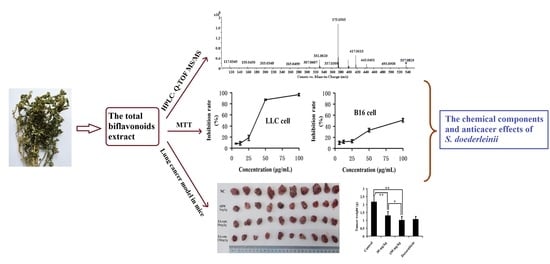
Analysis of the Total Biflavonoids Extract from Selaginella doederleinii by HPLC-QTOF-MS and Its In Vitro and In Vivo Anticancer Effects
Abstract
:1. Introduction
2. Results and Discussion
2.1. (−)ESI-QTOF MS/MS Analysis of Biflavonoid Reference Compounds
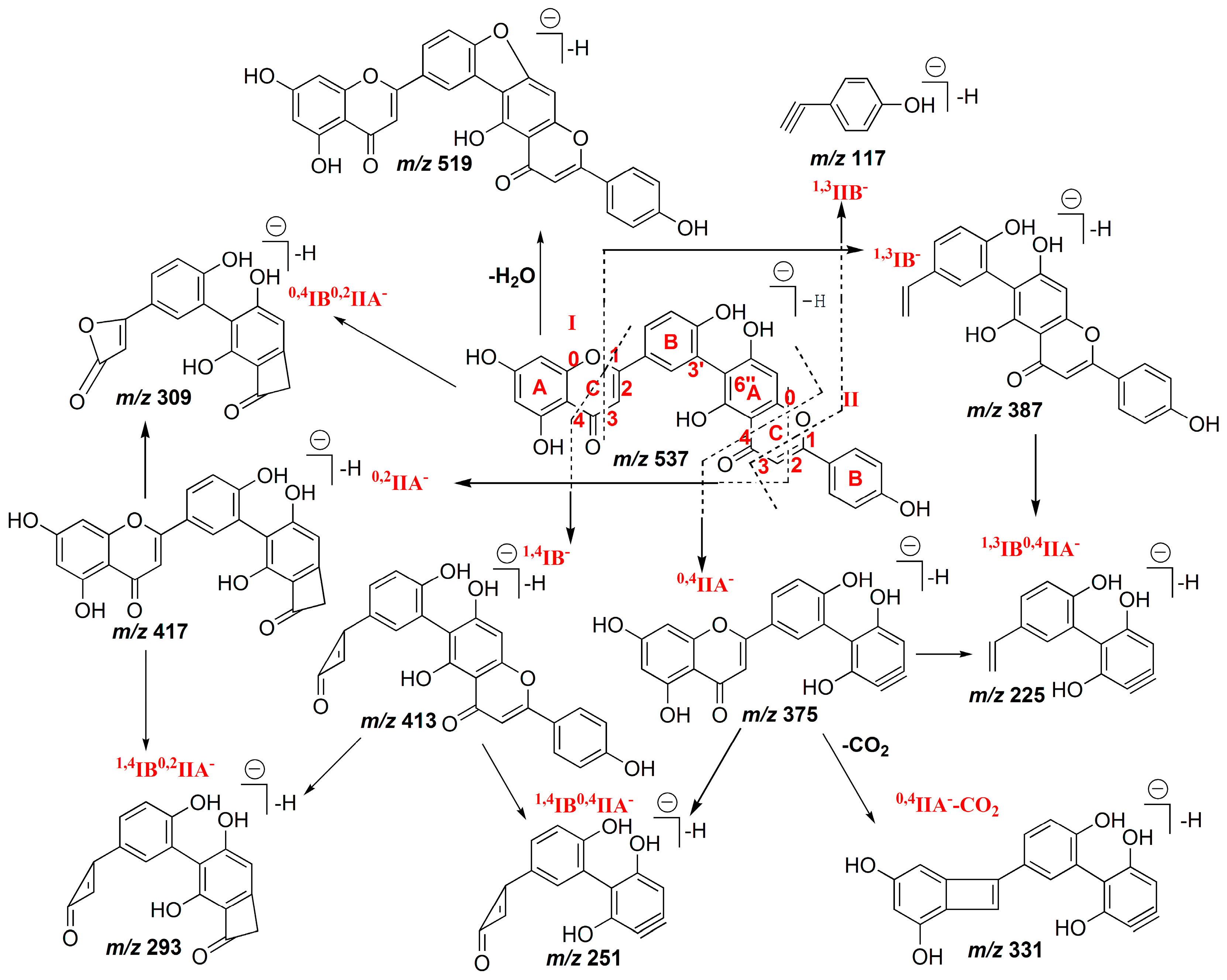
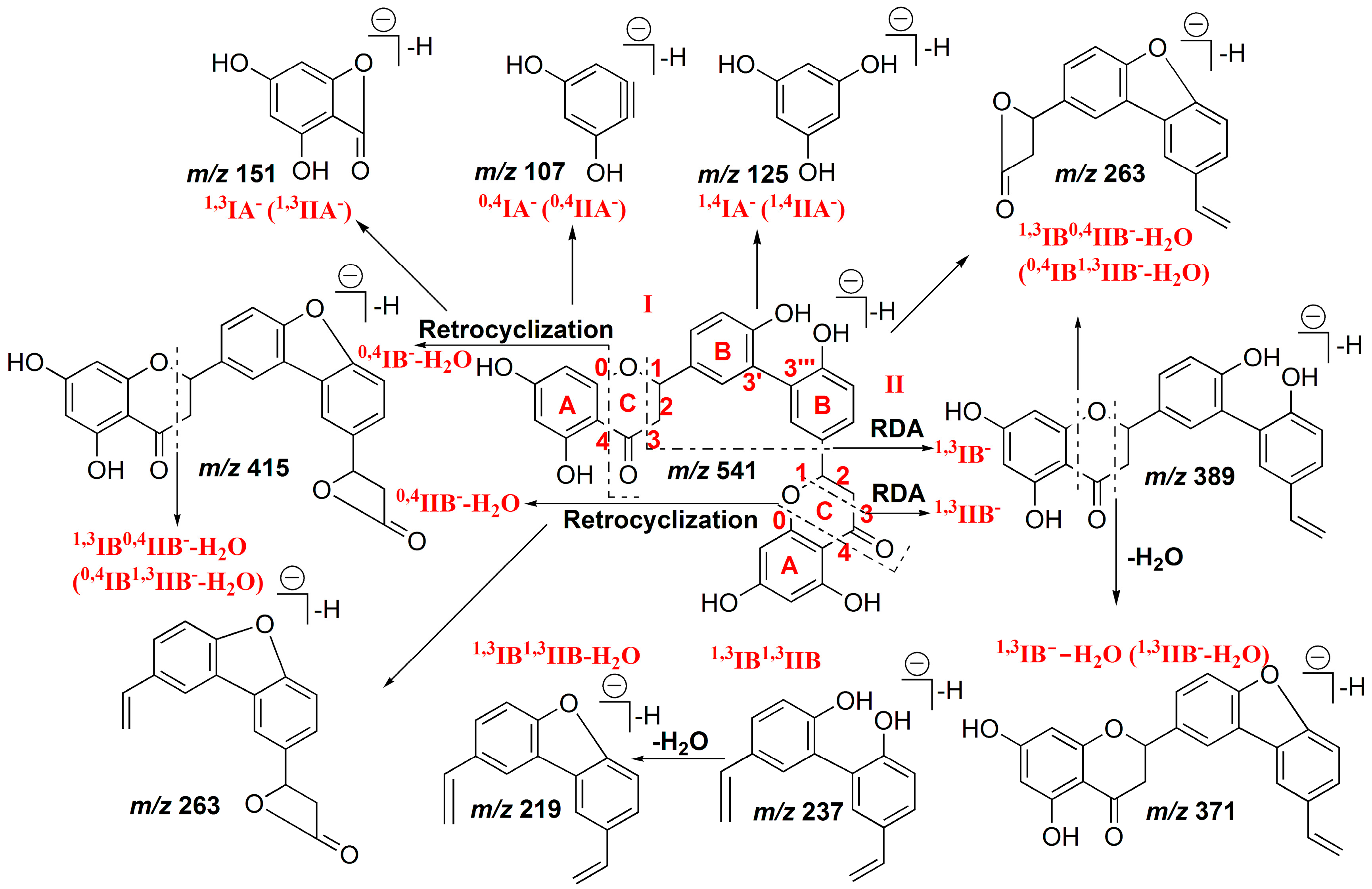
2.1.1. Characterization of IC3′–IIC8′′ Linked Biflavones
2.1.2. Characterization of IC3′–IIC6′′ Linked Biflavones
2.1.3. Characterization of IC3′–IIC3′′′ Linked Biflavonoids
2.1.4. Characterization of C–O Linked Biflavonoids
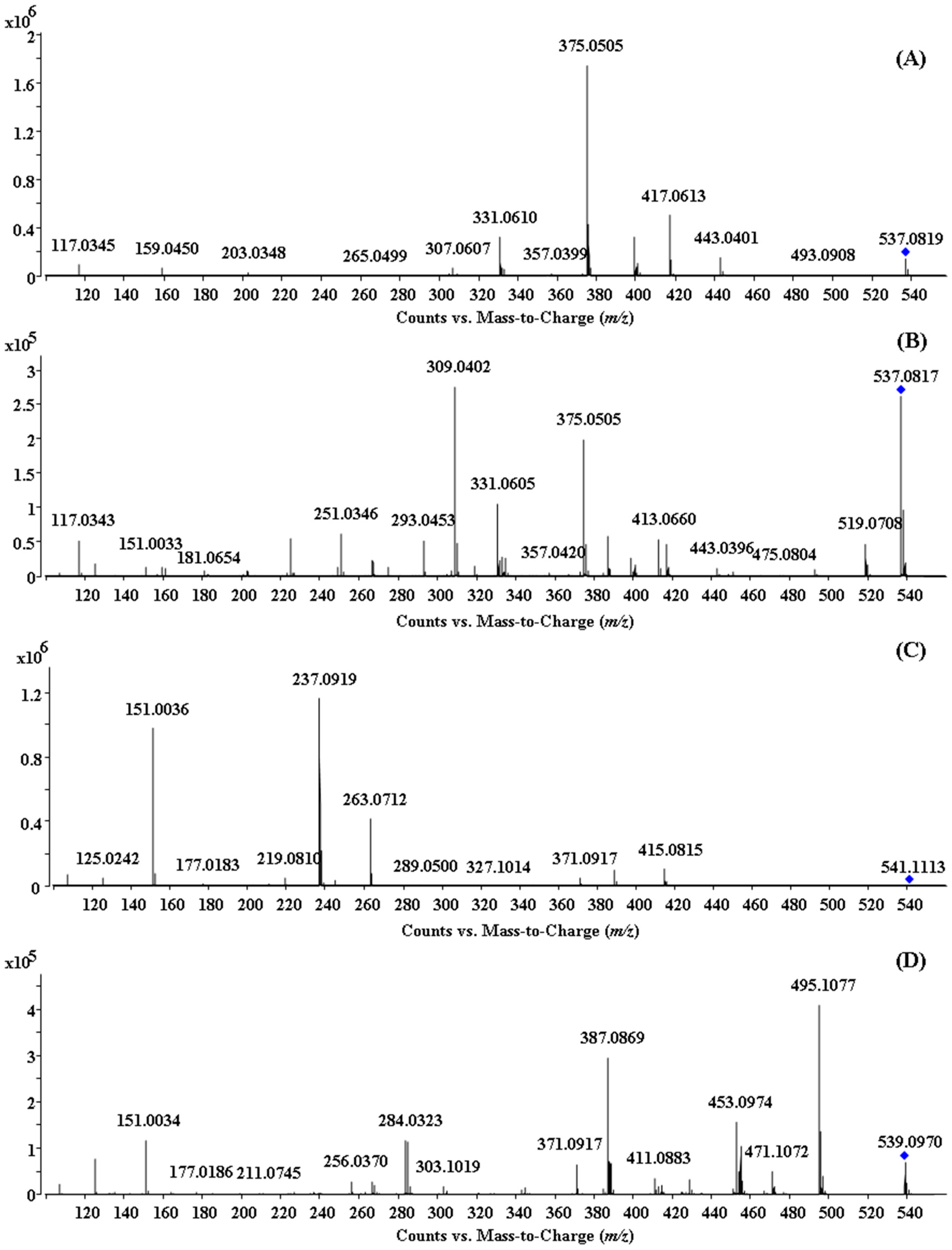
2.2. HPLC-ESI-QTOF MS/MS Analysis of the Biflavonoids from S. doederleinii
2.2.1. Characterization of IC3′–IIC8′′ Linked Biflavonoids from S. doederleinii
2.2.2. Characterization of IC3′–IIC6′′ Linked Biflavonoids from S. doederleinii
2.2.3. Characterization of IC3′–IIC3′′′ Linked Biflavonoids from S. doederleinii
2.2.4. Characterization of C–O Linked Biflavonoids from S. doederleinii
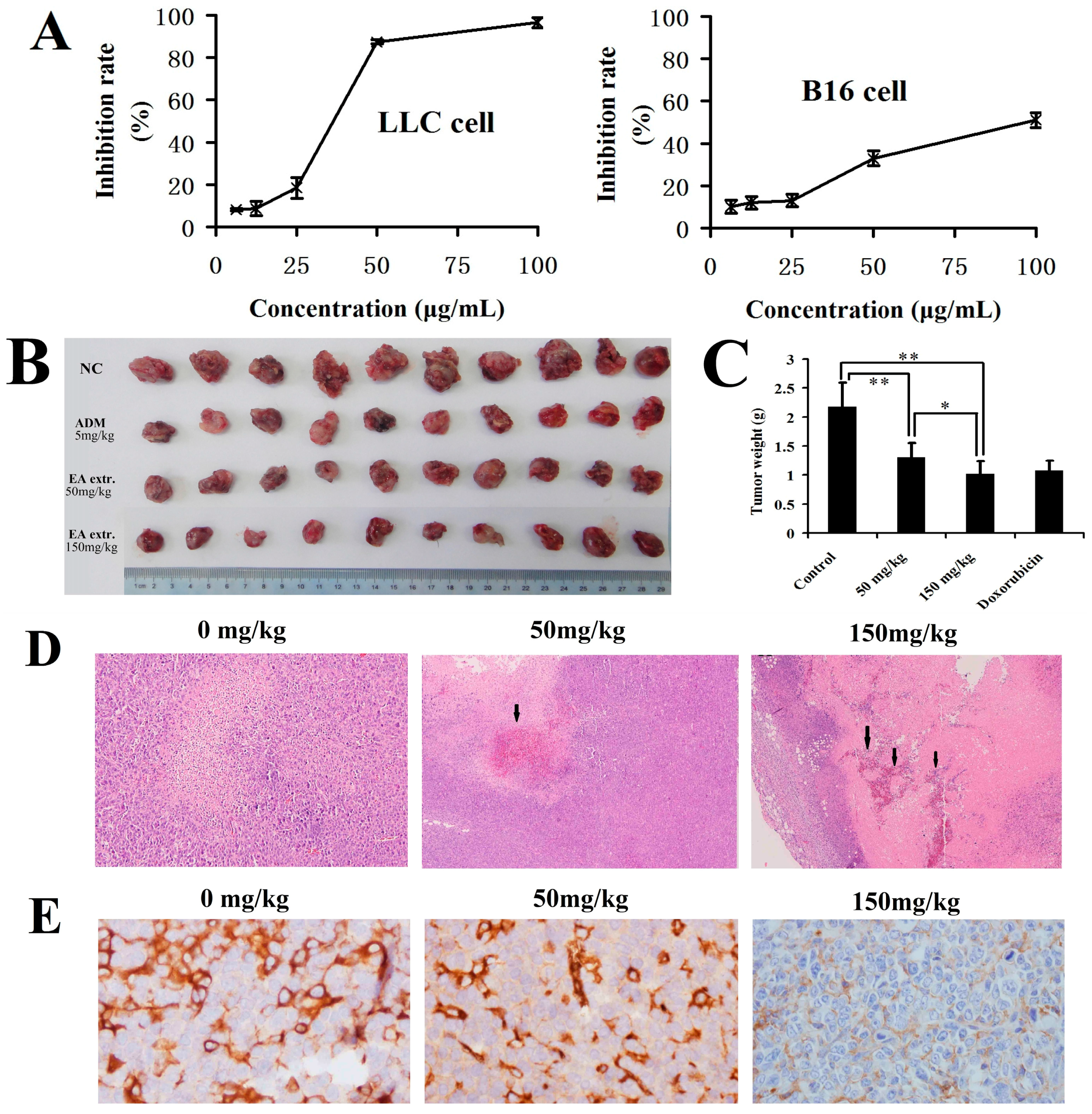
2.3. In Vitro and In Vivo Anticancer Activity of the Total Biflavonoids Extract
3. Experimental
3.1. Reagents and Materials
3.2. Standard Solutions and Sample Preparation
3.3. HPLC-ESI-QTOF MS/MS Analysis
3.4. Cell Lines and Culture
3.5. Cell Viability Assay
3.6. In Vivo Anticancer Test
3.7. Immunohistochemistry and Microvessel Density (MVD) Assessment
3.8. Determination of TNF-α and IFN-γ in Mice Serum
3.9. Data Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, G.P.; Dai, S.; Chen, R.S. Dictionary of Traditional Chinese Medicine; Shanghai Science and Technology Press: Shanghai, China, 2006; pp. 831–832. [Google Scholar]
- Setyawan, A.D. Review: Natural products from genus Selaginella (Selaginellaceae). Nusant. Biosci. 2011, 3, 44–58. [Google Scholar]
- Ahn, S.H.; Mun, Y.J.; Lee, S.W.; Kwak, S.; Choi, M.K.; Baik, S.K.; Kim, Y.M.; Woo, W.H. Selaginella tamariscina induces apoptosis via a caspase-3-mediated mechanism in human promyelocytic leukemia cells. J. Med. Food 2006, 9, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.K.; Li, Y.J.; Zhang, L.; Feng, W.S.; Zhang, X. Antihyperglycemic activity of Selaginella tamariscina (Beauv.) Spring. J. Ethnopharmacol. 