Abstract
Several novel methods, catalysts and reagents have been developed to improve organic synthesis. Synergistic effects between reactions, reagents and catalysts can lead to minor heats of reaction and occur as an inherent result of multicomponent reactions (MCRs) and their extensions. They enable syntheses to be performed at a low energy level and the number of synthesis steps to be drastically reduced in comparison with ‘classical’ two-component reactions, fulfilling the rules of Green Chemistry. The very high potential for variability, diversity and complexity of MCRs additionally generates an extremely diverse range of products, thus bringing us closer to the aim of being able to produce tailor-made and extremely low-cost materials, drugs and compound libraries.
1. Prologue
The great discrepancy between the traditional feasibility mania and rational efficiency assessment in chemical synthesis became immediately apparent to the author while working on his thesis and during everyday laboratory life. On the one hand, classic peptide synthesis with an inefficient, extremely high number of synthesis steps and on the other hand, elegant multicomponent reactions that can make such efforts unnecessary. This double strategy in chemical synthesis has given the author cause to reflect (Figure 1) on how to close this gap using a consistent procedure. Thereby significant systemic differences in syntheses of chemical products play an essential role (Figure 2).
Figure 1.
Problem in chemical syntheses relating to terms of producibility, ecology and economy.
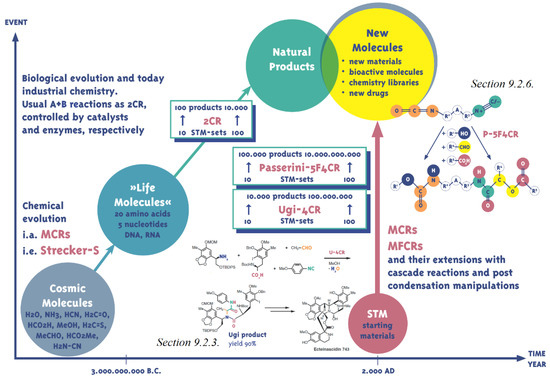
Figure 2.
Significant systemic differences in syntheses of chemical products by means of two- and multicomponent reactions, respectively.
2. Introduction
Special events in the author’s Vita runs synchronously alongside the scientific text and are indicated by alphabetically-ordered notes in Section 13. The scientific work carried out by the author spans a wide range and encompasses the development of new methods and reagents, as well as the defusing of hazardous substances. These methods and reagents flow into the section on syntheses by MCRs. Their applications in various areas of chemical synthesis include:
Tools, novel methods, catalysts and reagents to improve organic synthesis
- Vitamin B12 models. Structures, supernucleophilicity.
- Protective group techniques. Peptides such as β-lactam antibiotics, N-heterocyclic compounds and natural substances, such as camptothecin, using novel protective group techniques (2,2,2-trichloro-tert-butyloxycarbonyl (TCBOC) residue, cobalt-phthalocyanine).
- Metal phthalocyanines. Poisoning-resistant hydrogenation catalysts, palladium-phthalocyanine with three switchable, partially orthogonal catalysis patterns, alamethicine sequence.
- Peptide chemistry. Peptides such as human-β-endorphin sequences, Leu-enkephalin, polyamino acids and cyclopeptides, using the novel ferrocenylmethyl masking group.
- Triphosgene. Safe syntheses with triphosgene and their up-scaling processes.
- Synthesis efficiency. Quantitative assessment of a synthesis.
Concerted simplifying synthesis chemistry on MCR basis
- Multicomponent reactions (MCRs). Systemic and post-MCR extensions.
- MCR synthesis efficiency. Special preconditions. Algorithm on efficiency.
- MCRs’ future. Concerted simplification of chemical processes.
The individual sections are seamlessly meshed, and thus methodologically produce what are to some extent substantial synergistic effects. All these different advantageous methods and reagents listed in Section 3, Section 4, Section 5, Section 6, Section 7 and Section 8 are developed tools for efficient syntheses. They support multicomponent reactions in particular, Section 9, Section 10 and Section 11 through their systemic property which strongly reduces the number of synthesis steps and promoting simplification of synthesis chemistry. Nearly all referred literature in Section 9 and Section 11 are reviews about the relative domain.
3. Vitamin B12 Models
[A] The idea was to find a cobalt(I) complex [1] that demonstrated the reactive chemical properties of vitamin B12 and cobaloximes [2], as well as constituting a stable reagent or catalyst. The choice fell on the light- and color-fast pigment cobalt-phthalocyanin, CoPc, 1 [3] [B]. Its properties were then to be investigated, in particular the nucleophilicity of its anion [PcCoI]− 1a in the oxidation stage +1 of the metal [4].
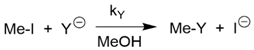
The nucleophilicity of [PcCoI]− was determined conductometrically [1]. Table 1 shows the comparison with standard strongly nucleophilic agents [5], vitamin B12s and its model cobaloxime(I), whereby 1a, together with the latter, has a value >10 and is therefore designated a supernucleophile.

Table 1.
Relative nucleophilicity nMeI = log (kY/kMeOH) for the following reaction at 25 °C [5].
4. Protective Group Techniques
Suitable protective groups play a central role in classic chemical peptide synthesis. Although the permanent protection of α-amino and carboxyl functions is well provided for by Z, BOC, ALOC, FMOC, TMS, benzyl and tert-butyl groups, there is still a need for selective orthogonal intermediary protective groups for the protection of amino acid third functions or special applications. A useful solution is provided here by using β-halogenalkyl and β-halogenalkoxy-carbonyl groups [6], which can be cleaved off by reduction in a weakly acidic environment, e.g., using zinc. The ideal protective group is one which is stable in acids and alkaline solutions, and not affected by catalytic hydrogenolysis, and which can be cleaved off under conditions that do not attack most of the other protective groups (orthogonality). This method was developed [7,8,9] and optimized [10,11,12,13] by Eckert and Ugi using selective reagents following the defined mechanism of reductive fragmentation as per Scheme 1.
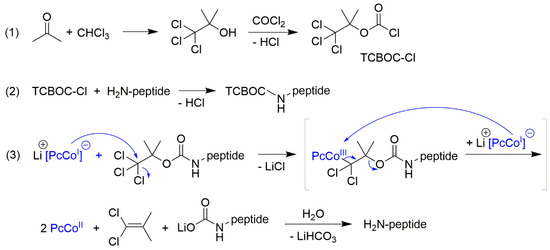
Scheme 1.
Introduction of TCBOC-residue (1) and its cleavage by means of 1b (3).
The lithium salt of Cobalt(I)-phthalocyanin, Li[CoIPc] 1b (Section 3), was the selected reagent which met all conditions: fast reaction at room temperature (RT) in neutral environments, no reaction with other functional groups (except for the nitro group), high product yields and complete regeneration capability of the fully stable reagent. The cleavage of the protective groups can also be carried out catalytically on CoIIPc 1 with NaBH4 [12]. As an optimal protective group, the acid and base-stable 2,2,2-trichloro-tert-butyloxycarbonyl-residue (TCBOC) was created, which is introduced via its stable chloride TCBOC-Cl and can be cleaved off in just 1 min using 1a (Scheme 1) [10]. Due to the extremely mild conditions, the method has been successfully used for the semi-synthesis of unstable β-lactam antibiotics, such as penicillin and cephalosporin derivatives (Figure 3) [13,14,15,16] [C].
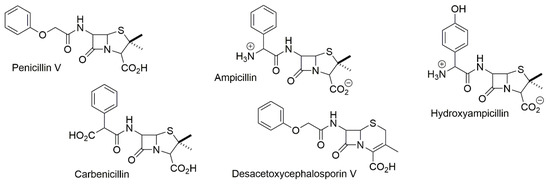
Figure 3.
Unstable β-lactam antibiotics synthesized using protective group technique of Scheme 1.
5. Metal-Phthalocyanines, Hydrogenation Catalysts, Palladium-Phthalocyanin
5.1. Cobalt-Phthalocyanine as Reagent
The reagent Li[CoIPc] stands out due to its almost unique selectivity: apart from the reductive fragmentation, practically only the nitro group is reduced to the primary amine function [17,18]. All other normal functional groups remain intact, which makes 1a particularly suitable for the in-situ preparation of aromatic o-amino-aldehydes in the Friedländer synthesis reaction. A significant improvement in the total synthesis of the frequently used anticancer drug camptothecin could be achieved (Scheme 2), whereby the yield of step 6 was increased from 20% to 62% [18] and up to 80%.
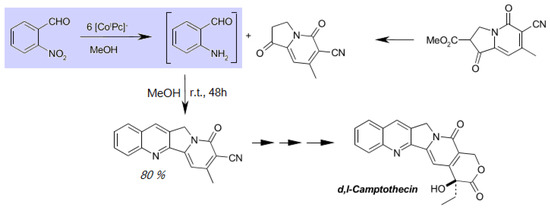
Scheme 2.
Camptothecin synthesis using 1a in a Friedländer synthesis forming o-amino-benzaldehyde.
5.2. Hydrogenation Catalysts
In order to determine the capabilities of the vitamin B12-related CoIIPc and analogous metal-phthalocyanines (MPc), the reactive and the reductive properties of MPc in particular were researched with the metals M = VO, Mn, Fe, Co and Pd as catalysts using the reduction agents hydrogen and sodium borohydride [19,20]. The pronounced poisoning resistance of CoIIPc against strong catalyst poisons such as I−, CN− or R-S− was impressive.
5.3. Palladium-Phthalocyanine [D]
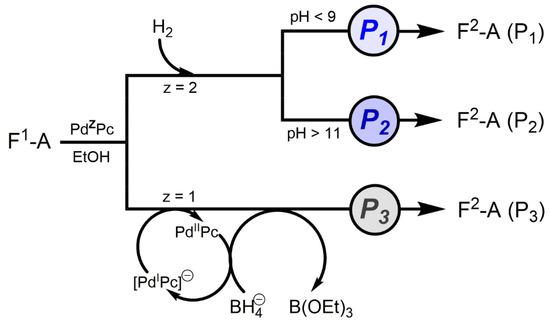
A prominent role is played here by palladium-phthalocyanin 2, which can act with three different switchable specificities as PdIIPc 2 and Na[PdIPc] 2a as well as 2a in alkaline environments (pH > 11, Figure 4), thereby generating three discrete catalyst patterns P1–P3 (Table 2 and Scheme 3) [20,21]. These are also partially orthogonal to each other and thus open up interesting new options for synthesis. This “catalytic doubling” has been successfully applied in a one-reactor reaction during the synthesis of the partial sequence [13-16], BOC-Aib-Pro-Val-Aib-OBz, of the peptide antibiotic alamethicin (Scheme 4).
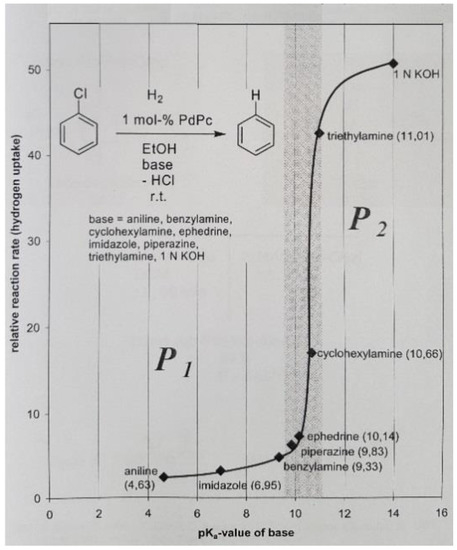
Figure 4.
Graph of the sharp crossover from P1 to P2 caused by the basicity of reaction medium.

Table 2.
Catalysis-pattern Pi (i = 1–3) of the catalyst PdZPc transforming A-F1 to A-F2 referred to Scheme 3. [a] Corbobenzoxy residue. [b] 2,2,2-Trichloro-tert-butoxycarbonyl residue. [c] Benzyl residue.
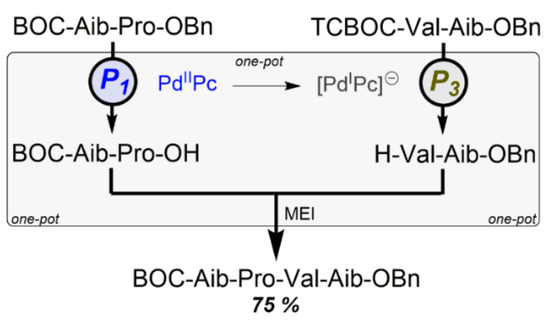
Scheme 4.
Reaction control by catalyst PdzPc in the one-pot synthesis of the alamethicin [13-16] sequence.
The starting substance was the protected dipeptide BOC-Aib-Pro-OBz. After P1, the benzyl residue is selectively cleaved off with H2 at 2. After adding of TCBOC-Val-Aib-OBn the TCBOC residue can be removed with inverse selectivity after P3 at 2a through the addition of NaBH4. This leaves the benzyl residue intact. The final coupling using morpholinoethyl isocyanide delivers the protected tetrapeptide with a yield of 75% via three steps in one reactor (Scheme 4). Synthesis efficiency (for this item see Section 8) is EffSynth = 75%, whereas that of the three single reactions with very good yields in [21] is EffSynth = 23% only. The 2/H2/EtOH system is air-stable and the catalyst PdIIPc can be quantitatively recovered and re-used without further treatment.
