm-level sulfur quantification for fuel cell applications. Appl. Catal. B Environ. 2005, 56, 137–147. [Google Scholar] [CrossRef]
- Yang, H.Y.; Sothen, R.; Cahela, D.R.; Tatarchuk, B.J. Breakthrough Characteristics of Reformate Desulfurization Using ZnO Sorbents for Logistic Fuel Cell Power Systems. Ind. Eng. Chem. Res. 2008, 47, 10064–10070. [Google Scholar] [CrossRef]
- Dasgupta, S.; Agnihotri, V.; Gupta, P.; Nanoti, A.; Garg, M.O.; Goswami, A.N. Simulation of a fixed bed absorber for thiophene removal. Catal. Today 2009, 141, 84–88. [Google Scholar] [CrossRef]
- Kim, J.H.; Ma, X.; Zhou, A.; Song, C. Ultra-deep desulfurization and denitrogenation of diesel fuel by selective adsorption over three different adsorbents: A study on adsorptive selectivity and mechanism. Catal. Today 2006, 111, 74–83. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, L.; Wu, D.; Yu, Q.; Tang, T.; Tang, T. Mesoporous Zeolite ZSM-5 Supported Ni2P Catalysts with High Activity in the Hydrogenation of Phenanthrene and 4,6-Dimethyldibenzothiophene. Ind. Eng. Chem. Res. 2016, 55, 7085–7095. [Google Scholar] [CrossRef]
- Vonortas, A.; Papayannakos, N. Hydrodesulphurization and hydrodeoxygenation of gasoil-vegetable oil mixtures over a Pt/γ-Al2O3 catalyst. Fuel Process. Technol. 2016, 150, 126–131. [Google Scholar] [CrossRef]
- Yang, R.T.; Takahashi, A.; Yang, F.H. New Sorbents for Desulfurization of Liquid Fuels by π-Complexation. Ind. Eng. Chem. Res. 2001, 40, 6236–6239. [Google Scholar] [CrossRef]
- Yang, R.T.; Hernandez-Maldonado, A.J.; Yang, F.H. Desulfurization of Transportation Fuels with Zeolites under Ambient Conditions. Science 2003, 301, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, A.; Song, C. A novel method for oxidative desulfurization of liquid hydrocarbon fuels based on catalytic oxidation using molecular oxygen coupled with selective adsorption. Catal. Today 2007, 123, 276–284. [Google Scholar] [CrossRef]
- Deshpande, A.; Bassi, A.; Prakash, A. Ultrasound-Assisted, Base-Catalyzed Oxidation of 4,6-Dimethyldibenzothiophene in a Biphasic Diesel–Acetonitrile System. Energy Fuels 2005, 19, 28–34. [Google Scholar] [CrossRef]
- Mohebali, G.; Ball, A.S. Biodesulfurization of diesel fuels—Past, present and future perspectives. Int. Biodeterior. Biodegrad. 2016, 110, 163–180. [Google Scholar] [CrossRef]
- Mohebali, G.; Ball, A.S. Biocatalytic desulfurization (BDS) of petrodiesel fuels. Microbiology 2008, 154, 2169–2183. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Gupta, P.; Nanoti, A.; Goswami, A.N.; Garg, M.O.; Tangstad, E.; Vistad, R.B.; Karlsson, A.; St Cker, M. Adsorptive desulfurization of diesel by regenerable nickel based adsorbents. Fuel 2013, 108, 184–189. [Google Scholar] [CrossRef]