2011, 133, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Peng, H.; Ji, Z.H.; Zhao, S.W.; Zhang, Y.F.; Wu, J.; Fan, J.P.; Liao, J.C. Reactive oxygen species-mediated mitochondrial dysfunction is involved in apoptosis in human nasopharyngeal carcinoma CNE cells induced by Selaginella doederleinii extract. J. Ethnopharmacol. 2011, 138, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Li, S.G.; Yao, H.; Zhao, M.F.; Li, Y.X.; Huang, L.Y.; Lin, X.H. Determination of seven biflavones of Selaginella doederleinii by high performance liquid chromatography. Anal. Lett. 2013, 46, 2835–2845. [Google Scholar] [CrossRef]
- Li, J.; Lei, X.; Chen, K.L. Comparison of cytotoxic activities of extract from Selaginella species. Pharmacogn. Mag. 2014, 10, 529–535. [Google Scholar] [PubMed]
- Li, S.G.; Zhao, M.F.; Li, Y.X.; Sui, Y.X.; Yao, H.; Huang, L.Y.; Lin, X.H. Preparative isolation of six antitumourbiflavonoids from Selaginella doederleinii Hieron by high-speed counter-current chromatography. Phytochem. Anal. 2014, 25, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.X.; Li, S.G.; Shi, P.Y.; Wu, Y.J.; Li, Y.X.; Chen, W.Y.; Huang, L.Y.; Yao, H.; Lin, X.H. Ethyl acetate extract from Selaginella doederleinii Hieron inhibits the growth of human lung cancer cells A549 via caspase-dependent apoptosis pathway. J. Ethnopharmacol. 2016, 190, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.F.; Shi, P.Y.; Huang, X.M.; Zhao, M.F.; Li, S.G.; Wu, Y.J.; Lin, X.H.; Yao, H. Pharmacokinetics, tissue distribution and protein binding studies of chrysocauloflavone I in rats. Planta Med. 2016, 82, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.X.; Li, S.G.; Shi, P.Y.; Wu, Y.J.; Li, Y.X.; Chen, W.Y.; Huang, L.Y.; Yao, H.; Lin, X.H. Delicaflavone induces autophagic cell death in lung cancer via Akt/mTOR/p70S6K signaling pathway. J. Mol. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Gattuso, G.; Laganà, G.; Leuzzi, U.; Bellocco, E. C- and O-glycosyl flavonoids in Sanguinello and Tarocco blood orange (Citrus sinensis (L.) Osbeck) juice: Identification and influence on antioxidant properties and acetylcholinesterase activity. Food Chem. 2016, 196, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.Z.; Zhai, Y.X.; Guo, C.; Liu, Y.Q.; Tang, D.Q.; Pan, Y.J. A new strategy to determine the protein mutation site using matrix-assisted laser desorption ionization in-source decay: Derivatization by ionic liquid. Anal. Chim. Acta 2015, 865, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Sun, Y.Q.; Ding, L.N.; Wang, Y.Y.; Gao, Z.; Wu, Z.; Wang, S.M.; Li, W.; Bi, Y.F. Mechanism evaluation of the interactions between flavonoids and bovine serum albumin based on multi-spectroscopy, molecular docking and Q-TOF HR-MS analyses. Food Chem. 2016, 203, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Ouyang, S.H.; Chang, Y.Q.; Wang, T.M.; Li, W.X.; Tian, H.Y.; Cao, H.; Kurihara, H.; He, R.R. A comparative analysis of chemical compositions in Camellia sinensis var. puanensis Kurihara, a novel Chinese tea, by HPLC and UFLC-Q-TOF-MS/MS. Food Chem. 2017, 216, 282–288. [Google Scholar] [CrossRef] [PubMed]
- You, Z.S.; Guo, C.; Pan, Y.J. An experimental and theoretical study on fragmentation of protonated N-(2-pyridinylmethyl)indole in electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 2509–2516. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Wei, W.H.; Dang, Q.K.; Ding, C.F.; Pan, Y.J. Negative charge induced dissociation: Fragmentation of deprotonated N-benzylidene-2-hydroxylanilines in electrospray ionization mass spectrometry. J. Mass Spectrom. 2014, 49, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Klavina, L.; Springe, G.; Nikolajeva, V.; Martsinkevich, I.; Nakurte, I.; Dzabijeva, D.; Steinberga, I. Chemical composition analysis, antimicrobial activity and cytotoxicity screening of Moss extracts (Moss phytochemistry). Molecules 2015, 20, 17221–17243. [Google Scholar] [CrossRef] [PubMed]
- Kicel, A.; Michel, P.; Owczarek, A.; Marchelak, A.; Żyżelewicz, D.; Budryn, G.; Oracz, J.; Olszewska, M.A. Phenolic profile and antioxidant potential of leaves from selected Cotoneaster Medik. species. Molecules 2016, 21, 688. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, H.; Zhou, C.; Jia, H.; Ma, Z.; Zou, Z. Identification of the chemical constituents in aqueous extract of Zhi-Qiao and evaluation of its antidepressant effect. Molecules 2015, 20, 6925–6940. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Zhang, Q.; Zhang, Y.H.; Lu, X.Y.; Fu, W.M.; He, J.Y. Chemical analysis of dietary constituents in Rosa roxburghii and Rosa sterilis fruits. Molecules 2016, 21, 1204. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wu, J.L.; Leung, E.L.; Zhou, H.; Liu, Z.; Yan, G.; Liu, Y.; Liu, L.; Li, N. Identification of oxygenated fatty acid as a side chain of lipo-alkaloids in Aconitum carmichaelii by UHPLC-Q-TOF-MS and a database. Molecules 2016, 21, 437. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yao, S.; Zhang, X.X.; Song, H. Rapid screening and structural characterisation of antioxidants from the extract of Selaginella doederleinii Hieron with DPPH-UPLC-Q-TOF/MS method. Int. J. Anal. Chem. 2015, 2015, 849769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Li, Q.Y.; Yan, L.L.; Shi, Y. Structural characterisation and identification of biflavones in Selaginella tamariscina by liquid chromatography-diode-array detection/electrospray ionization tandem mass spectrometry. Rapid Comm. Mass Spectrom. 2011, 25, 2173–2186. [Google Scholar] [CrossRef] [PubMed]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanoneaglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef]
- Sun, C.M.; Syu, W.J.; Huang, Y.T.; Chen, C.C.; Ou, J.C. Selective cytotoxicity of ginkgetin from Selaginella moellendorffii. J. Nat. Prod. 1997, 60, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.C.; Kuo, Y.C.; Chou, C.J. Cytotoxic biflavonoids from Selaginella delicatula. J. Nat. Prod. 2000, 63, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Swamy, R.C.; Kunert, O.; Schühly, W.; Bucar, F.; Ferreira, D.; Rani, V.S.; Kumar, B.R.; Rao, A.V.N.A. Structurally unique biflavonoids from Selaginella chrysocaulos and Selaginella bryopteris. Chem. Biodivers. 2006, 3, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.L.; Chai, H.; Gupta, M.P.; Farnsworth, N.R.; Cordell, G.A.; Pezzuto, J.M.; Beecher, C.W.W.; Kinghorn, A.D. Cytotoxic biflavonoids from Selaginella willdenowii. Phytochemistry 1995, 40, 129–134. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, C.X.; Li, Y.L.; Liu, C.Y.; Rong, Y.H. Chemical constituents from Selaginella doederleinii and their bioactivities. Chin. Trad. Herbal Drugs 2013, 44, 3270–3275. [Google Scholar]