This means the catalyst 2/2a has an unusually high synergistic potential: using the same catalyst means that orthogonal reactions in a one-reactor method can proceed without prior isolation and preparation, thus saving two out of three practical synthesis steps! Therefore, the application of protective group techniques may no longer be an efficiency brake in syntheses; e.g., this method could help make the eurystatin synthesis in Section 9.2.4 even more efficient by saving up to three steps.
6. Peptide Chemistry—The Ferrocenylmethyl (Fem) Masking Group
Peptide synthesis is often faced with the problem of difficult or practical insolubility of sequences in solvents if corresponding conformations of the sequences can be taken. Preventing this requires reversibly masking the peptide bonds. This is most favorably achieved using the ferrocenylmethyl (Fem) residue [22], which can simply be introduced via the easily accessible ferrocenaldehyde on the amino function of the respective amino acid in a reductive alkylation reaction with H2 at PdIIPc and removed with trifluoroacetic acid in dichloromethane. Various human-β-endorphin sequences [23], Leu-enkephalin and hexaglycine [24] and the cyclopeptides cyclo-triglycine and cyclo-pentaglycine [25] could be produced with this method under mild conditions with far better yields than previously possible (Figure 5). Also of interest is the further useful application of PdIIPc in selective reductive alkylation. The Fem residue transfers modified properties here to the peptides:
- -
- Lipophilisation of the peptide bonds. The Fem peptides mentioned above dissolve well in standard non-polar solvents such as ethyl acetate and ethyl acetate/hexane mixtures, even to some extent in pure hexane! This effect is particularly favorable on peptide bonds of glycine [23,24,25].
- -
- Strong chromophore. Due to their high lipophility, Fem peptides can be easily and inexpensively purified using silica gel chromatography and ethyl acetate/hexane mixtures. Implementation is facilitated by the strong intrinsic orange colour of all Fem derivatives.
- -
- Electrochemical Detection (ECD). Detection following chromatography can also be done with ECD. The detection limit here is very low with 10−15 M (femto-molar!) [26]. The FeII in the ferrocene of the Fem residue is the electrophore.
- -
- Conformational effects. Strong steric hindrance and high lipophilicity of the Fem residue have a significant influence on the conformational effects of the Fem peptide.
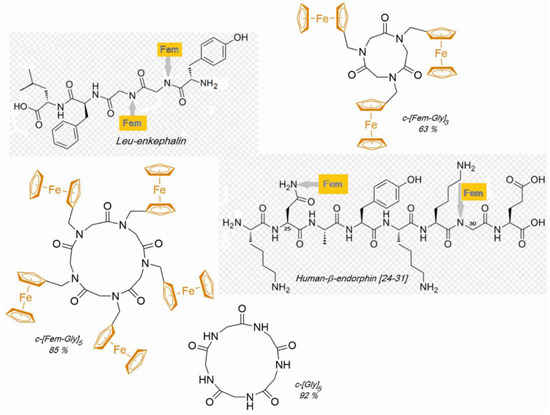
Figure 5.
Synthesis of peptides otherwise hardly accessible, via their Fem-derivatives.
During Leu-Enkephalin synthesis, masking of both peptide bonds on the Gly-Gly sequence prevents the formation of a side product that would occur more substantially without Fem masking [24]. In cyclo-triglycin synthesis, the strong effect of all-Fem masking is particularly evident: under mild reaction conditions (6 h at RT), the output is 63% cyclo-(Fem-Gly)3 from H-(Fem-Gly)3-OH. Cyclo-(FemGly)5 from H-(FemGly)5-OH runs with 85% yield, what from the free cyclo-(Gly)5 is obtained with 92% yield [25] (Figure 5). Also, a severe solubility problem during the synthesis of the octapeptide [24-31] sequence of human-β-endorphine has been solved successfully by using Fem-residues at [30] Gly and [25] Asn-positions (Figure 5) [23].
7. Triphosgene—A Safe Phosgene Substitute
[E] The elemental analysis of triphosgene suggested a molecular formula of COCl2, which was baffling and gave rise to the conclusion that this solid could be a solid phosgene. An IR spectrum provided information about the composition: it was not the cyclic trimeric hexachlor-1,3,5-trioxane, but rather bis(trichloromethyl)carbonate 3. The 13C-NMR spectrum with two signals confirmed this structure.
Synthesis of 3 was rather simple: radical chlorination of dimethyl carbonate (DMC, Scheme 5). The up-scaling of the reaction to a 20-L flask resulted in a yield per batch of approx. 20 kg (99%) triphosgene with a melting point of 80 °C [F]. DMC is a million-ton product that is industrially produced using methanol, CO and O2 (Scheme 5). Compound 3 could replace all actual reactions of phosgene [27,28,29], was therefore named as triphosgene and as a crystalline solid it is significantly safer to handle than the gaseous phosgene 4, as can be seen in Table 3.
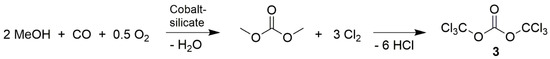
Scheme 5.
Triphosgene synthesis from dimethylcarbonate and chlorine.

Table 3.
Physical, chemical, thermochemical, and toxicity data of phosgene and triphosgene [28,29].
Triphosgene is preferably used to synthesize isocyanides applied in the production of important isocyanide-based IMCRs [27,28,29], as described in Section 9.2.
Contrary to most opinions, phosgene is not just produced during the thermal decomposition of triphosgene, instead there are two defined ways of transformation as per Scheme 6, whereby the thermal decomposition to phosgene, carbon dioxide and tetrachloromethane (pathway A) always occurs around approx. 200 °C and catalyses according to a 6-element mechanism, because as is thermodynamically determined, ΔH = −243 J·g−1. If the transformation is enabled at relatively lower temperatures of 80–120 °C, pure phosgene (pathway B) is output in a reconversion without reaction heat, ΔH = +9 J·g−1, if an appropriate catalyst is used that also blocks the 6-element mechanism [30].
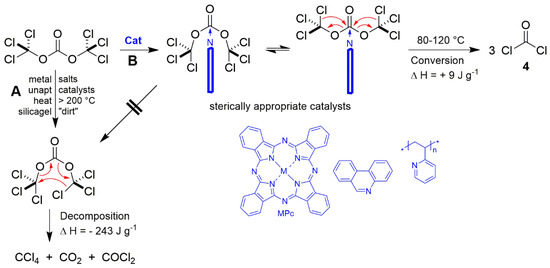
Scheme 6.
The two pathways of triphosgene transformation [29,30].
There are reactions and processes in synthesis chemistry that run better with phosgene than with triphosgene, as i.e., the production of high-chem isocyanato-isocyanides for MCR chemistry in Section 9.2.6 [28,29,30]. This process was easily up-scaled to the 5 kg range and a phosgene generator was designed for a 30 kg·h−1 throughput [29]. Thus, also the supply-chain of phosgene in bulk quantities becomes safer: triphosgene for transport and storage, phosgene as reagent. As a highly remarkable instance, MPc are also absolutely stable and re-usable catalysts for this process [G].
8. Synthesis Efficiency
The smallest unit, the cell, of chemical synthesis is the (synthesis) step. Its evaluation criteria are:
- -
- A logical synthesis plan with environmentally friendly processes and starting materials (STM), the least possible side products (additional reactions),
- -
- access to and price of starting materials,
- -
- cost of implementation and purifying the product and
- -
- foremost, the yield.
This is the sum criterion with a high intrinsic chemical component and the yardstick for every synthesis step. Its significance is well known by every chemist. The observer will drastically notice the relation between the overall yield yoa and the number of steps n as clearly shown in Table 4: To facilitate understanding, intermediate yields yav are applied on a case by case basis.

Table 4.
Overall yields yoa dependent on geometric average yields yav and numbers n of steps of syntheses.  highly profitable short syntheses,
highly profitable short syntheses,  usual pharma production,
usual pharma production,  high-chem research preparations.
high-chem research preparations.
 highly profitable short syntheses,
highly profitable short syntheses,  usual pharma production,
usual pharma production,  high-chem research preparations.
high-chem research preparations.
The number n of all product-generating steps is the second main criterion for assessing a synthesis. “The less, the better” is the motto here and most chemists do not realize how great the influence of the number of steps is on synthesis efficiency. As yield and step number are primarily factual criteria (mol or quantity), they provide a sound basis for evaluating a synthesis. In combination, they are a reliable criterion for the quantitative overall evaluation of a synthesis, i.e., its efficiency.
The synthesis efficiency EffSynth (Scheme 7) follows from the overall yield yoa and number of steps n of a synthesis, where the overall product yield equals the product of all the individual yields per step. A value which better reflects the yield is the average step yield yav. Scheme 7 represents the data for the camptothecin synthesis in Section 5.1 Scheme 2 [18] on the basis of these evaluation criteria.
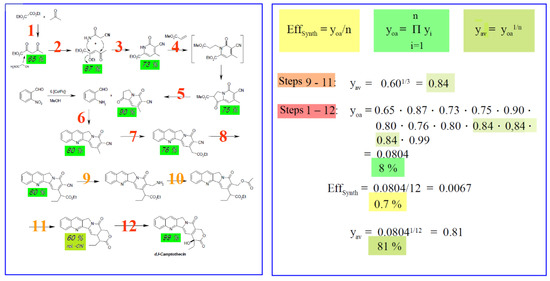
Scheme 7.
Efficiency of synthesis EffSynth of the camptothecin synthesis in Section 5.1 Scheme 2. yi: yield of one step; n: number of steps; yoa: over-all-yield over n steps; yav: geometric average yield over n steps; EffSynth: efficiency of synthesis.
Syntheses of a product D with various numbers of steps can vary greatly in efficiency, as shown in Scheme 8. Even when the yield of the MCR is only moderate, its synthesis efficiency still is twice as high as that of a 3-step synthesis with its good yield. The synthesis efficiency tool helps to assess and forecast synthesis schemes. Section 10 concerns the development of an algorithm relating to the efficiency of MCRs with regards to parallel reactions.

Scheme 8.
Case study of two ways of synthesis to form product D.
9. Multicomponent Reactions (MCRs)
The prominent role of Multi-Component Reactions (MCRs) in the chemical evolution of the range of cosmic molecules (Figure 2) to the molecules of life, such as amino acids and nucleobases, became clear as the selecting agents, the enzymes, were not yet available at that time. Biological evolution over billions of years consequently selected the pathway of the enzyme-controlled, highly selective two-component reactions. The domain of chemical synthesis that expanded exponentially over the past 200 years retained this scheme even though, since the middle of the 19th century, extremely important MCRs such as the Strecker and Hantzsch dihydropyridine syntheses, and the Biginelli reactions represented a yet small, but profoundly important part of the wealth of chemical reactions. Only the isocyanide-based Passerini and Ugi reactions (IMCRs) in the 20th century [31] gave access to a versatile and broad range of prospective syntheses. MCR chemistry has seen a very fast upswing (over 500 reviews) since the turn of the millennium, while the number of new MCRs and their application is increasing steadily, demonstrated in some reviews [32,33,34,35,36,37,38,39,40,41].
IMCRs [38,42,43,44,45,46,47,48,49,50], alkyne-based [38,51,52,53,54] and C-H-acidic compound [55,56,57,58,59,60]-based MCRs in particular have a high potential to be very diverse. Many novel MCRs [57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73] have meanwhile become part of the range of syntheses, e.g., boron-mediated [62,66], photo-induced [67] and carbene-based MCRs [72] such as N-heterocyclic carbenes (NHCs). They all increase the application options for MCRs, such as MCR nanosystems and mechanochemical reactions [65]. MCRs have been also applied in polymer [74], nucleoside [75] and carbohydrate [76] chemistry. Main field of application remains the production of heterocyclic compounds and many natural products by means of MCR [38,42,77,78,79,80,81,82,83,84,85,86,87,88], leading directly towards drug design and discovery [89,90,91,92,93]. Scheme 9 presents a current brief of well-known multicomponent reactions [35,40,55,90,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108].
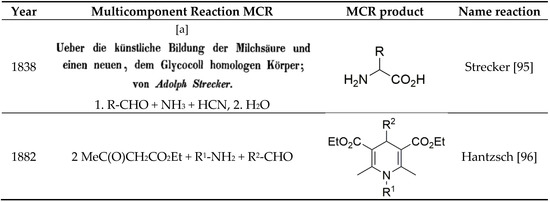
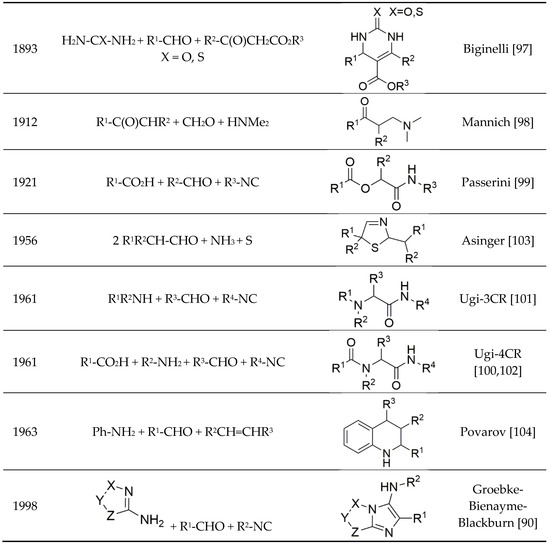
Scheme 9.
Well-known multicomponent reactions (MCRs). [a] On the Artificial Formation of Lactic Acid and a New Compound Homologous to Glycine; by Adolph Strecker.