- Xue, M.; Chitrakar, R.; Sakane, K.; Hirotsu, T.; Ooi, K.; Yoshimura, Y.; Toba, M.; Feng, Q. Preparation of cerium-loaded Y-zeolites for removal of organic sulfur compounds from hydrodesulfurizated gasoline and diesel oil. J. Colloid Interface Sci. 2006, 298, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, X.; Huang, H.; Wang, Y.; Zhao, L.; Cao, L.; Shen, B.; Gao, J.; Xu, C. Competitive adsorption desulfurization performance over K-Doped NiY zeolite. J. Colloid Interface Sci. 2016, 483, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Whitehurst, D.D.; Isoda, T.; Mochida, I. Present State of the Art and Future Challenges in the Hydrodesulfurization of Polyaromatic Sulfur Compounds. ChemInform 1998, 42, 345–471. [Google Scholar]
- Peralta, D.; Chaplais, G.; Simon-Masseron, A.; Barthelet, K.; Pirngruber, G.D. Metal–Organic Framework Materials for Desulfurization by Adsorption. Energy Fuels 2012, 26, 4953–4960. [Google Scholar] [CrossRef]
- Watanabe, S.; Ma, X.; Song, C. Selective sulfur removal from liquid hydrocarbons over regenerable CeO2-TiO2 adsorbents for fuel cell applications. ACS Div. Fuel Chem. 2004, 49, 511–513. [Google Scholar]
- Nejad, N.F.; Shams, E.; Amini, M.K.; Bennett, J.C. Ordered mesoporous carbon CMK-5 as a potential sorbent for fuel desulfurization: Application to the removal of dibenzothiophene and comparison with CMK-3. Microporous Mesoporous Mater. 2013, 168, 239–246. [Google Scholar] [CrossRef]
- Haji, S.; Erkey, C. Removal of Dibenzothiophene from Model Diesel by Adsorption on Carbon Aerogels for Fuel Cell Applications. Ind. Eng. Chem. Res. 2003, 42, 6933–6937. [Google Scholar] [CrossRef]
- Sano, Y. Adsorptive removal of sulfur and nitrogen species from a straight run gas oil over activated carbons for its deep hydrodesulfurization. Appl. Catal. B Environ. 2004, 49, 219–225. [Google Scholar] [CrossRef]
- Nuntang, S.; Prasassarakich, P.; Ngamcharussrivichai, C. Comparative Study on Adsorptive Removal of Thiophenic Sulfurs over Y and USY Zeolites. Ind. Eng. Chem. Res. 2008, 47, 7405–7413. [Google Scholar] [CrossRef]
- Hernández-Maldonado, A.J.; Yang, R.T. Desulfurization of Liquid Fuels by Adsorption via π Complexation with Cu(I)-Y and Ag-Y Zeolites. Ind. Eng. Chem. Res. 2003, 42, 123–129. [Google Scholar] [CrossRef]
- Montazerolghaem, M.; Seyedeyn-Azad, F.; Rahimi, A. The performance of pelletized Ce-Y and Ni-Y zeolites for removal of thiophene from model gasoline solutions. Korean J. Chem. Eng. 2015, 32, 328–334. [Google Scholar] [CrossRef]
- Tian, F.; Shen, Q.; Fu, Z.; Wu, Y.; Jia, C. Enhanced adsorption desulfurization performance over hierarchically structured zeolite Y. Fuel Process. Technol. 2014, 128, 176–182. [Google Scholar] [CrossRef]
- Hernández-Maldonado, A.J.; Yang, F.H.; Qi, G.; Yang, R.T. Desulfurization of transportation fuels by π-complexation sorbents: Cu(I)-, Ni(II)-, and Zn(II)-zeolites. Appl. Catal. B Environ. 2005, 56, 111–126. [Google Scholar] [CrossRef]