- Lin, L.C.; Chou, C.J. Three new biflavonoids from Selaginella delicatula. Chin. Pharm. J. 2000, 52, 211–218. [Google Scholar]
- Sample Availability: Samples of the compounds 1, 2, 4, 5, 8, 11, 18 and 20 are available from the authors.
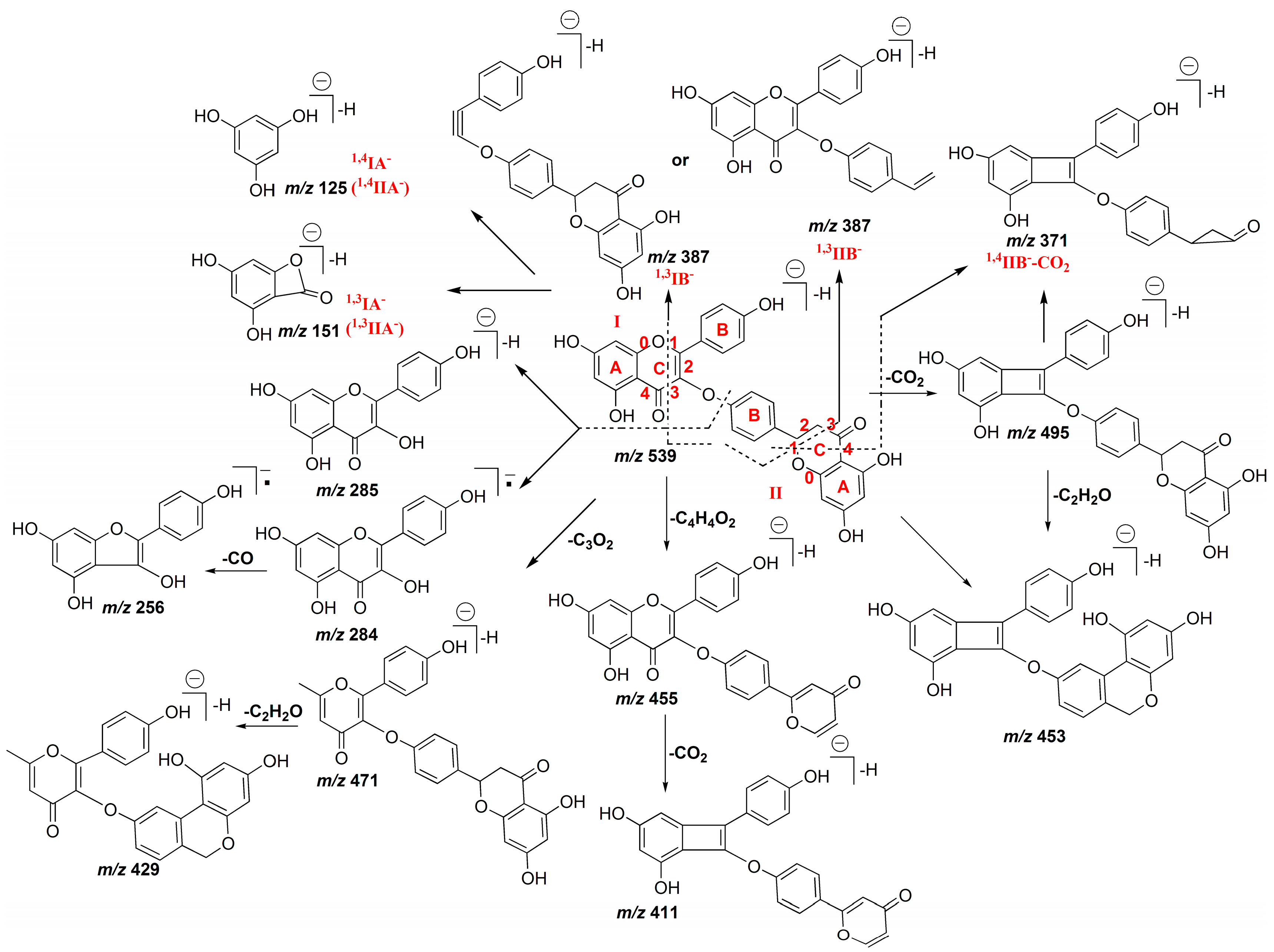
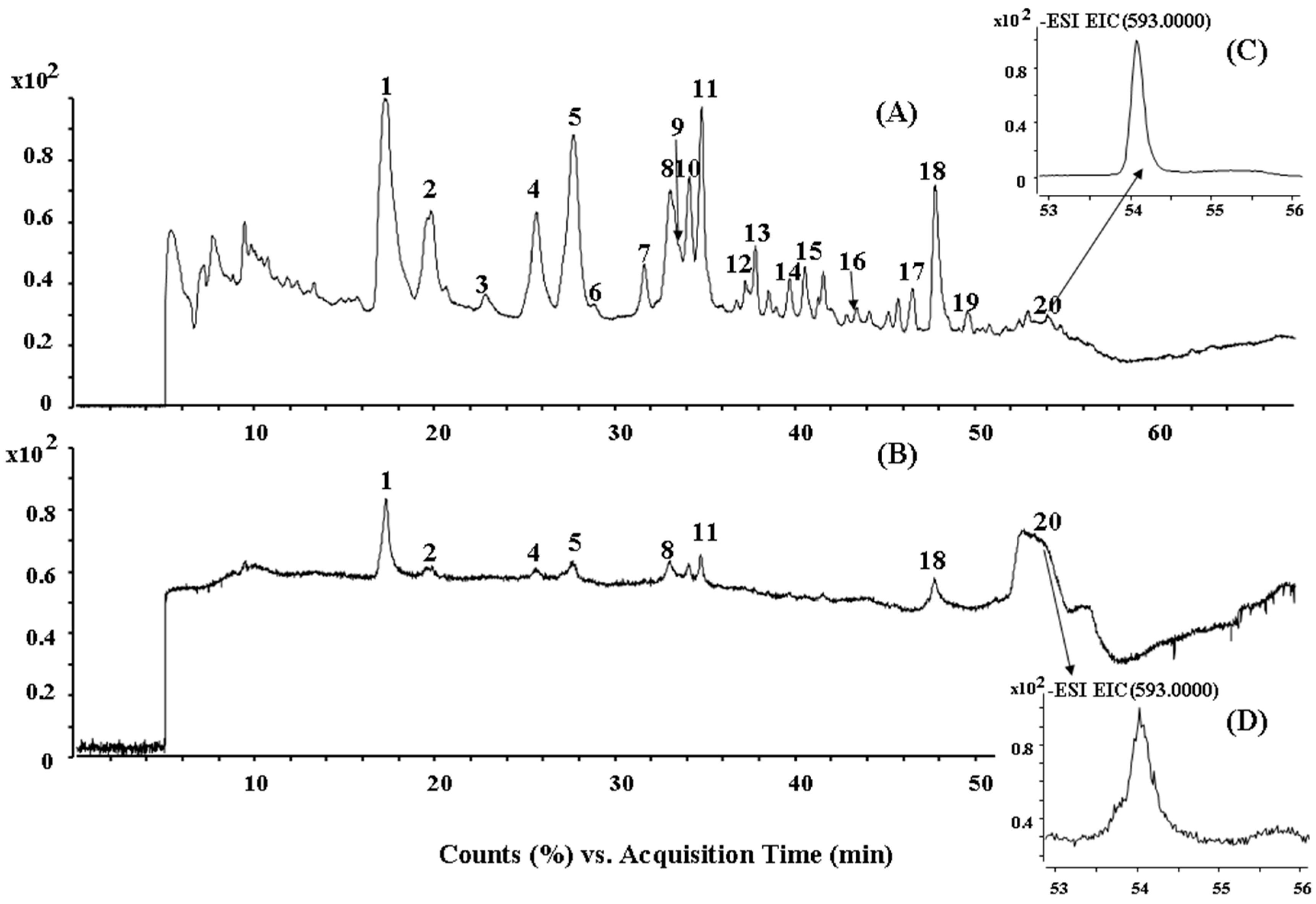
| Peak No. | tR (min) | Identification | (−)ESI–MS m/z | Formula | |
|---|---|---|---|---|---|
| Observed | Calculated (Δppm) | ||||
| 1 | 17.4 | Amentoflavone | 537.082 | 537.0827 (−1.30) | C30H18O10 |
| 2 | 19.6 | Robustaflavone | 537.0815 | 537.0827 (−2.23) | C30H18O10 |
| 3 | 22.8 | 2′,8′′-Biapigenin | 537.0824 | 537.0827 (−0.56) | C30H18O10 |
| 4 | 25.6 | 2′′,3′′-Dihydro-3′, 3′′′-biapigenin | 539.0974 | 539.0984 (−1.85) | C30H20O10 |
| 5 | 27.9 | 3′,3′′′-Binaringenin | 541.113 | 541.114 (−1.85) | C30H22O10 |
| 6 | 28.9 | Bilobetin | 551.0977 | 551.0984 (−1.27) | C31H20O10 |
| 7 | 31.6 | 4′′′-Dehydroxyamentoflavone a | 521.0871 | 521.0878 (−1.34) | C30H18O9 |
| 8 | 33.0 | Delicaflavone | 537.0822 | 537.0827 (−0.93) | C30H18O10 |
| 9 | 33.6 | Hinokiflavone | 537.0819 | 537.0827 (−1.49) | C30H18O10 |
| 10 | 34.2 | 2,3-Dihydrohinokiflavone a | 539.0974 | 539.0984 (−1.85) | C30H20O10 |
| 11 | 34.8 | Chrysocauloflavone I | 539.0976 | 539.0984 (−1.48) | C30H20O10 |
| 12 | 37.2 | 2′′,3′′-Dihydro-3′,3′′′-biapigenin methyl ether a | 553.1134 | 553.114 (−1.08) | C31H22O10 |
| 13 | 37.8 | 3′,3′′′-Binaringenin methyl ether a | 555.1285 | 555.1297 (−2.16) | C31H24O10 |
| 14 | 39.7 | Isoginkgetin | 565.1133 | 565.114 (−1.24) | C32H22O10 |
| 15 | 40.5 | Robustaflavone 7,4′-dimethyl ether a | 565.1135 | 565.114 (−0.88) | C32H22O10 |
| 16 | 43.4 | 2,3-Dihydroisocryptomerin a | 553.1132 | 553.114 (−1.45) | C31H22O10 |
| 17 | 46.5 | 4′,7′′,4′′′-Trimethylamentoflavone a | 579.1289 | 579.1297 (−1.38) | C33H24O10 |
| 18 | 47.7 | Heveaflavone | 579.1286 | 579.1297 (−1.90) | C33H24O10 |
| 19 | 49.6 | 2′′,3′′-Dihydroheveaflavone a | 581.1435 | 581.1453 (−3.10) | C33H26O10 |
| 20 | 54.1 | 7,4′,7′′,4′′′-Tetra-O-methyl-amentoflavone | 593.1446 | 593.1453 (−1.18) | C34H26O10 |