9.1. Definition of MCR, Extensions and Optimal Reaction Conditions
Formally, every reaction with three or more reaction partners is a multicomponent reaction. The difference from 1CR and 2CR is easily understood, while the overall chemical reactions almost completely occur in the latter. Nature chose this pathway at a very early stage of evolution (Figure 2). All MCRs are characterised by the integration of all reaction partners (components C) in just one reaction product and, to put it succinctly, thus generate addition reactions which inherently do not show by-products. MCRs do not easily fit into the conventional way of thinking. Small molecules such as H2O can for example be released in the MCR. An exact definition for this effect still does not exist today. MCR mechanisms have been partly clarified but cannot be easily reproduced [35,109]. Statistical models for the kinetics fail because while third or higher grade collisions are highly improbable, MCRs nevertheless run smoothly and rapidly. The preferred interactions of the individual components with each other are often not yet fully understood but do deliver overall very functional reactions such as the Gewald reaction. The thermodynamic assessment of the MCRs [110,111] generally results in a “temperate balance” (cf. Bach’s “Well-Tempered Clavier”) where the reaction energies are slightly negative, reactions are self-propelled and do not require intensive thermal control. The atomic balance of the MCRs is usually excellent because all molecular parts remain present in the target molecule as structural elements. Variability, diversity and complexity of the target molecules are high [112,113] and can be controlled via the arrangement and nature of the MCRs. The distinct capacity for one-reactor syntheses also render MCRs fit for large-volume productions.
The efficiency of MCRs can be increased by extensions without adding additional reaction partners, because functional groups are present in the STM that react in Domino-reactions with the newly created functional groups in the MCR as post-condensation modification (PCM) and post-condensation cyclisation (PCC) [87,112,113,114,115,116,117,118,119,120,121,122]. Scheme 10 shows examples of several Tandem-U-4CR/Diels-Alder, Knoevenagel, Heck or UDC reactions as PCCs [87].
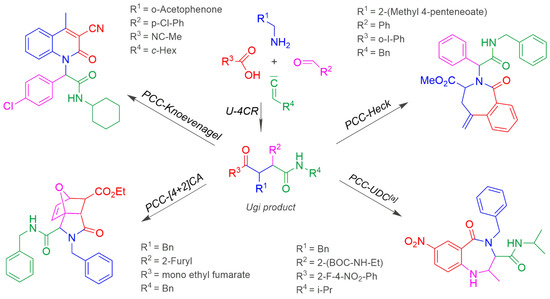
Scheme 10.
Post-condensation cyclisations (PCCs) of an Ugi-reaction. [a] UDC: Ugi deprotection cyclization. BOC (tert-butoxycarbonyl) protective group is cleaved by trifluoracetic acid.
Suitable solvents for MCRs are alcohols such as methanol and ethanol, water [123,124,125] and ionic liquids (ILs) [126,127,128,129,130,131], PEG-based ILs [132]. Unconventional solvents [133] such as trifluoroethanol [134] were also investigated. Solvent-free MCRs [135,136] also provide good results.
Even though MCRs normally run without catalysts, there are special applications for both various transition metal catalysts [79,116,137,138,139,140,141,142,143,144] and organic catalysts [79,145,146,147,148].
High pressure can at times improve the results of a few MCRs, in particular when domino-[4 + 2] or domino-[3 + 2] cyclic additions follow [149].
Various forms of the energy can be transmitted by excitation of certain bonds such as carbonyl or hydroxy and amino functional groups via: microwaves (MW) [38,150,151,152,153], infrared (IR) [154] and ultrasound (US) [38,155]. They can cause rather substantial effects on the MCRs. In this regard, there are some astonishing examples in connection to thermal energy [38].
Green concepts, based on rational considerations, are increasingly included in MCR chemistry [124,133,156,157,158]. There is a renaissance of catalyst-free synthesis [124].
All these criteria provide very good conditions for saving resources and energy, as well as environmentally-safe and friendly technology with regards to the environment and process and safety engineering. The atom balance is usually ideal. The key criterion in practice, synthesis efficiency, is calculated and evaluated in the following appropriate examples.
9.2. Several Natural Products and Drugs Syntheses via MCRs, Their Features and Comparisons
Some syntheses of natural products should contribute as examples to understanding the simplification of chemical synthesis. Efficiencies are calculated following the method in Section 8 and Section 10, the features of some individual syntheses are discussed and amazing initiatives to finding solutions for the concerted simplification of the synthesis chemistry are proposed. First, we will look at examples with a strong reduction of the number of steps.
9.2.1. Nifedipine and Xylocain Syntheses
In the ideal case, synthesis chemistry should produce a compound in one step. With nifedipine 5 this succeeded via Hantzsch-3CR (Scheme 11, Reaction (1)) [159]. An efficiency >50% is now possible for the first time. In the synthesis of xylocaine (6) the conventional two-step synthesis (2) via Ugi-3CR is reduced to one step (Reaction (3)); with half the number of steps, the yield of 6 is 80% [101].
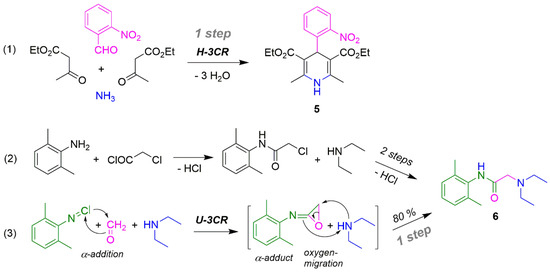
Scheme 11.
Simple drugs syntheses (1) and (3) via MCR and analogous reactions (2).
9.2.2. Shortened Crixivan Synthesis
Another example for a strong reduction of steps using an MCR is present in the synthesis of the HIV protease inhibitor crixivan. Using Ugi-4CR the important piperazine pharmacophore 8 could be used as a component in the large scale synthesis of crixivan (7) by the Merck Company and so the number of steps was reduced by six steps from 18 down to 12 (33%) (Scheme 12) [160,161].
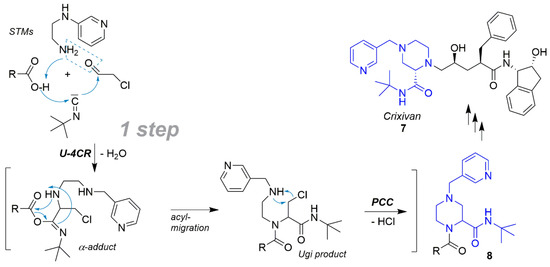
Scheme 12.
Important piperazine pharmacophore 8 for shortened Crixivan synthesis.
In both the following syntheses in Section 9.2.3 and Section 9.2.4, the focus is on the number of steps and yield of the synthesis with rational efficiency calculation.
9.2.3. Eicteinascidin 743 Total Synthesis
The key step of the synthesis of the natural compound ecteinascidin-743 (12) is a U-4CR based on Scheme 13 [162], delivering the Ugi product 11 with a 90% yield.
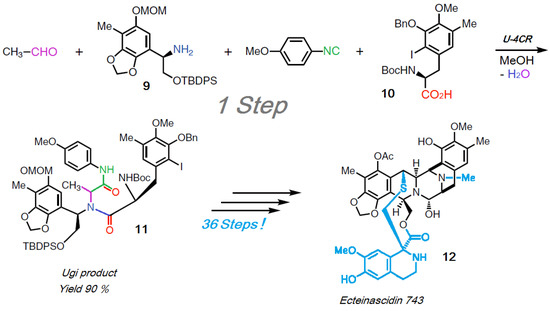
Scheme 13.
Total synthesis of eicteinascidin 743.
2/3 (24 C-atoms) and a greater part of the scaffold of the target product 12 (36 C-atoms) are generated in one step, presented together with the concentrated synthesis capacity of this U-4CR. For the rest of the synthesis another 36 traditional steps (!) are needed. Luckily, all synthesis data were complete and available in the publication [162] so the efficiency assessment based on this was exact.
The complete synthesis of 12 via 45 steps gives an overall yield of yoa = 0.076% and an efficiency of EffSynth = 0.0017%. That is extremely low, but the completely distinct distribution of the numbers on the synthesis sections is more interesting: while it brings the synthesis of 9 and 10 and their coupling by means of U-4CR to 11 (and so 2/3 of the TM 12) with nine and eight steps at yoa = 44% and EffSynth = 5%, the values practically dwindle in the following 36 steps towards 12, yoa = 1.9%, EffSynth = 0.053%. One recognises very clearly the steep decline caused by the outright destructive influence of the high number of steps. It would be urgently necessary to consider whether and how the number of synthesis steps could be extremely reduced using higher order MCRs [163] and in particular their combined applications [164] and tools, such as multi-catalysis (Section 5.3).
9.2.4. Smart Eurystatin Synthesis
This synthesis of natural product eurystatin (13) [165] is well matured and reasonably short thanks to intelligent chemical reactions considerations. Except for an additional carbonyl function group there is a cyclopeptide-derivative from alanine, leucine and ornithine, derivatized with 2-isoocteneoic acid. The retrosynthesis already indicates a P-3CR being the precursor molecule in the synthesis (Scheme 14).
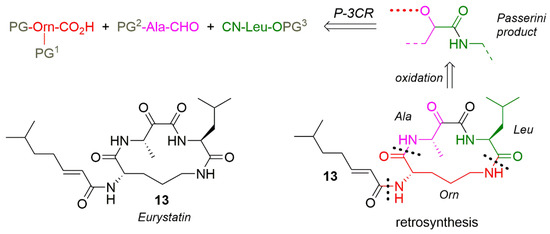
Scheme 14.
Retrosynthesis of eurystatin, indication for P-3CR.
The smart application of the thermodynamically driven O-N-acyl migration of the ornithin derivative from the hydroxyl group in the Passerini product 14 to the vicinal amino group of the alaninol to 15 saves several conventional synthesis steps (Scheme 15). Cyclisation of 15 by coupling after cleavage of Bn- and Z-residues succeed directly the ring closure of the eurystatin scaffold. The efficiency balance can be presented as: overall yield yoa = 29%, eight steps, synthesis efficiency EffSynth = 3.6% and average yield yav = 86%.
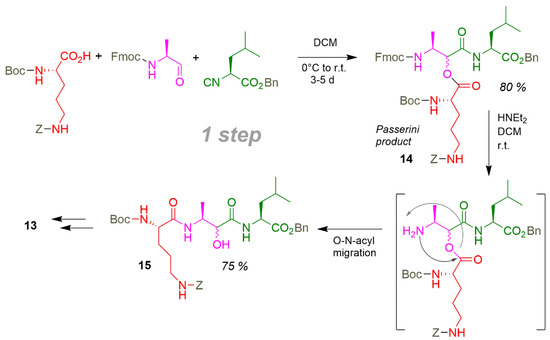
Scheme 15.
Smart P-3CR/deprotection/acyl-migration sequence in the synthesis of eurystatin.
As the greater part of the synthesis consists of a classical protective groups based peptide synthesis, application of multi-functional catalysis from Section 5.3 (Scheme 4) could result in the addition deletion of three steps down to five.
9.2.5. Tandem-U-4CR/IMDA/ROM-RCM Synthesis
This synthesis is an example of highest diversity and complexity of the target molecule, which through suitable combination (Scheme 16) of a U-4CR, Domino-Diels-Alder and a ROM-RCM reaction in three steps via the products 16 and 17 generated two annulated azepinone, furan and pyrrole heterocyclic compounds in product 18 [166] and thus delivered the starting approach for a reliable concerted simplification methodology of the synthesis chemistry. The efficiency data, yoa = 41%, EffSynth = 14%, are very good with regards to the outstanding structural result.
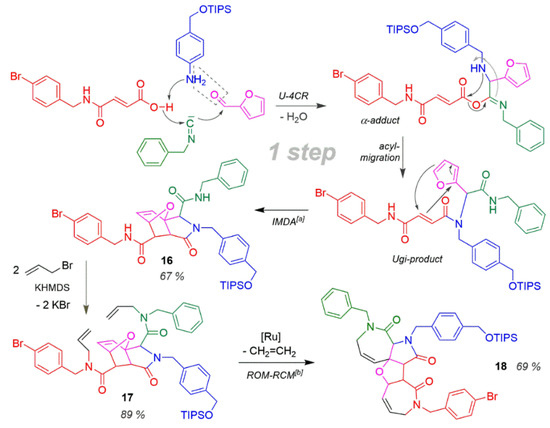
Scheme 16.
Tandem-U-4CR/IMDA/ROM-RCM synthesis. [a] Intramolecular Diels-Alder reaction. [b] Ring opening metathesis—ring closure metathesis.
9.2.6. Multi-Function-Component Reactions (MFCRs) [H,I]
The intrinsic extension of MCRs leads to an increase of functional groups of the MCR and hence an increase in functional density. When auto-balancing functional groups such as amino and carboxylic functional groups in amino acids (AA) are concerned, the respective functional group for a reaction must first be released or activated [48,167].
This is different from the orthogonal highly active functional isocyanate group (strong electrophile) and isocyanide (strong nucleophile) which within one molecule I-I (isocyanato-isocyanide) are both looking for suitable partners for the MFCR (multifunctional component reaction). For the nomenclature of the MFCR refer to [38,39]. Scheme 17 introduces the I-I 19, which is produced from formamido-amine by means of phosgene (Scheme 5) [H]. Syntheses of Lysin-derivatives 20 und 21 succeed in the same manner. 19 reacts in an additions/Passerini reaction P-5F4CR and delivers the additions/Passerini product 22 with a 92% yield [39,168]. The increase of density of functions is accompanied by a grave decrease of synthesis steps compared with a sequential synthesis [I].