- Hernández-Maldonado, A.J.; Yang, R.T. Desulfurization of Commercial Liquid Fuels by Selective Adsorption via π-Complexation with Cu(I)-Y Zeolite. Ind. Eng. Chem. Res. 2003, 42, 3103–3110. [Google Scholar] [CrossRef]
- Velu, S.; Ma, X.; Song, C. Selective Adsorption for Removing Sulfur from Jet Fuel over Zeolite-Based Adsorbents. Ind. Eng. Chem. Res. 2003, 42, 5293–5304. [Google Scholar] [CrossRef]
- Oliveira, M.L.M.; Miranda, A.A.L.; Barbosa, C.M.B.M.; Cavalcante, C.L.; Azevedo, D.C.S.; Rodriguez-Castellon, E. Adsorption of thiophene and toluene on NaY zeolites exchanged with Ag(I), Ni(II) and Zn(II). Fuel 2009, 88, 1885–1892. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Shi, T.B.; Jia, C.Z.; Ji, W.J.; Chen, Y.; He, M.Y. Adsorptive removal of aromatic organosulfur compounds over the modified Na-Y zeolites. Appl. Catal. B Environ. 2008, 82, 1–10. [Google Scholar] [CrossRef]
- Tawara, K.; Nishimura, T.; Iwanami, H.; Nishimoto, T.; Hasuike, T. New Hydrodesulfurization Catalyst for Petroleum-Fed Fuel Cell Vehicles and Cogenerations. Ind. Eng. Chem. Res. 2001, 40, 2367–2370. [Google Scholar] [CrossRef]
- And, Z.L.; Flytzanistephanopoulos, M. Cu-Cr-O and Cu-Ce-O Regenerable Oxide Sorbents for Hot Gas Desulfurization. Ind. Eng. Chem. Res. 1997, 36, 187–196. [Google Scholar]
- Gaspergalvin, L.D.; Atimtay, A.T.; Gupta, R.P. Zeolite-Supported Metal Oxide Sorbents for Hot-Gas Desulfurization. Ind. Eng. Chem. Res. 1998, 37, 4157–4166. [Google Scholar] [CrossRef]
- Yang, X.; Cao, C.; Kenneth, J.K.; And, K.L.H.; Larry, E.E. Adsorptive Desulfurization with Xerogel-Derived Zinc-Based Nanocrystalline Aluminum Oxide. Ind. Eng. Chem. Res. 2007, 46, 4819–4823. [Google Scholar] [CrossRef]
- Jiang, J.; Ng, F.T.T. Production of low sulfur diesel fuel via adsorption: An equilibrium and kinetic study on the adsorption of dibenzothiophene onto NaY zeolite. Adsorption 2010, 16, 549–558. [Google Scholar] [CrossRef]
- Song, H.; Chang, Y.; Wan, X.; Dai, M.; Song, H.; Jin, Z. Equilibrium, Kinetic, and Thermodynamic Studies on Adsorptive Desulfurization onto CuICeIVY Zeolite. Ind. Eng. Chem. Res. 2014, 53, 5701–5708. [Google Scholar] [CrossRef]
- Nouri, L.; Ghodbane, I.; Hamdaoui, O.; Chiha, M. Batch sorption dynamics and equilibrium for the removal of cadmium ions from aqueous phase using wheat bran. J. Hazard. Mater. 2007, 149, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Richardeau, D.; Joly, G.; Canaff, C.; Magnoux, P.; Guisnet, M.; Thomas, M.; Nicolaos, A. Adsorption and reaction over HFAU zeolites of thiophene in liquid hydrocarbon solutions. Appl. Catal. A Gen. 2004, 263, 49–61. [Google Scholar] [CrossRef]
- Sample Availability: Not Available.