| (−)ESI-MS2 m/z (% Base Peak) | (−)ESI-MS2 m/z (% Base Peak) | ||||||
|---|---|---|---|---|---|---|---|
| Peak | Observed Mass | Calculated Mass (Δppm) | Proposed Formula | Peak | Observed Mass | Calculated Mass (Δppm) | Proposed Formula |
| IC3′ (I2′)–IIC8′′Linked Biflavonoids | |||||||
| 3 | MS2[537]: | 7 | MS2[521]: | ||||
| 537.0824 (100) | 537.0827 (−0.56) | C30H17O10− | 375.05 (100) | 375.051 (−2.67) | C21H11O7− | ||
| 385.071 (25) | 385.0718 (−2.08) | C23H13O6− | 331.0595 (15) | 331.0612 (−5.14) | C20H11O5− | ||
| 519.0715 (14) | 519.0722 (−1.35) | C30H15O9− | 521.0871 (6) | 521.0878 (−1.34) | C30H17O9− | ||
| 151.0028 (10) | 151.0037 (−5.96) | C7H3O4− | 14 | MS2[565]: | |||
| 375.05 (7) | 375.051 (−2.67) | C21H11O7− | 533.0868 (100) | 533.0878 (−1.88) | C31H17O9− | ||
| 6 | MS2[551]: | 518.063 (22) | 518.0643 (−2.51) | C30H14O9−• | |||
| 457.0586 (100) | 457.0565 (4.59) | C25H13O9− | 507.0713 (12) | 507.0722 (−1.77) | C29H15O9− | ||
| 431.0793 (93) | 431.0772 (4.87) | C24H15O8− | 565.1133 (10) | 565.114 (−1.24) | C32H21O10− | ||
| 389.0688 (64) | 389.0667 (5.40) | C22H13O7− | 389.0654 (10) | 389.0667 (−3.34) | C22H13O7− | ||
| 151.0036 (27) | 151.0037 (−0.66) | C7H3O4− | 415.0445 (6) | 415.0459 (−3.37) | C23H11O8− | ||
| 442.0342 (25) | 442.0330 (2.71) | C24H10O9−• | 374.0427 (5) | 374.0432 (−1.34) | C21H10O7−• | ||
| 413.0689 (23) | 413.0667 (5.33) | C24H13O7− | 388.0577 (5) | 388.0589 (−3.09) | C22H12O7−• | ||
| 551.0977 (18) | 551.0984 (−1.27) | C31H19O10− | 151.003 (4) | 151.0037 (−4.64) | C7H3O4− | ||
| 17 | MS2[579]: | 19 | MS2[581]: | ||||
| 533.086 (100) | 533.0878 (−3.38) | C31H17O9− | 403.0836 (100) | 403.0823 (−1.49) | C23H15O7− | ||
| 579.1289 (42) | 579.1297 (−1.38) | C33H23O10− | 581.1435 (53) | 581.1453 (−3.10) | C33H25O10− | ||
| 388.0573 (7) | 388.0589 (−4.12) | C22H12O7−• | 165.0193 (46) | 165.0193 (0) | C8H5O4− | ||
| 403.0817 (6) | 403.0823 (−1.49) | C23H15O7− | 383.0934 (40) | 383.0925 (2.35) | C24H15O5− | ||
| 547.101 (4) | 547.1035 (−4.57) | C32H19O9− | |||||
| 415.0472 (2) | 415.0459 (3.13) | C23H11O8− | |||||
| IC3′–IIC6′′Linked Biflavonoid | |||||||
| 15 | MS2[565]: | ||||||