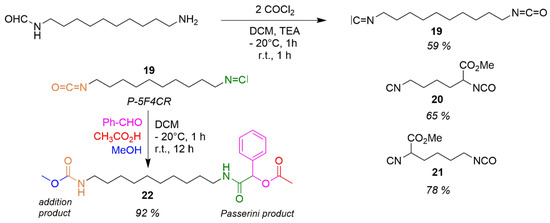
Scheme 17.
Multi-function-component reactions (MFCRs) [39].
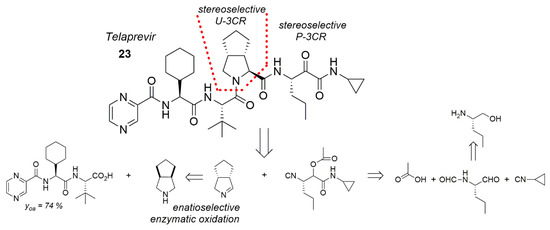
9.2.7. Telaprevir, Combined MCRs for a 50% Shorter Synthesis
The HCV NS3 protease inhibitor telaprevir (23) used against hepatitis C could be synthesized in less steps by combining a Ugi-3CR, Passerini-3CR and a biocatalysis (Scheme 18) [169]. Thus, the synthesis route could be shortened by more than 50% vs. hitherto predominantly peptide-chemical syntheses [170]. Particularly the “right” section of 23 could be led by stereoselective U-3CR and stereoselective P-3CR as well as enantioselective enzymatic oxidation on the shorter road to success.
Scheme 18.
Telaprevir synthesis by combined U-3CR/P-3CR/enzymatic catalysis.
9.2.8. One-step Syntheses of Oxazepinones from Carbohydrates and α-Amino Acids
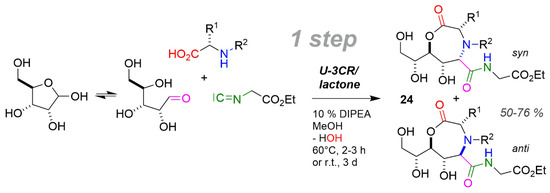
The strong dependence of carbohydrate chemistry on protective groups is well known [171], but MCRs like Ugi reactions are insensitive to hydroxy groups and thus appropriate for application with carbohydrates as carbonyl component to save material synthesis steps. This has been achieved in a one-step U-3CR/lactonisation of D-ribose with α-amino acids (AA) and ethyl isocyanoacetate (Scheme 19) [76,172], furnishing oxazepinones 24 in yields of around 50% up to 76%. The syn/anti values of 24 are 67/33 to 91/9. For the mechanism of the U-3CR see Scheme 11 Reaction (3). The reward of this high-performance synthesis is an exceptionally high efficiency of up to 76%.
Scheme 19.
Carbohydrate carbonyl in U-3CR/lactonisation one-step synthesis of oxazepinones.
9.2.9. Mild Esterification by P-3CR
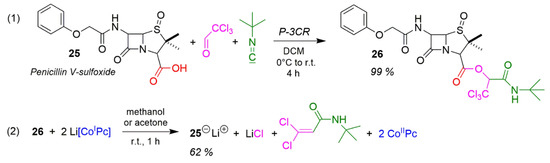
The presentation of the diversity of methodologies in the MCR domain is rounded off by the application of the Passerini reaction which can solve so many practical problems, notably the esterification under extremely mild conditions according to Scheme 20 Reaction (1) [13,173]. This reaction enables simple and high-yield esterification of readily decomposing β-Lactam antibiotics with excellent yields. The reaction between penicillin V-sulfoxide, chloral and tert-butyl isocyanide produces the penicillin-V-sulfoxide 1′-tert-butylaminocarbonyl-2′,2′,2′-trichlorethylester 26 as colourless crystals with a yield of 99%. Cleaving off the alkyl residue (Reaction (2)) furnishes 25 with a yield of 62% [13]. The mechanism of removal works according to Scheme 1.
Scheme 20.
Application of P-3CR for esterification reactions.
10. Synthesis-Efficiency of MCRs
Naturally, all calculations in Section 8 apply fully to the MCR syntheses. Parallel reactions originating from the synthesis of the precursors of the components C > 1 can be added. One 3CR can therefore have up to two, and one 4CR up to three, parallel reactions in their precursor syntheses. As the parallel reactions are not sequential, they do not have a multiplier character regarding yields and efficiency, but must be suitably included in the overall calculation, which is a summation. The partial sections (of the parallel reactions) are incorporated into the algorithm as weight-averaged yields of the arithmetic mean values of the parallel reactions. The number of steps is simply the sum of all steps in the respective section. The algorithm can be expressed as a mathematical equation for the synthesis efficiency as shown in Figure 6.
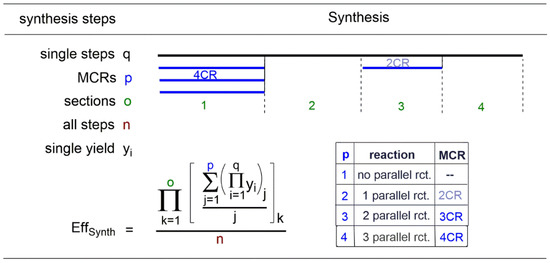
Figure 6.
Algorithm “Sythesis efficiencies including MCRs” phrased as mathematical equation.
The quantitative synthesis efficiency EffSynth specifically refers only to the concrete criteria of yield and number of steps, and is therefore particularly reliable and independent of other “soft” evaluation criteria that can complement this value. Regarding the reaction work-up, MCR products can mostly be easily isolated and purified. The lack of akin products could be explained by MCRs nature as formal addition reactions and their “well-tempered” reaction enthalpies. The latter are the result of a balanced system of several reaction-parts acting with each other (see Section 9.1).
Evaluating syntheses in Section 9.2.1, Section 9.2.2, Section 9.2.3, Section 9.2.4, Section 9.2.5, Section 9.2.6, Section 9.2.7, Section 9.2.8 and Section 9.2.9 in cumulo with regards to their over-all and embedded MCRs efficiencies and in comparison with each other, it is significantly evident, that MCRs are much more able than 2CRs, to generate main parts of complex products, as demonstrated in Section 9.2.2, Section 9.2.3, Section 9.2.4, Section 9.2.5 and Section 9.2.7. Also, complete products can be synthesized in one-step MCRs, as shown in Section 9.2.1. Even highly diverse products are easily produced from simple and low-cost STMs by a one-step MCR, as evidenced in Section 9.2.8. Another progress has happened by synchronously running of two orthogonal reactions at the same molecule, presented in Section 9.2.6.
The requirements of Green Chemistry are fulfilled, such as excellent atom-efficiency, the avoidance of waste, better performing compounds, eco-compatible solvents, renewable materials, and energy reduction.
11. Multicomponent Chemistry—The Future
Section 9 presents the diversity of prospects for MCR chemistry in a systematic (Scheme 9 and Scheme 10) and a perspective manner using illustrative examples (Scheme 11, Scheme 12, Scheme 13, Scheme 14, Scheme 15, Scheme 16, Scheme 17, Scheme 18, Scheme 19 and Scheme 20 The multitude of reactions and their combinations with each other and within biology opens up the exponentially growing scope of reactions for this methodology [36,109,113,174].
If one links the exponential growth of potential MCRs with their intrinsic exponentially increasing numbers of products, one can quickly discern the unique potential of this methodology which enables the production and synthesis at will of molecules of whatever structure and quantity. Syntheses via the MCR methodology allow us to achieve what Nature has already been doing for a very long time in its tried and tested manner. In comparison, the possibly virtually unlimited number of products will be attained relatively fast. Part 2 of the chemical evolution (Figure 2) lies ahead and this time humans are involved. How do we achieve the afore mentioned opportunities and goals?
- Creation of further MCRs and MFCRs that can create numerous structural elements of chemical compounds and show high diversity.
- Creation of further MCRs of a higher order (with as many components as possible) that will exponentially increase the variability and the quantity of products.
- Creation of further MFCRs with as many functional groups as possible that will strongly increase the complexity of products in particular.
- Combining MCRs with each other in a rational way and number thus making it feasible to extremely decrease the number of synthesis steps.
- Creating an algorithm in order to verify procedures 1 through 4.
- New tandem, domino and cascade reactions to extend the MCRs or their combinations, which will further strongly decrease the number of synthesis steps. This will result in double the reduction in steps in conjunction with the MCR combinations.
- Process development of one-reactor synthesis as a tool to reduce the number of synthesis steps.
- Multifunctional catalysts and multi-catalysis cascades (MCC) as tools to reduce the number of synthesis steps.
- High pressure as tool for increasing scope and yield of certain MCRs.
- Identifying and developing new tools to simplify production.
- Rational and practice-based efficiency calculation in the prognosis of planned and the analysis of syntheses performed with a feed-back effect on synthesis optimisation.
In order to support the aforementioned concept, important known data will be determined:
- 12.
- Databases (e.g., SciFinder) with information about the target products and intermediates of the synthesis plan.
- 13.
- Databases (e.g., SciFinder) about the applications of the planned MCRs and their scope and limitations. Synthesis data and synthesis protocols, references and details.
- 14.
- Drug Design & Discovery.
- 15.
- Specialist literature for understanding MCRs and their mechanisms, including probable mechanisms, in order to assess undesired side reactions.
With respect to 1: New and in particular novel MCRs [38,45,61,81,82,112,113,154,157,174,175,176,177,178] are the basis and guarantee for high diversity and therewith the capacity to produce many structurally-different compounds. A method has been found to design MCRs going from libraries of compounds to libraries of reactions [176].
With respect to 2: In order to obtain very high product quantities using the MCRs (libraries), MCRs of a higher order with component numbers >4 are particularly suitable [163], such as 5CR [179], 7CR [180] and 8CR [181]. Table 5 shows the relationship between exponential components and product numbers.

Table 5.
Calculated products as function of component C or function F numbers in MCRs.
With respect to 3: As much as possible high complexity can be obtained by as many as possible functional groups on the components of the MCR. Just one additional functional group already causes a new dimension in the scope of reactions [39,112,113,174,182]. Further to the functional density, one has to look at the reactivity. While passive “pairs” such as amino and carboxylic groups (AAs) first have to be activated, active “pairs” such as isocyanate-isocyanide are able to enter 2 different MCRs or reactions in a highly selective way (Scheme 17) [39].
With respect to 4: The well-planned combination of MCRs (unions) [170,182,183,184,185] opens up a further method for reducing the number of synthesis steps with simultaneous structural assembly based on a modular building concept, such as Scheme 18.
With respect to 5: The relative reactivities of the functional groups of the MCRs are important criteria for an algorithm which is useful when looking for new MCRs including computer-aided methods [186]. Unexpected products lead to new MCRs. Algorithm-based methods for the discovery of novel multicomponent reactions [187,188].
With respect to 6: During MCR development, suitable domino reactions are also designed, creating indole and steroid alkaloids [122]. Functional π-systems [189], norbornene [190] and A3-coupling [115] with MCRs/domino reactions were deployed for DOS. Proline-catalysed domino reactions are used for the synthesis of heterocyclic groups using MCRs [117]. MCR/domino processes develop through rational design and serendipity [114].
With respect to 7: The advantages of MCR one-pot processes are demonstrated in several examples of the production of pharmaceutical products [164,191].
With respect to 7 + 8: Suitable multifunctional catalysis can greatly contribute to make low-step syntheses using MCR. This has been performed in one-pot sequential reactions [164].
Scheme 4 shows another convincing example. The process reduces a classical synthesis sequence of the peptide chemistry from three steps down to one! The efficiency of the synthesis is three times higher than the 3-step synthesis. This is achieved using the switchable catalyst palladium-phthalocyanin. The method chosen can be generally and widely used for the synthesis of peptides, and can be repeated successively in a single reactor if necessary.
With respect to 9: Some MCRs could only be performed when high pressure catalysis was applied [149].
With respect to 11: Three multicomponent reactions, among them the Strecker reaction (without any catalyst), have proven to be very efficient in the generation of a diversity of polyfunctionalized molecules [192]. A review provides an overview of the exploitation of multicomponent reactions for the synthesis of nonsteroidal anti-inflammatory drugs: Multicomponent reactions are more efficient, cost effective and economical than traditional methods [193,194].
In Section 8 and Section 10 an algorithm has been developed to calculate synthesis efficiency based on hard criteria overall yield and steps of a synthesis. The algorithm also takes parallel reactions and MCRs and delivers an exact result of the efficiency even on comprehensive and complex syntheses.
With respect to 14: Many capabilities and properties of MCRs make them particularly suitable for Drug Design and Discovery [91,177,188,195,196] primarily with the higher order MCRs, which can easily generate very large libraries as Table 5 clearly indicates. The availability of MCRs has become so diverse that, in combination with each other and with post condensation modifications such as tandem, cascade or domino reactions, we can now look at an extremely high structural diversity of products.
12. Conclusions
Research results relating to the aforementioned points are piling up in the literature. The time has now come to coordinate these highly valuable individual results from the most diverse domains of organic (Section 3, Section 4, Section 5, Section 6, Section 7 and Section 8) and MCR chemistry (Section 9, Section 10 and Section 11) in accordance with the above-mentioned 15 points-scheme, to bundle the already strong individual effects and to concentrate on the actual synthesis. Under the fulfilment of Green Chemistry requirements (Section 10) the concerted simplification of the chemical/biological synthesis (Figure 7) will subsequently take great strides, providing mankind, science and the economy with tailor-made and keenly low-cost materials, drugs and chemistry libraries.