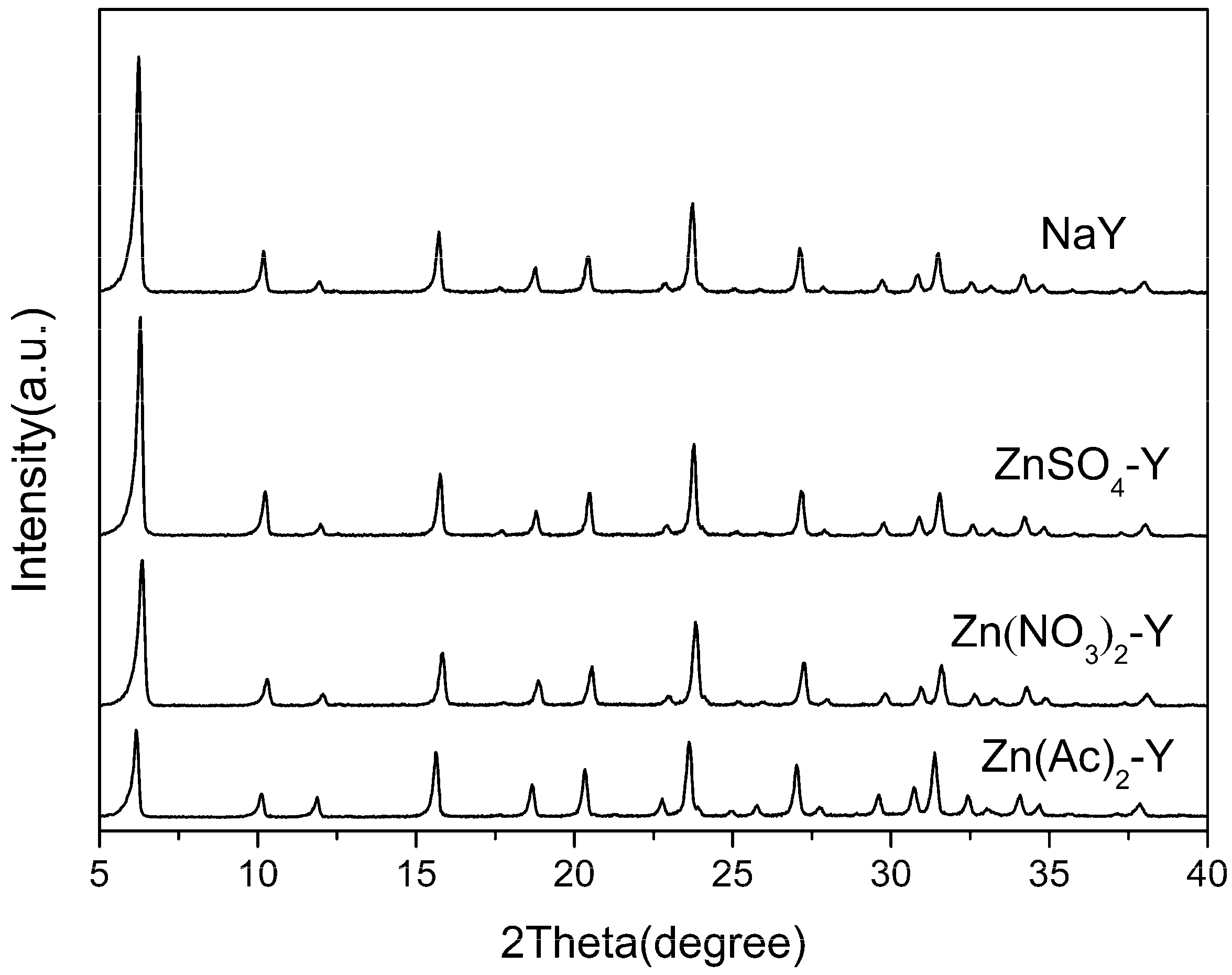
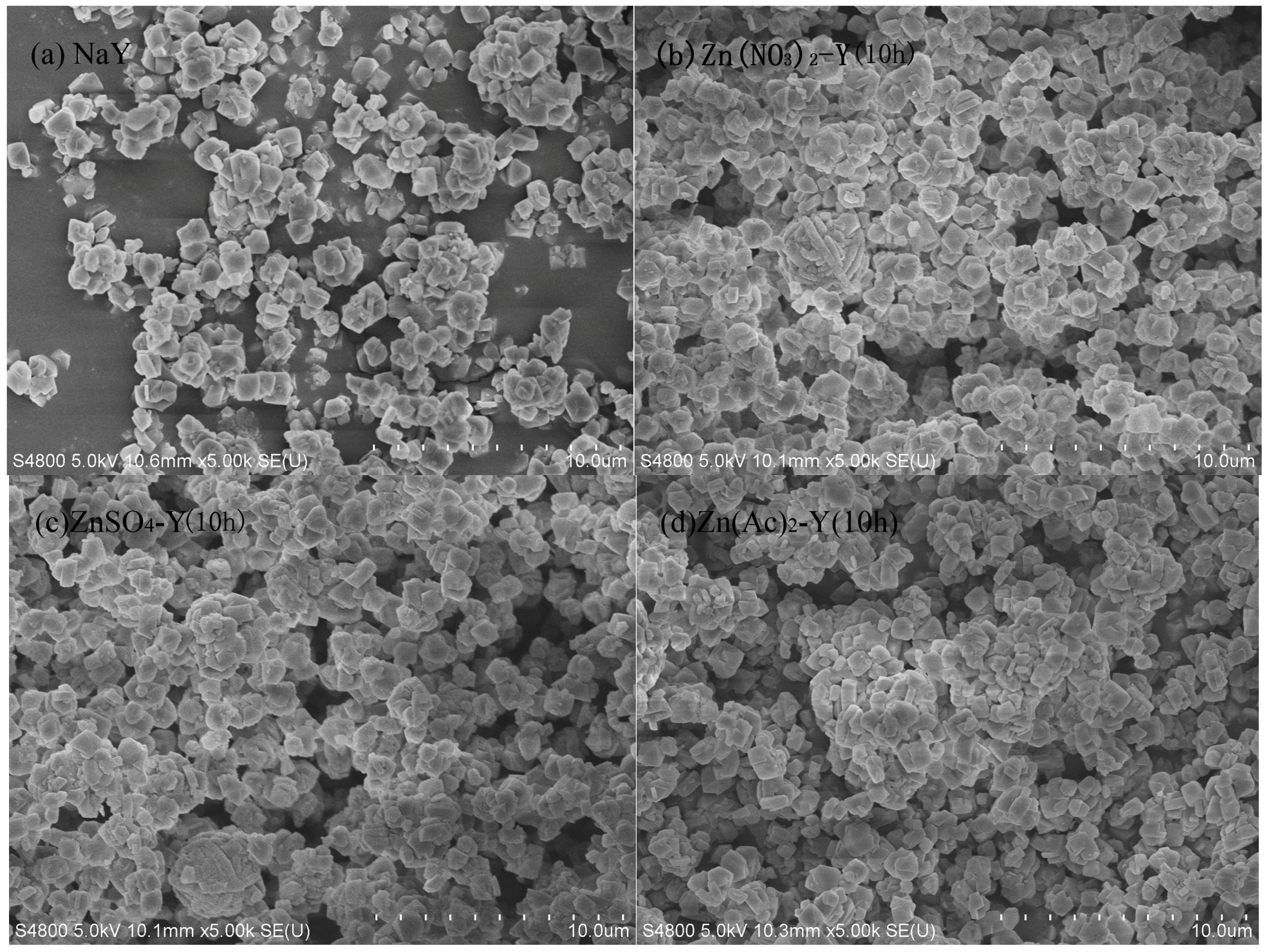
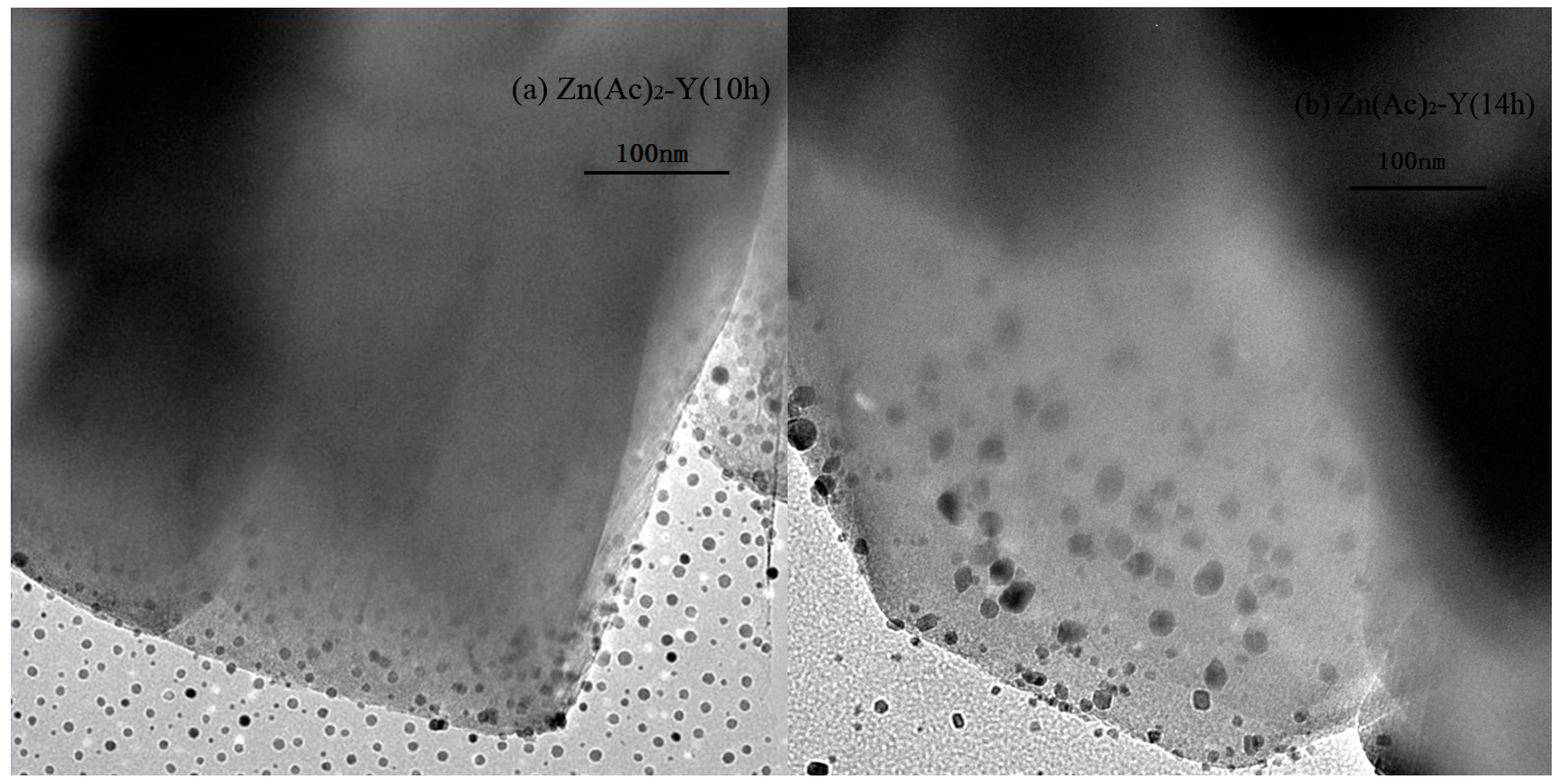
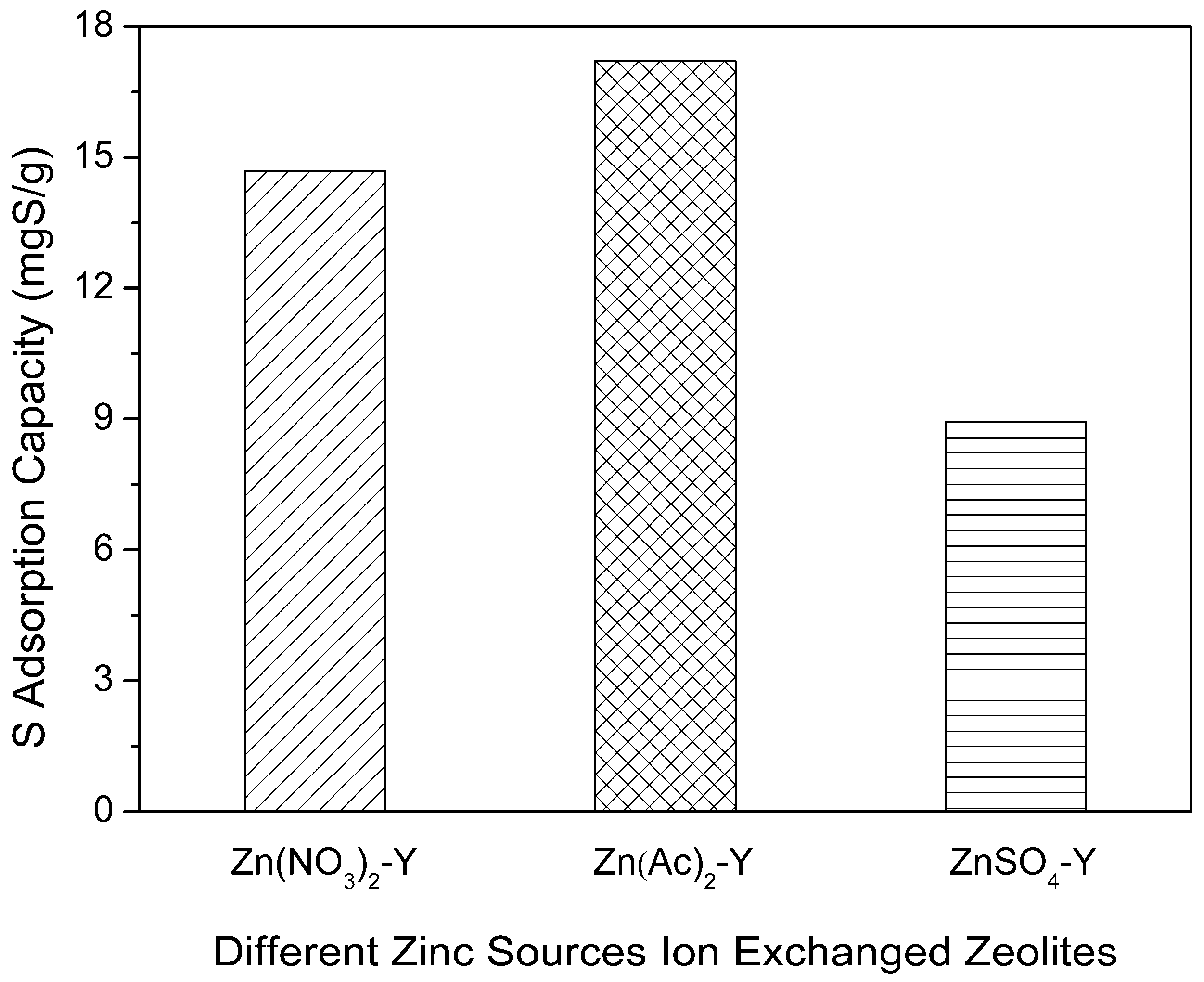

| Sample | Preparation Condition | Chemical Content (mol/g) | Molar Ratio | Na Exchange Degree (%) |
|---|---|---|---|---|
| Ion Exchange Time (h) | Zn | Si/Al | ||