| 445.0919 (100) | 445.0929 (−2.25) | C25H17O8− | 430.0683 (36) | 430.0694 (−2.56) | C24H14O8−• | ||
| 388.058 (86) | 388.0589 (−2.32) | C22H12O7−• | 372.0629 (29) | 372.0639 (−2.69) | C22H12O6−• | ||
| 403.0813 (80) | 403.0823 (−2.48) | C23H15O7− | 412.0575 (27) | 412.0589 (−3.40) | C24H12O7−• | ||
| 456.0477 (50) | 456.0487 (−2.19) | C25H12O9−• | 117.0343 (23) | 117.0346 (−2.56) | C8H5O− | ||
| 471.0709 (43) | 471.0722 (−2.76) | C26H15O9− | 533.0854 (16) | 533.0878 (−4.50) | C31H17O9− | ||
| 427.0812 (42) | 427.0823 (−2.58) | C25H15O7− | |||||
| IC3′–IIC3′′′Linked Biflavonoids | |||||||
| 12 | MS2[553]: | 13 | MS2[555]: | ||||
| 387.0869 (100) | 387.0874 (−1.29) | C23H15O6− | 237.0921 (100) | 237.0921 (0) | C16H13O2− | ||
| 369.0763 (8) | 369.0768 (−1.35) | C23H13O5− | 151.0036 (14) | 151.0037 (−0.66) | C7H3O4− | ||
| 413.0657 (2) | 413.0667 (−2.42) | C24H13O7− | 403.1174 (13) | 403.1187 (−3.22) | C24H19O6− | ||
| 151.0032 (2) | 151.0037 (−3.31) | C7H3O4− | 263.0711 (10) | 263.0714 (−1.14) | C17H11O3− | ||
| 165.0187 (5) | 165.0193 (−3.64) | C8H5O4− | |||||
| 219.0808 (4) | 219.0815 (−3.20) | C16H11O− | |||||
| 429.0969 (3) | 429.098 (−2.56) | C25H17O7− | |||||
| C–O Linked Biflavonoids | |||||||
| 9 | MS2[537]: | 16 | MS2[553]: | ||||
| 537.0819 (100) | 537.0827 (−1.49) | C30H17O10− | 401.102 (100) | 401.1031 (−2.74) | C24H17O6− | ||
| 284.0318 (14) | 284.0326 (−2.82) | C15H8O6−• | 469.0931 (29) | 469.0929 (0.43) | C27H17O8− | ||
| 269.0443 (11) | 269.0455 (−4.46) | C15H9O5− | 225.0063 (27) | 225.0041 (9.78) | C9H5O7− | ||
| 151.0034 (10) | 151.0037 (−1.99) | C7H3O4− | 467.1128 (26) | 467.1136 (−1.71) | C28H19O7− | ||
| 285.0392 (10) | 285.0405 (−4.56) | C15H19O6− | 299.0534 (24) | 299.0561 (−9.03) | C16H11O6− | ||
| 469.0918 (9) | 469.0929 (−2.34) | C27H17O8− | 553.1132 (23) | 553.114 (−1.45) | C31H21O10− | ||
| 385.0709 (8) | 385.0718 (−2.34) | C23H13O6− | 509.1231 (21) | 509.1242 (−2.16) | C30H21O8− | ||
| 256.0365 (7) | 256.0377 (−4.69) | C14H8O5−• | 386.078 (19) | 386.0796 (−4.14) | C23H14O6−• | ||
| 493.0920 (5) | 493.0929 (−1.83) | C29H17O8− | 298.047 (15) | 298.0483 (−4.36) | C16H10O6−• | ||
| 10 | MS2[539]: | 151.0035 (11) | 151.0037 (−1.32) | C7H3O4− | |||