Figure 7.
Solution of the discrepancy in Section Prologue in performing syntheses by concerted simplification in an elegant and highly efficient way.
13. Biographical Data of Heiner Eckert
A. During my search for a thesis in the Faculty of Chemistry at the Technical University of Munich, the newly appointed Professor Ivar Ugi, who wished to be on first-name basis, immediately offered me several topics for selection, all based around his 4CC method, the Ugi four-component condensation. A novel protective group technique needed to be developed and integrated within the 4CC-based synthesis of peptides. Ugi had just brought this idea with him from California where he was still supervising several PhD theses at his former base, the University of Southern California. While there, he met his old friend Gerhard Schrauzer (Professor at the University of California) who was researching the synthetic function-analogous complexes of vitamin B12, the cobaloximes. As this interdisciplinary project fascinated me as a “greenhorn”, I enthusiastically accepted, not realising what it meant to gain a foothold as the sole participant in this project—a valuable experience!
B. I had the idea, to employ the dye-stuff cobalt-phthalocyanine as “technical vitamin B12” into the project, and this opened self-reliant work for me, which was strongly supported by Ugi.
C. The ability of the new protecting groups technology which I developed to produce highly sensitive compounds such as β-lactam antibiotics (Figure 3) made me very proud. These particular characteristics of the reduced metal-phthalocyanines, and their exceptional chemically reactive features, drew the interest of Bayer AG, who have since, together with Ugi and myself, registered various patents, both in the area of pigment production [14] and in the field of chemical semi-synthesis of β-lactam antibiotics [15,16]. The respective work at Bayer was stopped after the biochemical synthesis processes were established. This decision taken by the global player already shows the limits of practicability of classic chemical multi-stage synthesis. Meanwhile I achieved my doctorate as “Dr. rer. nat.” with the grade “summa cum laude” with Ugi.
D. Up to this day I consider the switchable specificity of the catalyst PdPc between three partly orthogonal catalyst patterns with their multiple application patterns as a special highlight of my work in this domain. It constitutes the basis of the “Eckert Hydrogenation Catalysts” [20].
E. One of the first preparations on the agenda at the start of my thesis work was the production of several 100 g of a chlorocarbonic acid ester from phosgene and an alcohol. Concern was manifest and initial considerations for a substitute began to arise. But it took another 10 years before an opportunity appeared. During the production of diphosgene, a substitute for phosgene that had just arrived on the market, a small amount of white solid remained following distillation and I decided to analyze it instead of discarding it.
F. These results encouraged me to sell triphosgene as a commercial phosgene substitute via my newly founded company Dr. Eckert GmbH and the chemicals trade reacted very rapidly, increasingly taking over distribution. After several years, China entered production and currently provides the entire world with thousands of tons of triphosgene.
G. In order to be able to greatly extend the business activities of the company, I took an interested investor on board who shortly afterwards wanted to proceed doing business on his own. And thus, at the end of the millennium, I sold the company for a share in its profits to the investor.
H. The very first and successful production of isocyanato-isocyanide really was the most exciting experience of my work history in chemistry. The contradictory existence of these compounds within one molecule was assumed to be impossible by many colleagues. Two such strong, but incompatible functional groups should not be able to coexist and would react with each other at very moment of formation. This made the analytical confirmation feel even sweeter.
I. After a comprehensive and diverse research in chemistry done over three decades I had time to perform the overdue habilitation [25] achieving the “venia legendi” in chemistry at Technical University of Muenchen (TUM) at the age of 59 years (Figure 8).

Figure 8.
Heiner Eckert at the laboratory [J].
J. After two years in pension I accepted the call of the biochemistry start-up-company BSAZ Biotech (Hangzhou) Ltd. in China to become its Vice President. In August 2015, I was presented with the “Quianjiang Friendship Award 2015” for foreign experts in the city of Hangzhou and the “West Lake Friendship Award 2015” of the government of Zheijang Province in October.
Acknowledgments
Thanks to Fine Heininger (Denken & Handeln) and Madeleine P. Potganski (Kleine Einheit) for technical support with the manuscript.
Conflicts of Interest
The author declares no conflict of interest.
References
- Eckert, H.; Ugi, I. Phthalocyaninecobalt(I) Salts, “Supernucleophilic” Vitamin B12 Models Stable in Neutral Medium. Angew. Chem. Int. Ed. 1975, 14, 825–826. [Google Scholar] [CrossRef]
- Schrauzer, G.N.; Deutsch, E. Reactions of cobalt(I) supernucleophiles. The alkylation of vitamin B12s, cobaloximes(I), and related compounds. J. Am. Chem. Soc. 1969, 91, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- Lever, A.B.P. The phthalocyanines. Adv. Inorg. Chem. Radiochem. 1965, 7, 27–105. [Google Scholar]
- Taube, R.; Drevs, H.; Tran, D.-H. Organometallic phthalocyanines of iron and cobalt. Z. Chem. 1969, 9, 115–116. [Google Scholar] [CrossRef]
- Pearson, R.G.; Sobel, H.; Songstad, J. Nucleophilic reactivity constants toward methyl iodide and trans-dichlorodi(pyridine)platinum(II). J. Am. Chem. Soc. 1968, 90, 319–326. [Google Scholar] [CrossRef]
- Woodward, R.B. Neueste Fortschritte in der Chemie der Naturstoffe. Die Totalsynthese des Cephalosporins C. Nobel-Vortrag am 11 Dezember 1965. Angew. Chem. 1966, 78, 557–564. [Google Scholar] [CrossRef]
- Eckert, H.; Ugi, I. A New Technique of Protecting Groups—Cleavage of ß-Halogenated Alkyl Esters with Supernucleophilic Cobalt(I)-phthalocyanin. Angew. Chem. Int. Ed. 1976, 15, 681–682. [Google Scholar] [CrossRef]
- Eckert, H.; Lagerlund, I.; Ugi, I. β-Haloalkyl Groups as Functional Protection in Peptide Synthesis. A Kinetic Study of the Reaction of the Cobalt-(I)-phthalocyanine Anion with Organic Halides. Tetrahedron 1977, 33, 2243–2247. [Google Scholar] [CrossRef]
- Kellner, H.A.; Schneiderwind, R.G.K.; Eckert, H.; Ugi, I. Bis(2,2,2-trichlor-1,1-dimethylethyl)monochlorphosphat, ein selektives Reagens fuer Phosphorylierung und Schutz der 5′-OH-Gruppe von Nucleosid Derivaten. Angew. Chem. Int. Ed. 1981, 20, 577–578. [Google Scholar] [CrossRef]
- Eckert, H.; Listl, M.; Ugi, I. The 2,2,2-Trichloro-tert-butyloxycarbonyl Group (TCBOC)-An Acid- and Base-Resistant Protecting Group Removable under Mild Conditions. Angew. Chem. Int. Ed. 1978, 17, 361–362. [Google Scholar] [CrossRef]
- Eckert, H.; Ugi, I. Spaltung β-halogenierter Urethane mit Kobalt(I)-phthalocyanin; eine neue Schutzgruppentechnik fuer Peptid-Synthesen. Liebigs Ann. Chem. 1979, 278–295. [Google Scholar] [CrossRef]
- Eckert, H.; Kiesel, Y. Redox-Katalysator Kobalt-phthalocyanin: Deblockierung von 2-Halogenalkyl-Verbindungen. Synthesis 1980, 947–949. [Google Scholar] [CrossRef]
- Eckert, H. Aeusserst selektive und schonende Abspaltung von β-Halogenalkyl-Gruppen mittels Cobalt(I)phthalocyanin-anion bei Semi-synthesen von β-Lactam- Antibiotica. Z. Naturforsch. 1990, 45b, 1715–1724. [Google Scholar]
- Seidler, H.; Wunderlich, K.; Eckert, H.; Bayer, A.G. Verfahren zur Herstellung von Komplexverbindungen der Kobaltphthalocyanin-Reihe. German Patent DE 2555243, 9 December 1975. Chem. Abstr. 1977, 87, 153424. [Google Scholar]
- Eckert, H.; Kabbe, H.-J.; Ugi, I.; Bayer, A.G. Verfahren zum Schutz Reaktionsfaehiger Amino-, Hydroxyl- und/oder Carboxyl-Gruppen. German Patent DE 2619247, 30 April 1976. Chem. Abstr. 1978, 88, 61674. [Google Scholar]
- Ugi, I.; Eckert, H.; Bayer, A.G. Verfahren zum Schutz Funktioneller Gruppen. German Patent DE 2747724, 25 October 1977. Chem. Abstr. 1979, 91, 91954. [Google Scholar]
- Eckert, H.; Schier, A. Cobalt(I)-phthalocyanine Anion as Vitamin B12s Model: Selektivity in Reactions with Electrophiles. Angew. Chem. Int. Ed. 1979, 18, 794–796. [Google Scholar] [CrossRef]
- Eckert, H. Selective Reduction of the Nitro to the Amino Functional Group by means of the phthalocyanin-Cobalt(I) Anion; Synthesis of N-Heterocycles and Alkaloids. Angew. Chem. Int. Ed. 1981, 20, 208–210. [Google Scholar] [CrossRef]
- Eckert, H.; Kiesel, Y. Stable Metal-Phthalocyanines as Poison-Resistant Catalysts in homogenous Catalysis: Reduction of organic compounds with NaBH4. Angew. Chem. Int. Ed. 1981, 20, 473–475. [Google Scholar] [CrossRef]
- Eckert, H. Eckert Hydrogenation Catalysts. In Organic Syntheses Based on Name Reactions; Tetrahedron Organic Chemistry Series; Hassner, A., Stumer, C., Eds.; Pergamon (Elsevier Science): Amsterdam, The Netherlands; New York, NY, USA, 2002; Volume 22, p. 97. [Google Scholar]
- Eckert, H.; Fabry, G.; Kiesel, Y.; Raudaschl, G.; Seidel, C. Reaction Control by Catalysts with Variable Specificity: Stable Palladium-Phthalocyanin as Hydrogenation Catalyst with Three Catalytic Patterns. Angew. Chem. Int. Ed. 1983, 22, 881–882, Angew. Chem. Suppl. 1983, 1291–1314. [Google Scholar] [CrossRef]
- Eckert, H.; Seidel, C. The Ferrocenylmethyl (Fem) Group as a Highly Lipophilic and Chromophoric Group for the Masking of Peptide Bonds. Angew. Chem. Int. Ed. 1986, 25, 159–160. [Google Scholar] [CrossRef]
- Eckert, H.; Kiesel, Y.; Seidel, C.; Kaulberg, C.; Brinkmann, H. New Strategy for Peptide Synthesis Using Highly Lipophilic and Chromophoric Groups. Synthesis of Octapetide Sequence [24-31] H-Lys-Asn-Ala-Tyr-Lys-Lys-Gly-Glu-OH of Human ß-Endorphin. In Chemistry of Peptides and Proteins, Volume 3, Proceedings of the 5th USSR-FRG Symposium on Chemistry of Peptides and Proteins, Odessa, USSR, 16–21 May 1985; Voelter, W., Bayer, E., Ovchinnikov, Y.A., Ivanov, T., Eds.; De Gruyter: Berlin, Germany; New York, NY, USA, 1986; pp. 19–28. [Google Scholar]
- Eckert, H.; Forster, B.; Seidel, C. Vollstaendige Maskierung der -Gly-Bindungen mit dem stark lipophilen und chromophoren Ferrocenylmethyl-[Fem]-Rest bei Peptidsynthesen von Hexaglycin und Leu-Enkephalin. Z. Naturforsch. 1991, 46b, 339–352. [Google Scholar]
- Eckert, H. Old and Novel Reagents and Reactions—Results from Methods Development; Habilitationschrift. Technische Universitaet Muenchen 2005. [Google Scholar]
- Koller, M.; Eckert, H. Derivatization of Peptides for their Determination by Chromtographic Methods. Anal. Chim. Acta 1997, 352, 31–59. [Google Scholar] [CrossRef]
- Eckert, H.; Forster, B. Triphosgene, a Crystalline Phosgene Substitute. Angew. Chem. Int. Ed. 1987, 26, 894–895. [Google Scholar] [CrossRef]
- Cotarca, L.; Eckert, H. Phosgenations—A Handbook; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Eckert, H. Phosgenation reactions with phosgene from triphosgene. Chim. Oggi 2011, 29, 40–46. [Google Scholar]
- Eckert, H.; Auerweck, J. Solvent-free and safe process for the quantitative production of phosgene from triphosgene by deactivated imino-based catalysts. Org. Process Res. Dev. 2010, 14, 1501–1505. [Google Scholar] [CrossRef]
- Ugi, I. (Ed.) Isonitrile Chemistry; Academic Press: New York, NY, USA, 1971.
- Zhu, J.; Bienayme, H. (Eds.) Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005.