| NaY | - | - | 2.341 | - |
| Zn(NO3)2-Y | 10 | 0.894 | 2.346 | 56.70 |
| ZnSO4-Y | 10 | 0.702 | 2.384 | 43.17 |
| Zn(Ac)2-Y | 6 | 0.672 | 2.342 | 39.62 |
| 10 | 0.969 | 2.386 | 59.47 | |
| 14 | 1.206 | 2.391 | 82.82 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rui, J.; Liu, F.; Wang, R.; Lu, Y.; Yang, X. Adsorptive Desulfurization of Model Gasoline by Using Different Zn Sources Exchanged NaY Zeolites. Molecules 2017, 22, 305. https://doi.org/10.3390/molecules22020305
Rui J, Liu F, Wang R, Lu Y, Yang X. Adsorptive Desulfurization of Model Gasoline by Using Different Zn Sources Exchanged NaY Zeolites. Molecules. 2017; 22(2):305. https://doi.org/10.3390/molecules22020305
Chicago/Turabian StyleRui, Jingwei, Fei Liu, Rijie Wang, Yanfei Lu, and Xiaoxia Yang. 2017. "Adsorptive Desulfurization of Model Gasoline by Using Different Zn Sources Exchanged NaY Zeolites" Molecules 22, no. 2: 305. https://doi.org/10.3390/molecules22020305