| 495.1081 (100) | 495.1085 (−0.81) | C29H19O8− | 164.9846 (11) | 164.9829 (10.30) | C7HO5− | ||
| 453.0973 (27) | 453.0980 (−1.54) | C27H17O7− | 425.1014 (10) | 425.1031 (−4.00) | C26H17O6− | ||
| 284.0325 (26) | 284.0326 (−0.35) | C15H8O6−• | 284.0315 (9) | 284.0326 (−3.87) | C15H8O6−• | ||
| 387.0869 (22) | 387.0874 (−1.29) | C23H15O6− | 255.0296 (7) | 255.0299 (−1.18) | C14H7O5− | ||
| 151.0035 (15) | 151.0037 (−1.32) | C7H3O4− | 283.0261 (6) | 283.0248 (4.59) | C15H7O6− | ||
| 455.0784 (14) | 455.0772 (2.64) | C26H15O8− | 485.1225 (6) | 485.1242 (−3.50) | C28H21O8− | ||
| 539.0974 (14) | 539.0984 (−1.85) | C30H19O10− | 125.0239 (5) | 125.0244 (−4.00) | C6H5O3− | ||
| 190.9985 (13) | 190.9986 (−0.52) | C9H3O5− | |||||
| 256.0371 (10) | 256.0377 (−2.34) | C14H8O5−• | |||||
| 255.0298 (10) | 255.0299 (−0.39) | C14H7O5− | |||||
| 411.0864 (9) | 411.0874 (−2.43) | C25H15O6− | |||||
| 471.108 (9) | 471.1085 (−1.06) | C27H19O8− | |||||
| 268.037 (7) | 268.0377 (−2.61) | C15H8O5−• | |||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, H.; Chen, B.; Zhang, Y.; Ou, H.; Li, Y.; Li, S.; Shi, P.; Lin, X. Analysis of the Total Biflavonoids Extract from Selaginella doederleinii by HPLC-QTOF-MS and Its In Vitro and In Vivo Anticancer Effects. Molecules 2017, 22, 325. https://doi.org/10.3390/molecules22020325
Yao H, Chen B, Zhang Y, Ou H, Li Y, Li S, Shi P, Lin X. Analysis of the Total Biflavonoids Extract from Selaginella doederleinii by HPLC-QTOF-MS and Its In Vitro and In Vivo Anticancer Effects. Molecules. 2017; 22(2):325. https://doi.org/10.3390/molecules22020325
Chicago/Turabian StyleYao, Hong, Bing Chen, Yanyan Zhang, Huigen Ou, Yuxiang Li, Shaoguang Li, Peiying Shi, and Xinhua Lin. 2017. "Analysis of the Total Biflavonoids Extract from Selaginella doederleinii by HPLC-QTOF-MS and Its In Vitro and In Vivo Anticancer Effects" Molecules 22, no. 2: 325. https://doi.org/10.3390/molecules22020325
APA StyleYao, H., Chen, B., Zhang, Y., Ou, H., Li, Y., Li, S., Shi, P., & Lin, X. (2017). Analysis of the Total Biflavonoids Extract from Selaginella doederleinii by HPLC-QTOF-MS and Its In Vitro and In Vivo Anticancer Effects. Molecules, 22(2), 325. https://doi.org/10.3390/molecules22020325