- Müller, T. Science of Synthesis. Multicomponent Reactions 1; Thieme: Stuttgart, Germany, 2014. [Google Scholar]
- Müller, T. Science of Synthesis. Multicomponent Reactions 2; Thieme: Stuttgart, Germany, 2014. [Google Scholar]
- Alvim, H.G.O.; da Silva, E.N., Jr.; Neto, B.A.D. What do we know about multicomponent reactions? Mechanisms and trends for the Biginelli, Hantzsch, Mannich, Passerini and Ugi MCRs. RSC Adv. 2014, 4, 54282–54299. [Google Scholar] [CrossRef]
- Domling, A.; Wang, W.; Wang, K. Chemistry and Biology of Multicomponent Reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef] [PubMed]
- De Graaff, C.; Ruijter, E.; Orru, R.V.A. Recent developments in asymmetric multicomponent reactions. Chem. Soc. Rev. 2012, 41, 3969–4009. [Google Scholar] [CrossRef] [PubMed]
- Eckert, H. Diversity oriented syntheses of conventional heterocycles by smart multi component reactions (MCRs). Molecules 2012, 17, 1074–1102. [Google Scholar] [CrossRef] [PubMed]
- Eckert, H. From Multi-Component-Reactions (MCRs) towards Multi-Function-Component-Reactions (MFCRs). Heterocycles 2007, 73, 149–158. [Google Scholar] [CrossRef]
- Tuch, A.; Walle, S. Multicomponent reactions. In Handbook of Combinatorial Chemistry; Nicolaou, K.C., Hanko, R., Hartwig, W., Eds.; Wiley-VCH: Weinheim, Germany, 2002; Volume 2, pp. 685–705. [Google Scholar]
- Domling, A.; Ugi, I. Multicomponent reactions with isocyanides. Angew. Chem. Int. Ed. 2000, 39, 3168–3210. [Google Scholar] [CrossRef]
- Sadjadi, S.; Heravi, M.M.; Nazari, N. Isocyanide-based multicomponent reactions in the synthesis of heterocycles. RSC Adv. 2016, 6, 53203–53272. [Google Scholar] [CrossRef]
- Koopmanschap, G.; Ruijter, E.; Orru, R.V.A. Isocyanide-based multicomponent reactions towards cyclic constrained peptidomimetics. Beilstein J. Org. Chem. 2014, 10, 544–598. [Google Scholar] [CrossRef] [PubMed]
- Shaabani, A.; Sarvary, A.; Shaabani, S. Multicomponent reactions with isocyanide participation. In Science of Synthesis, Multicomponent Reactions; Mueller, T.J.J., Ed.; Thieme: Stuttgart, Germany, 2014; Volume 2, pp. 55–102. [Google Scholar]
- Ayaz, M.; De Moliner, F.; Dietrich, J.; Hulme, C. Applications of isocyanides in IMCRs for the rapid generation of molecular diversity. In Isocyanide Chemistry; Nenajdenko, V., Ed.; Wiley-VCH: Weinheim, Germany, 2012; pp. 335–384. [Google Scholar]
- Kazemizadeh, A.R.; Ramazani, A. Synthetic applications of Passerini reaction. Curr. Org. Chem. 2012, 16, 418–450. [Google Scholar] [CrossRef]
- Van Berkel, S.S.; Boegels, B.G.M.; Wijdeven, M.A.; Westermann, B.; Rutjes, F.P.J.T. Recent Advances in Asymmetric Isocyanide-Based Multicomponent Reactions. Eur. J. Org. Chem. 2012, 2012, 3543–3559. [Google Scholar] [CrossRef]
- Doemling, A. Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef] [PubMed]
- Eckert, H.; Ugi, I. The Role of Isocyanides in the Synthesis of ß-Lactam Antibiotics and Related Compounds. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands; London, UK; New York, NY, USA; Tokyo, Japan, 1993; pp. 113–143. [Google Scholar]
- Ugi, I.; Eberle, G.; Eckert, H.; Lagerlund, I.; Marquarding, D.; Skorna, G.; Urban, R.; Wackerle, L.; von Zychlinski, H. The Present Status of Peptide Synthesis by Four-Component Condensation and Related Chemistry. In Peptides, Proceedings of the 5th American Peptide Symposium; Goodman, M., Meienhofer, J., Eds.; Halsted Press/Wiley & Sons: New York, NY, USA, 1977; pp. 484–487. [Google Scholar]
- Rostamnia, S. In situ generation and protonation of the isocyanide/acetylene adduct: A powerful catalyst-free strategy for multicomponent synthesis of ketenimines, aza-dienes, and heterocycles. RSC Adv. 2015, 5, 97044–97065. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Q.; Ye, F.; Xia, Y.; Wu, G.; Hossain, M.L.; Zhang, Y.; Wang, J. Copper(I)-Catalyzed Three-Component Coupling of N-Tosylhydrazones, Alkynes and Azides: Synthesis of Trisubstituted 1,2,3-Triazoles. Adv. Synth. Catal. 2015, 357, 2277–2286. [Google Scholar] [CrossRef]
- Shaabani, A.; Sarvary, A.; Shaabani, S. Electron-deficient alkynes as electrophiles. In Science of Synthesis, Multicomponent Reactions; Mueller, T.J.J., Ed.; Thieme: Stuttgart, Germany; New York, NY, USA, 2014; Volume 2, pp. 103–193. [Google Scholar]
- De Moliner, F.; Banfi, L.; Riva, R.; Basso, A. Beyond Ugi and Passerini reactions: Multicomponent approaches based on isocyanides and alkynes as an efficient tool for diversity oriented synthesis. Comb. Chem. High Throughput Screen. 2011, 14, 782–810. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Wadhwa, P.; Sharma, A. Arylsulfonylmethyl isocyanides: A novel paradigm in organic synthesis. RSC Adv. 2015, 5, 52769–52787. [Google Scholar] [CrossRef]
- Dandia, A.; Singh, R.; Maheshwari, S. Malononitrile as a Key Reagent in Multicomponent Reactions for the Synthesis of Pharmaceutically Important Pyridines. Curr. Org. Chem. 2014, 18, 2513–2529. [Google Scholar] [CrossRef]
- Azim, Z.H.; Irishi, N.N.; Seyyed, E.H. Nitroalkenes in the synthesis of carbocyclic compounds. RSC Adv. 2014, 4, 31261. [Google Scholar]
- Di Mola, A.; Massa, A. The aldol reactions of active methylene compounds. Curr. Org. Chem. 2012, 16, 2290–2301. [Google Scholar] [CrossRef]
- Bonne, D.; Coquerel, Y.; Constantieux, T.; Rodriguez, J. 1,3-Dicarbonyl compounds in stereoselective domino and multicomponent reactions. Tetrahedron 2010, 21, 1085–1109. [Google Scholar] [CrossRef]
- Simon, C.; Constantieux, T.; Rodriguez, J. Utilisation of 1,3-dicarbonyl derivatives in multicomponent reactions. Eur. J. Org. Chem. 2004, 4957–4980. [Google Scholar] [CrossRef]
- Giustiniano, M.; Novellino, E.; Tron, G.C. Nitrile N-Oxides and Nitrile Imines as New Fuels for the Discovery of Novel Isocyanide-Based Multicomponent Reactions. Synthesis 2016, 48, 2721–2731. [Google Scholar] [CrossRef]
- Szabo, K.J. Boron-mediated multicomponent reactions. In Science of Synthesis, Multicomponent Reactions; Mueller, T.J.J., Ed.; Thieme: Stuttgart, Germany; New York, NY, USA, 2014; Volume 2, pp. 345–376. [Google Scholar]
- Fusano, A.; Ryu, I. Free-radical-mediated multicomponent reactions involving carbon monoxide. In Science of Synthesis, Multicomponent Reactions; Mueller, T.J.J., Ed.; Thieme: Stuttgart, Germany; New York, NY, USA, 2014; Volume 2, pp. 409–437. [Google Scholar]
- Zhakenovich, A.E.; Stepanovna, Y.V.; Vladimirovna, S.T.; Kuandykovich, T.N.; Vladimirovich, B.E. Search for new three-, four-component Petasis, Passerini, Hantzsch, Kabachnic-Fields, Ugi reactions with organic compounds of phosphorus, arsenic, antimony and bismuth. J. Chem. Chem. Eng. 2014, 8, 428–432. [Google Scholar] [CrossRef]
- Balaz, P.; Achimovicova, M.; Balaz, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutkova, E.; Gaffet, E.; Gotor, F.J. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- Pyne, S.G.; Tang, M. The boronic acid Mannich reaction. Org. React. 2014, 83, 211–498. [Google Scholar]
- Protti, S.; Garbarino, S.; Ravelli, D.; Basso, A. Photoinduced Multicomponent Reactions. Angew. Chem. Int. Ed. 2016, 55, 15476–15484. [Google Scholar]
- Guo, X.; Hu, W. Novel Multicomponent Reactions via Trapping of Protic Onium Ylides with Electrophiles. Acc. Chem. Res. 2013, 46, 2427–2440. [Google Scholar] [CrossRef] [PubMed]
- Tron, G.C. Off the Beaten Track: The Use of Secondary Amines in the Ugi Reaction. Eur. J. Org. Chem. 2013, 2013, 1849–1859. [Google Scholar] [CrossRef]
- Quesnel, J.S.; Arndtsen, B.A. Transition-metal-catalyzed multicomponent coupling reactions with imines and carbon monoxide. Pure Appl. Chem. 2013, 85, 377–384. [Google Scholar] [CrossRef]
- Bhojgude, S.S.; Biju, A.T. Arynes in Transition-Metal-Free Multicomponent Coupling Reactions. Angew. Chem. Int. Ed. 2012, 51, 1520–1522. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Menon, R.S.; Sreekumar, V. Multicomponent reactions based on nucleophilic carbenes and their applications in organic synthesis. Pure Appl. Chem. 2005, 77, 1191–1198. [Google Scholar] [CrossRef]
- Doemling, A. The discovery of new isocyanide-based multicomponent reactions. In Multicomponent Reactions; Zhu, J., Bienayme, H., Eds.; Wiley-VCH: Weinheim, Germany, 2005; pp. 76–94. [Google Scholar]
- Kakuchi, R. Multicomponent Reactions in Polymer Synthesis. Angew. Chem. Int. Ed. 2014, 53, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Koszytkowska-Stawinska, M.; Buchowicz, W. Multicomponent reactions in nucleoside chemistry. Beilstein J. Org. Chem. 2014, 10, 1706–1732. [Google Scholar] [CrossRef] [PubMed]
- Mahrwald, R. The long underestimated carbonyl function of carbohydrates—An organocatalyzed shot into carbohydrate chemistry. Chem. Commun. 2015, 51, 13868–13877. [Google Scholar] [CrossRef] [PubMed]
- Orru, R.V.A.; Ruijter, E. (Eds.) Synthesis of Heterocycles via Multicomponent Reactions I; Topics in Heterocyclic Chemistry; Springer: New York, NY, USA, 2010; Volume 23.
- Orru, R.V.A.; Ruijter, E. (Eds.) Synthesis of Heterocycles via Multicomponent Reactions II; Topics in Heterocyclic Chemistry; Springer: New York, NY, USA, 2010; Volume 25.
- Keiko, N.A.; Vchislo, N.V. α,β-Unsaturated Aldehydes in the Synthesis of Five-Membered Heterocyclic Compounds with One Heteroatom: Recent Advances from Developments in Metal- and Organocatalysis. Asian J. Org. Chem. 2016, 5, 439–461. [Google Scholar] [CrossRef]
- Varadi, A.; Palmer, T.C.; Dardashti, R.N.; Majumdar, S. Isocyanide-based multicomponent reactions for the synthesis of heterocycles. Molecules 2016, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-M.; Yan, S.-J.; Lin, J. Heterocyclic Ketene Aminals: Scaffolds for Heterocycle Molecular Diversity. Eur. J. Org. Chem. 2014, 1129–1145. [Google Scholar] [CrossRef]
- Rotstein, B.H.; Zaretsky, S.; Rai, V.; Yudin, A.K. Small Heterocycles in Multicomponent Reactions. Chem. Rev. 2014, 114, 8323–8359. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.R.; Elwahy, A.H.M. Synthesis of Oxazolo-, Thiazolo-, Pyrazolo-, and Imidazo-Fused Heterocycles by Multi-Component Reactions. Curr. Org. Synth. 2014, 11, 471–525. [Google Scholar] [CrossRef]
- Elwahy, A.H.M.; Shaaban, M.R. Synthesis of Pyrido- and Pyrimido- Fused Heterocycles by Multi-Component Reactions. Curr. Org. Synth. 2014, 11, 835–873. [Google Scholar] [CrossRef]
- Martin, S.F. Strategies for the synthesis of alkaloids and novel nitrogen heterocycles. Adv. Heterocycl. Chem. 2013, 110, 73–117. [Google Scholar]
- Huang, Y.; Domling, A. Isocyanide-based multicomponent reactions towards benzodiazepines. In Isocyanide Chemistry; Nenajdenko, V., Ed.; Wiley-VCH: Weinheim, Germany, 2012; pp. 431–449. [Google Scholar]
- Sunderhaus, J.D.; Martin, S.F. Applications of multicomponent reactions to the synthesis of diverse heterocyclic scaffolds. Chem. Eur. J. 2009, 15, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Toure, B.B.; Hall, D.G. Natural Product Synthesis Using Multicomponent Reaction Strategies. Chem. Rev. 2009, 109, 4439–4486. [Google Scholar] [CrossRef] [PubMed]
- Akritopoulou-Zanze, I. Isocyanide-based multicomponent reactions in drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.; Abdel-Wahab, B.F. Groebke-Blackburn-Bienayme multicomponent reaction: Emerging chemistry for drug discovery. Mol. Div. 2016, 20, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, C.; Umkehrer, M.; Weber, L.; Kolb, J.; Burdack, C.; Ross, G. On the industrial applications of MCRs: Molecular diversity in drug discovery and generic drug synthesis. Mol. Divers. 2010, 14, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Guanti, G.; Banfi, L.; Basso, A.; Riva, R. Diversity-oriented synthesis via Passerini and Ugi multicomponent reactions. Chimica e l’Industria (Milan, Italy) 2006, 88, 82–86. [Google Scholar]
- Hulme, C.; Gore, V. Multi-component reactions: Emerging chemistry in drug discovery from xylocain to crixivan. Curr. Med. Chem. 2003, 10, 51–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Q. Two Neglected Multicomponent Reactions: Asinger and Groebke Reaction for Constructing Thiazolines and Imidazolines. Curr. Org. Synth. 2015, 12, 20–60. [Google Scholar] [CrossRef]
- Strecker, A. Über die künstliche Bildung der Milchsäure und einen neuen, dem Glycocoll homologen Körper. Justus Liebigs Ann. Chem. 1850, 75, 27–45. [Google Scholar] [CrossRef]
- Van den Eynde, J.J.; Mayence, A. Reactions involving a carbonyl compound as electrophilic component. Third component 1,3 dicarbonyl compound (with amines: Hantzsch pyridine synthesis). In Science of Synthesis, Multicomponent Reactions; Mueller, T.J.J., Ed.; Thieme: Stuttgart, Germany; New York, NY, USA, 2014; Volume 1, pp. 67–98. [Google Scholar]
- Chebanov, V.A.; Gorobets, N.Y.; Sedash, Y.V. Reactions involving a carbonyl compound as electrophilic component. Third component 1,3 dicarbonyl compound (with ureas: Biginelli reaction). In Science of Synthesis, Multicomponent Reactions; Mueller, T.J.J., Ed.; Thieme: Stuttgart, Germany; New York, NY, USA, 2014; Volume 1, pp. 29–66. [Google Scholar]
- Bernardi, L.; Ricci, A. Reactions involving a carbonyl compound as electrophilic component. Third components enolizable carbonyl compound (Mannich reaction). In Science of Synthesis, Multicomponent Reactions; Mueller, T.J.J., Ed.; Thieme: Stuttgart, Germany; New York, NY, USA, 2014; Volume 1, pp. 123–164. [Google Scholar]
- Riva, R.; Banfi, L.; Basso, A. Reactions involving a carbonyl compound as electrophilic component with an isocyanide as one component and third component carboxylic acid (Passerini reaction). In Science of Synthesis, Multicomponent Reactions; Mueller, T.J.J., Ed.; Thieme: Stuttgart, Germany; New York, NY, USA, 2014; Volume 1, pp. 327–413. [Google Scholar]
- Wessjohann, L.A.; Kaluderovic, G.N.; Filho, R.A.W.; Morejon, M.C.; Lemanski, G.; Ziegler, T. Reactions involving a carbonyl compound as electrophilic component with further components carboxylic acid and amine (Ugi reaction). In Science of Synthesis, Multicomponent Reactions; Mueller, T.J.J., Ed.; Thieme: Stuttgart, Germany; New York, NY, USA, 2014; Volume 1, pp. 415–502. [Google Scholar]
- Ugi, I.; Steinbrueckner, C. Reaktion von Isonitrilen mit Carbonylverbindungen, Aminen und Stickstoffwasserstoffsaeure. Chem. Ber. 1961, 94, 734–742. [Google Scholar] [CrossRef]
- Ugi, I.; Steinbrueckner, C. α-Addition von Immonium-Ionen und Carbonsame-Anionen an Isonitrile. Chem. Ber. 1961, 94, 2802–28014. [Google Scholar] [CrossRef]
- Asinger, F. Über die gemeinsame Einwirkung von Schwefel und Ammoniak auf Ketone. Angew. Chem. 1956, 68, 413. [Google Scholar] [CrossRef]
- Bello, D.; Ramon, R.; Lavilla, R. Mechanistic variations of the Povarov multicomponent reaction and related processes. Curr. Org. Chem. 2010, 14, 332–356. [Google Scholar] [CrossRef]
- Fuchter, M.J. Betti reaction. In Name Reactions in Heterocyclic Chemistry II; Li, J.J., Ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 352–359. [Google Scholar]
- Zhao, M.-X.; Wei, Y.; Shi, M. Morita-Baylis-Hillman reaction. RSC Catal. Ser. 2011, 8, 1–78. [Google Scholar]
- Huang, Y.; Doemling, A. The Gewald multicomponent reaction. Mol. Divers. 2011, 15, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Kielland, N.; Lavilla, R. Recent developments in Reissert-type multicomponent reactions. Top. Heterocycl. Chem. 2010, 25, 127–168. [Google Scholar]
- Liu, Z.-Q. Ugi and Passerini Reactions as Successful Models for Investigating Multicomponent Reactions. Curr. Org. Chem. 2014, 18, 719–739. [Google Scholar] [CrossRef]
- Darijani, M.; Habibi-Khorassani, S.M.; Shahraki, M. A Thermodynamic and Kinetic Insight into the Pathways Leading to a Highly Functionalized Ketenimine: A Computational Study. Int. J. Chem. Kinet. 2015, 47, 751–763. [Google Scholar] [CrossRef]
- Ocone, R.; Astarita, G. Kinetics and Thermodynamics in Multicomponent Mixtures. Adv. Chem. Eng. 1998, 24, 1–78. [Google Scholar]
- Van der Heijden, G.; Ruijter, E.; Orru, R.V.A. Efficiency, diversity, and complexity with multicomponent reactions. Synlett 2013, 24, 666–685. [Google Scholar]
- Ruijter, E.; Scheffelaar, R.; Orru, R.V.A. Multicomponent Reaction Design in the Quest for Molecular Complexity and Diversity. Angew. Chem. Int. Ed. 2011, 50, 6234–6246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhu, J. Multicomponent domino process: Rational design and serendipity. In Domino Reactions; Tietze, L.F., Ed.; Wiley-VCH: Weinheim, Germany, 2014; pp. 579–610. [Google Scholar]
- Liu, Y. Recent advances on diversity-oriented heterocycle synthesis via multicomponent tandem reactions based on A3-coupling. ARKIVOC 2014, 1–20. [Google Scholar] [CrossRef]
- Pellissier, H. Asymmetric domino reactions based on the use of chiral organocatalysts. RSC Catal. Ser. 2013, 10, 251–467. [Google Scholar]
- Indumathi, S.; Menendez, J.C.; Perumal, S. L-Proline Catalyzed Domino Reactions for the Synthesis of Heterocycles. Curr. Org. Chem. 2013, 17, 2038–2064. [Google Scholar] [CrossRef]
- Bugaut, X.; Bonne, D.; Coquerel, Y.; Rodriguez, J.; Constantieux, T. Michael Addition-initiated Sequential Reactions from 1,3-dicarbonyls for the Synthesis of Polycyclic Heterocycles. Curr. Org. Chem. 2013, 17, 1920–1928. [Google Scholar] [CrossRef]
- Pellissier, H. Stereocontrolled Domino Reactions. Chem. Rev. 2013, 113, 442–524. [Google Scholar] [CrossRef] [PubMed]
- Clavier, H.; Pellissier, H. Recent developments in enantioselective metal-catalyzed domino reactions. Adv. Synth. Catal. 2012, 354, 3347–3403. [Google Scholar] [CrossRef]
- Vlaar, T.; Ruijter, E.; Orru, R.V.A. Recent Advances in Palladium-Catalyzed Cascade Cyclizations. Adv. Synth. Catal. 2011, 353, 809–841. [Google Scholar] [CrossRef]
- Tietze, L.F.; Modi, A. Multicomponent domino reactions for the synthesis of biologically active natural products and drugs. Med. Res. Rev. 2000, 20, 304–322. [Google Scholar] [CrossRef]
- Rahmati, A.; Pashmforoush, N. Synthesis of various heterocyclic compounds via multi-component reactions in water. J. Iran. Chem. Soc. 2015, 12, 993–1036. [Google Scholar] [CrossRef]
- Gawande, M.B.; Bonifacio, V.D.B.; Luque, R.; Branco, P.S.; Varma, R.S. Benign by design. Catalyst-free in-water, on-water green chemical methodologies in organic synthesis. Chem. Soc. Rev. 2013, 42, 5522–5551. [Google Scholar] [CrossRef] [PubMed]
- Kumaravel, K.; Vasuki, G. Multicomponent reactions in water. Curr. Org. Chem. 2009, 13, 1820–1841. [Google Scholar] [CrossRef]
- Scholten, J.D.; Neto, B.A.D.; Suarez, P.A.Z.; Dupont, J. Ionic liquids as versatile media for chemical reactions. In Environment. Friendly Synthesis Using Ionic Liquids; Dupont, J., Ed.; CRC Press, Taylor & Francis Group: Bocca Raton, FL, USA, 2015; pp. 109–138. [Google Scholar]
- Liu, P.; Hao, J.-W.; Mo, L.-P.; Zhang, Z.-H. Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in organic reactions. RSC Adv. 2015, 5, 48675–48704. [Google Scholar] [CrossRef]
- Chaudhary, A.; Saluja, P.; Aggarwal, K.; Khurana, J.M. Applications of acidic and basic TSIL in multicomponent reactions. J. Indian Chem. Soc. 2014, 91, 1399–1409. [Google Scholar]
- Otaibi, A.A.; McCluskey, A. Multicomponent reactions in ionic liquids. In Ionic Liquids: New Aspects for the Future; Kadokawa, J., Ed.; InTech: Vienna, Austria, 2013; pp. 457–498. [Google Scholar]
- Dommaraju, Y.; Prajapati, D. Ionic liquids—An effective green media for cyclization and multicomponent reactions for the synthesis of N-heterocycles. Recent Res. Dev. Org. Chem. 2012, 12, 33–110. [Google Scholar]
- Isambert, N.; Duque, M.; Plaquevent, J.-C.; Genisson, Y.; Rodriguez, J.; Constantieux, T. Multicomponent reactions and ionic liquids: A perfect synergy for eco-compatible heterocyclic synthesis. Chem. Soc. Rev. 2011, 40, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, M.M.; Charnay, C.; DeAngelis, F.; Lamaty, F.; Martinez, J.; Colacino, E. Poly-(ethylene glycol)-based ionic liquids. Properties and uses as alternative solvents in organic synthesis and catalysis. ChemSusChem. 2014, 7, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y. Multicomponent reactions in unconventional solvents: State of the art. Green Chem. 2012, 14, 2091–2128. [Google Scholar] [CrossRef]
- Dandia, A.; Singh, R.; Joshi, J.; Kumari, S. 2,2,2-Trifluoroethanol as Green Solvent in Organic Synthesis: A Review. Mini-Rev. Org. Chem. 2014, 11, 462–476. [Google Scholar] [CrossRef]
- Singh, M.S.; Chowdhury, S. Recent developments in solvent-free multicomponent reactions: A perfect synergy for eco-compatible organic synthesis. RSC Adv. 2012, 2, 4547–4592. [Google Scholar] [CrossRef]
- Shearouse, W.C.; Waddell, D.C.; Mack, J. Alternative solvent-free methodologies in the synthesis of pharmaceutical drugs. Curr. Opin. Drug Discov. Dev. 2009, 12, 772–783. [Google Scholar]
- Hassan, S.; Mueller, T.J.J. Multicomponent Syntheses based upon Copper-Catalyzed Alkyne-Azide Cycloaddition. Adv. Synth. Catal. 2015, 357, 617–666. [Google Scholar] [CrossRef]
- Standley, E.A.; Tasker, S.Z.; Jensen, K.L.; Jamison, T.F. Nickel Catalysis: Synergy between Method Development and Total Synthesis. Acc. Chem. Res. 2015, 48, 1503–1514. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, J.J.; Nikolaev, V.A. Recent advances in the chemistry of Rh carbenoids: Multicomponent reactions of diazocarbonyl compounds. Russ. Chem. Rev. 2015, 84, 737–757. [Google Scholar] [CrossRef]
- Odom, A.L.; McDaniel, T.J. Titanium-Catalyzed Multicomponent Couplings: Efficient One-Pot Syntheses of Nitrogen Heterocycles. Acc. Chem. Res. 2015, 48, 2822–2833. [Google Scholar] [CrossRef] [PubMed]
- Arndtsen, B.A.; Tjutrins, J. Catalytic metal participation. In Science of Synthesis, Multicomponent Reactions; Mueller, T.J.J., Ed.; Thieme: Stuttgart, Germany; New York, NY, USA, 2014; Volume 2, pp. 475–533. [Google Scholar]
- Xi, C.; Chen, C. Metal-mediated multicomponent reactions. Stoichiometric metal participation. In Science of Synthesis, Multicomponent Reactions; Mueller, T.J.J., Ed.; Thieme: Stuttgart, Germany; New York, NY, USA, 2014; Volume 2, pp. 439–474. [Google Scholar]
- Lang, S. Unraveling the labyrinth of palladium-catalysed reactions involving isocyanides. Chem. Soc. Rev. 2013, 42, 4867–4880. [Google Scholar] [CrossRef] [PubMed]
- Abbiati, G.; Rossi, E. Silver and gold-catalyzed multicomponent reactions. Beilstein J. Org. Chem. 2014, 10, 481–513. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, S.; Tailor, Y.; Kumar, M. L-Proline Catalyzed Multicomponent Reactions. Curr. Organocat. 2016, 3, 176–204. [Google Scholar] [CrossRef]
- Rahimifard, M.; Mohammadi, Z.G.; Malekzadeh, L.B. Application of guanidine and its salts in multicomponent reactions. Turkish J. Chem. 2014, 38, 345–371. [Google Scholar] [CrossRef]
- Qu, M.; He, J. Progress of N-heterocyclic carbenes as organocatalysts. Youji Huaxue 2011, 31, 1388–1394. [Google Scholar]
- Guillena, G.; Ramon, D.J.; Yus, M. Organocatalytic enantioselective multicomponent reactions (OEMCRs). Tetrahedron Asymmetry 2007, 18, 693–700. [Google Scholar] [CrossRef]
- Van Berkom, L.W.A.; Kuster, G.J.T.; Scheeren, H.W. High pressure: A promising tool for multicomponent reactions. Mol. Divers. 2003, 6, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Kruithof, A.; Ruijter, E.; Orru, R.V.A. Microwave-assisted multicomponent reactions in the synthesis of heterocycles. In Microwaves in Organic Synthesis, 3rd ed.; De la Hoz, A., Loupy, A., Eds.; Wiley VCH: Weinheim, Germany, 2012; Volume 2, pp. 1099–1171. [Google Scholar]
- Bariwal, J.B.; Trivedi, J.C.; Van der Eycken, E.V. Microwave irradiation and multicomponent reactions. Top. Heterocycl. Chem. 2010, 25, 169–230. [Google Scholar]
- Scheffelaar, R.; Ruijter, E.; Orru, R.V.A. Multicomponent reaction design strategies: Towards scaffold and stereochemical diversity. Top. Heterocycl. Chem. 2010, 25, 95–126. [Google Scholar]
- Hugel, H.M. Microwave multicomponent synthesis. Molecules 2009, 14, 4936–4972. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.; Noguez, O.; Velasco, B.; Arroyo, G.; Penieres, G.; Martinez, J.O.; Delgado, F. Infrared irradiation: An alternative for reaction activation and its contribution to Green Chemistry. Educ. Quim. 2009, 20, 421–425. [Google Scholar]
- Chebanov, V.A.; Desenko, S.M. Multicomponent heterocyclization reactions with controlled selectivity. Chem. Heterocycl. Comp. 2012, 48, 566–583. [Google Scholar] [CrossRef]
- Elnagdi, M.H.; Moustafa, M.S.; Al-Mousawi, S.M.; Mekheimer, R.A.; Sadek, K.U. Recent developments in utility of green multi-component reactions for the efficient synthesis of polysubstituted pyrans, thiopyrans, pyridines, and pyrazoles. Mol. Divers. 2015, 19, 625–651. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, Q.; Wang, M.-X. Development and application of isocyanide-based multicomponent reactions. In Handbook Green Chemistry; Anastas, P.T., Ed.; Wiley: Hoboken, NJ, USA, 2012; Volume 7, pp. 121–157. [Google Scholar]
- Huang, Y.; Yazbak, A.; Domling, A. Multicomponent reactions. In Green Techniques for Organic Synthesis and Medicinal Chemistry; Zhang, W., Cue, B.W., Jr., Eds.; Wiley: Hoboken, NJ, USA, 2012; pp. 497–522. [Google Scholar]
- Bossert, F.; Vater, W. Bayer AG. 4-Aryl-1,4-Dihydropyridin. U.S. Patent US3485847, 23 December 1969. [Google Scholar]
- Rossen, K.; Pey, P.J.; DiMichele, L.M.; Volante, R.P.; Reider, P.J. An efficient asymmetric hydrogenation approach to the synthesis of the crixivan piperazine intermediate. Tetrahedron Lett. 1998, 39, 6823–6826. [Google Scholar] [CrossRef]
- Domling, A.; Huang, Y. Piperazine scaffolds via isocyanide-based multicomponent reactions. Synthesis 2010, 17, 2859–2883. [Google Scholar] [CrossRef]
- Endo, A.; Yanagisawa, A.; Abe, M.; Tohma, S.; Kan, T.; Fukuyama, T. Total Synthesis of Ecteinascidin 743. J. Am. Chem. Soc. 2002, 124, 6552–6554. [Google Scholar] [CrossRef] [PubMed]
- Brauch, S.; van Berkel, S.S.; Westermann, B. Higher-order multicomponent reactions: Beyond four 7 ujreactants. Chem. Soc. Rev. 2013, 42, 4948–4962. [Google Scholar] [CrossRef] [PubMed]
- Ramachary, D.B.; Jain, S. Sequential one-pot combination of multi-component and multi-catalysis cascade reactions: An emerging technology in organic synthesis. Org. Biomol. Chem. 2011, 9, 1277–1300. [Google Scholar] [CrossRef] [PubMed]
- Owens, T.D.; Araldi, G.-L.; Nutt, R.F.; Semple, J.E. Concise total synthesis of the prolyl endopeptidase inhibitor eurystatin A via a noval Passerini raction-deprotection-acyl-migration strategy. Tetrahedron Lett. 2001, 42, 6271–6274. [Google Scholar] [CrossRef]
- Zhu, J.; Bienayme, H. (Eds.) Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005; pp. 24–25.
- Sena, M.M.; Kleber, C.; Andrade, Z.; Rocha, R.O. Ugi Reaction of Natural Amino Acids Promoted by NbCl5. In Proceedings of the 14th Brazilian Meeting on Organic Synthesis (14th BMOS), Brasilia, Brazil, 1–5 September 2011.
- Cotarca, L.; Eckert, H. Phosgenations—A Handbook; Cotarca, L., Eckert, H., Eds.; Wiley-VCH: Weinheim, Germany; New York, NY, USA, 2004; pp. 449–451. [Google Scholar]
- Znabet, A.; Polak, M.M.; Janssen, E.; de Kanter, F.J.J.; Turner, N.J.; Orru, R.V.A.; Ruijter, E. A highly efficient synthesis of telaprevir by strategic use of biocatalysis and multicomponent reactions. Chem. Commun. 2010, 46, 7918–7920. [Google Scholar] [CrossRef] [PubMed]
- Zarganes-Tzitzikas, T.; Doemling, A. Modern multicomponent reactions for better drug syntheses. Org. Chem. Front. 2014, 1, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, K.; Muthusamy, K.; Prasad, Y.S.; Vemula, P.K.; Nagarajan, S. Recent developments in β-C-glycosides: Synthesis and applications. Carbohydr. Res. 2015, 402, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Voigt, B.; Linke, M.; Rainer Mahrwald, R. Multicomponent Cascade Reactions of Unprotected Carbohydrates and Amino Acids. Org. Lett. 2015, 17, 2606–2609. [Google Scholar] [CrossRef] [PubMed]
- Eckert, H. Einfuehrung von schonend abspaltbaren Carboxyl-Schutzgruppen mittels der Passerini-Reaktion. Synthesis 1977, 1977, 332–334. [Google Scholar] [CrossRef]
- Ganem, B. Strategies for Innovation in Multicomponent Reaction Design. Acc. Chem. Res. 2009, 42, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H.; Wang, J. Recent Advances in Diversity Oriented Synthesis through Isatin-based Multicomponent Reactions. Asian J. Org. Chem. 2013, 2, 374–386. [Google Scholar] [CrossRef]
- Mironov, M.A. Design of multi-component reactions: From libraries of compounds to libraries of reactions. QSAR Comb. Sci. 2006, 25, 423–431. [Google Scholar] [CrossRef]
- Weber, L.; Doemling, A. Evolutionary multi-component reaction (MCR) chemistry provides a boon/boost to efficiency and diversity in drug discovery. PharmaChem 2004, 3, 31–34. [Google Scholar]
- Burke, M.D.; Schreiber, S.L. A planning strategy for diversity-oriented synthesis. Angew. Chem. Int. Ed. 2004, 43, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gong, F.-M.; Wen, L.-R.; Li, Z.-R. Expeditious construction of spiro-pyrrolidines by an autocatalytic one-pot, five-component, 1,3-dipolar cycloaddition of in situ generated azo-methine ylides and olefinic dipolarophiles. Eur. J. Org. Chem. 2011, 3482–3490. [Google Scholar] [CrossRef]
- Doemling, A.; Herdtweck, E.; Ugi, I. The seven-component reaction. Acta Chem. Scand. 1998, 52, 107–113. [Google Scholar] [CrossRef]
- Enders, N.; van der Born, D.; Hendrickx, L.J.D.; Timmer, B.J.J.; Krause, A.; Janssen, E.; de Kanter, F.J.J.; Ruijter, E.; Orru, R.V.A. The efficient one-pot reaction of up to eight components by the union of multicomponent reactions. Angw. Chem. Int. Ed. 2009, 48, 5856–5859. [Google Scholar] [CrossRef] [PubMed]
- Tejedor, D.; Garcia-Tellado, F. Chemo-differentiating ABB’ multicomponent reactions. Privileged building blocks. Chem. Soc. Rev. 2007, 36, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Wessjohann, L.A.; Neves, F.; Ricardo, A.W.; Rivera, D.G. Multiple multicomponent reactions with isocyanides. In Isocyanide Chemistry; Nenajdenko, V., Ed.; Wiley-VCH: Weinheim, Germany, 2012; pp. 233–262. [Google Scholar]
- Pando, O.; Stark, S.; Denkert, A.; Porzel, A.; Preusentanz, R.; Wessjohann, L.A. The Multiple Multicomponent Approach to Natural Product Mimics: Tubugis, N-Substituted Anticancer Peptides with Picomolar Activity. J. Am. Chem. Soc. 2011, 133, 7692–7695. [Google Scholar] [CrossRef] [PubMed]
- Wessjohann, L.A.; Rivera, D.G.; Vercillo, O.E. Multiple Multicomponent Macrocyclizations (MiBs): A Strategic Development toward Macrocycle Diversity. Chem. Rev. 2009, 109, 796–814. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.J.J. Relative reactivities of functional groups as the key to multicomponent reactions. In Science of Synthesis, Multicomponent Reactions 1; Mueller, T.J.J., Ed.; Thieme: Stuttgart, Germany; New York, NY, USA, 2014; Volume 1, pp. 5–27. [Google Scholar]
- Weber, L. Algorithm-based methods for the discovery of novel multicomponent reactions. In Multicomponent Reactions; Zhu, J., Bienayme, H., Eds.; Wiley-VCH: Weinheim, Germany, 2005; pp. 300–310. [Google Scholar]
- Weber, L. Multi-component reactions and evolutionary chemistry. Drug Discov. Today 2002, 7, 143–147. [Google Scholar] [CrossRef]
- Mueller, T.J.J.; D’Souza, D.M. Diversity-oriented syntheses of functional π-systems by multicomponent and domino reactions. Pure Appl. Chem. 2008, 80, 609–620. [Google Scholar] [CrossRef]
- Basso, A.; Banfi, L.; Riva, R. A Marriage of Convenience: Combining the Power of Isocyanide- Based Multicomponent Reactions with the Versatility of (Hetero)norbornene Chemistry. Eur. J. Org. Chem. 2010, 1831–1841. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, F.-E. One-pot synthesis and its practical application in pharmaceutical industry. Curr. Org. Synth. 2012, 9, 873–897. [Google Scholar] [CrossRef]
- Alonso, F.; Foubelo, F.; Gonzalez-Gomez, J.C.; Martinez, R.; Ramon, D.J.; Riente, P.; Yus, M. Efficiency in chemistry: From hydrogen autotransfer to multicomponent catalysis. Mol. Divers. 2010, 14, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.R.; Nazeruddin, G.M. Multi component reactions and non-steroidal anti-inflammatory drugs. J. Chem. Pharm. Res. 2014, 6, 505–534. [Google Scholar]
- Bienayme, H.; Hulme, C.; Oddon, G.; Schmitt, P. Maximizing synthetic efficiency: Multi- component transformations lead the way. Chem. Eur. J. 2000, 6, 3321–3329. [Google Scholar] [CrossRef]
- Taylor, A.P.; Robinson, R.P.; Fobian, Y.M.; Blakemore, D.C.; Fadeyi, O.; Jones, L.H. Modern advances in heterocyclic chemistry in drug discovery. Org. Biomol. Chem. 2016, 14, 6611–6637. [Google Scholar] [CrossRef] [PubMed]
- Hulme, C. Applications of multicomponent reactions in drug discovery—Lead generation to process development. In Multicomponent Reactions; Zhu, J., Bienayme, H., Eds.; Wiley-VCH: Weinheim, Germany, 2005; pp. 311–341. [Google Scholar]
- Sample Availability: Samples of the compounds Enkephalines: BOC-Tyr(tBu)-FemGly-FemGly-Phe-Leu-OtBu; H-Tyr-Gly-Gly-Phe-Leu-OH (Leu-enkephaline); Human-β-endorphin-sequences: BOC-Ala-Tyr-Lys(BOC)-Lys(BOC)-FemGly-Glu(tBu)-OtBu; H-Ala-Tyr-Lys-Lys-Gly-Glu-OH; H-Lys-Asp-Ala-Tyr-Lys-Lys-Gly-Glu-OH (Octapeptide [24-31]); Cyclopeptides: H-(FemGly)3-OH; TCBOC-(FemGly)5-OH; cyclo-(FemGly)5; cyclo-(Gly)5; Organo-cobaltphthalocyanines: PcCo-CH2CH2-OH; PcCo-CH2CH2-OCO-C6H5; are available from the author.
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).