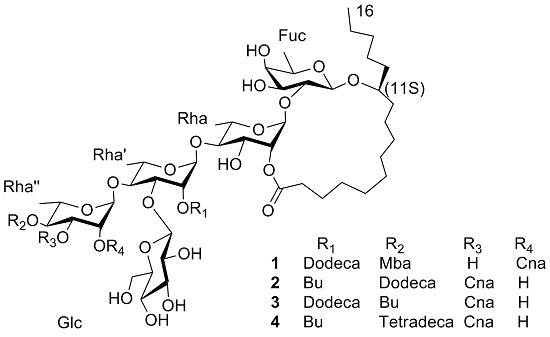
Four Pentasaccharide Resin Glycosides from Argyreia acuta
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectral Data
- Acutacoside F (1): White amorphous powder, −24.4° (c 0.09, MeOH); UV (MeOH) λmax (log ε) 278 (0.67) nm; IR (KBr) νmax: 3429, 2929, 2859, 1730, 1684, 1141 and 1061 cm−1, 1H-NMR and 13C-NMR data, see Table 1; HR-TOF-MS m/z 1419.7766 [M + Na]+ (calcd. for C72H116O26Na, 1419.7653).
- Acutacoside G (2): White amorphous powder; −18.0° (c 0.25, MeOH); UV (MeOH) λmax (log ε) 217 (0.74), 279 (1.02) nm; IR (KBr) νmax: 3451, 2930, 1733 and 1064 cm−1, 1H-NMR and 13C-NMR data, see Table 1; HR-TOF-MS m/z 1405.7697 [M + Na]+ (calcd. for C71H114O26Na, 1405.7496).
- Acutacoside H (3): White amorphous powder; −23.7° (c 0.19, MeOH); UV (MeOH) λmax (log ε) 217 (0.37), 280 (0.42) nm; IR (KBr) νmax: 3424, 2929, 1728 and 1067 cm−1, 1H-NMR and 13C-NMR data, see Table 1; HR-TOF-MS m/z 1405.7466 [M + Na]+ (calcd. for C71H114O26Na, 1405.7496).
- Acutacoside I (4): White amorphous powder; −11.3° (c 0.15, MeOH); UV (MeOH) λmax (log ε) 217 (0.62), 280 (0.87) nm; IR (KBr) νmax: 3453, 2930, 1734 and 1066 cm−1, 1H-NMR and 13C-NMR data, see Table 1; HR-TOF-MS m/z 1433.8016 [M + Na]+ (calcd. for C73H118O26Na, 1433.7809).
3.5. Hydrolysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pan, J.T.; Yu, B.W.; Yin, Y.Q.; Li, J.H.; Wang, L.; Guo, L.B.; Shen, Z.B. Four new pentasaccharide resin glycosides from Ipomoea cairica with strong α-glucosidase inhibitory activity. Molecules 2015, 20, 6601–6610. [Google Scholar] [CrossRef] [PubMed]
- Noda, N.; Tsuji, K.; Kawasaki, T.; Miyahara, K.; Hanazono, H.; Yang, C.R. A novel resin glycoside, merremin (tuguajalapin X dimmer) from Merremia hungaiensis. Chem. Pharm. Bull. 1995, 43, 1061–1063. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Noda, N.; Kawasaki, T.; Miyahara, K. Reinvestigation of the component organic and glycosidic acids of pharbitin, the crude ether-soluble resin glycoside (“Convolvulin”) of Pharbitidis semen (seeds of Pharbitis nil). Chem. Pharm. Bull. 1990, 38, 1892–1897. [Google Scholar] [CrossRef]
- Pereda-Miranda, R.; Mata, R.; Tricolorin, A. Major phytogrowth inhibitor from Ipomoea Tricolor. J. Nat. Prod. 1993, 56, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.C.; Smalley, M.K.; Manfredi, K.P.; Kindscher, K.; Loring, H.; Sheeley, D.M. Characterization of an Anti-tuberculosis Resin Glycoside from the Prairie Medicinal Plant Ipomoea leptophylla. J. Nat. Prod. 2003, 66, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Castaneda-Gómez, J.; Figueroa-González, G.; Jacobo, N.; Pereda-Miranda, R. Purgin II a resin glycoside ester-type dimer and inhibitor of multidrug efflux pumps from Ipomoea purge. J. Nat. Prod. 2013, 76, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Pereda-Miranda, R.; Kaatz, G.W.; Gibbons, S. Polyacylated Oligosaccharides from Medicinal Mexican Morning Glory Species as Antibacterials and Inhibitors of Multidrug Resistance in Staphylococcus aureus. J. Nat. Prod. 2006, 69, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Leon, I.; Miron-Lopez, G.; Molina-Salinas, G.M.; Herrera-Ruiz, M.; Estrada-Soto, S.; Gutierrez, M.C.; Alonso-Cortes, D.; Navarrete-Vazquez, G.; Ríos, M.H.; Said-Fernandez, S. Tyrianthinic acids from Ipomoea tyrianthina and their antimycobacterial activity, cytotoxicity, and effects on the central nervous system. J. Nat. Prod. 2008, 2008. 71, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Cherigo, L.; Pereda-Miranda, R.; Fragoso-Serrano, M.; Jacobo-Herrea, N.; Kaatz, G.W.; Gibbons, S. Inhibitors of Bacterial Multidrug Efflux Pumps from the Resin Glycosides of Ipomoea murucoides. J. Nat. Prod. 2008, 71, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Cherigo, L.; Pereda-Miranda, R.; Gibbons, S. Bacterial resistance modifying tetrasaccharide agents from Ipomoea murucoides. Phytochemistry. 2009, 70, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Martínez, C.; Cruz Morales, S.; Fragoso-Serrano, M.; Rahman, M.M.; Gibbons, S.; Pereda Miranda, R. Characterization of a xylose containing oligosaccharide, an inhibitor of multidrug resistance in Staphylococcus aureus, from Ipomoea pes-caprae. Phytochemistry. 2010, 71, 1796–1801. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.L. Studies on chemical xonstituents of Argyreia acute Lour. Asia Pac. Tradit. Med. 2013, 9, 31–32. [Google Scholar]
- Zeng, F.L.; Shen, Y.F.; Zhang, X.J. Pre-experiment of chemical composition from Argyreia acute. Guide Chin. Med. 2015, 13, 49–50. [Google Scholar]
- Yin, Y.Q.; Pan, J.T.; Yu, B.W.; Cui, H.H.; Yan, Y.S.; Chen, Y.F. Two pentasaccharide resin glycosides from Argyreia acuta. Nat. Prod. Res. 2015, 30, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yan, Y.S.; Cui, H.H.; Yin, Y.Q.; Pan, J.T.; Yu, B.W. Three new resin glycosides compounds from Argyreia acuta and their α-glucosidase inhibitory activity. Nat. Prod. Res. 2016. [Google Scholar] [CrossRef]
- Yin, Y.Q.; Huang, X.F.; Kong, L.Y.; Niva, M. Three New Pentasaccharide resin glycosides from the roots of sweet potato (Ipomoea batatas). Chem. Pharm. Bull. 2008, 56, 1670–1674. [Google Scholar] [CrossRef] [PubMed]
- Gerothanassis, I.P.; Exarchou, V.; Lagouri, V.; Troganis, A.; Tsimidou, M.; Boskou, D. Methodology for Identification of Phenolic Acids in Complex Phenolic Mixtures by High-Resolution Two-Dimensional Nuclear Magnetic Resonance. Application to Methanolic Extracts of Two Oregano Species. J. Agric. Food. Chem. 1998, 46, 4185–4192. [Google Scholar] [CrossRef]
- Exarchou, V.; Troganis, A.; Gerothanassis, I.P.; Tsimidou, M.; Boskou, D. Identification and Quantification of Caffeic and Rosmarinic Acid in Complex Plant Extracts by the Use of Variable-Temperature Two-Dimensional Nuclear Magnetic Resonance Spectroscopy. J. Agric. Food. Chem. 2001, 49, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.Q.; Kong, L.Y. Research on structure of resin glycoside-macrolide compounds. Lishizhen Med. Mater. Med. Res. 2009, 20, 517–518. [Google Scholar]
- Harris, R.K.; Grant, D.M.; Grant. Carbohydrates and glycoconjugates. Encyclopedia Nuclear Magnetic Resonance 2002, 1107–1134. [Google Scholar]
- Sample Availability: Samples of the compounds 1–4 are available from the authors.
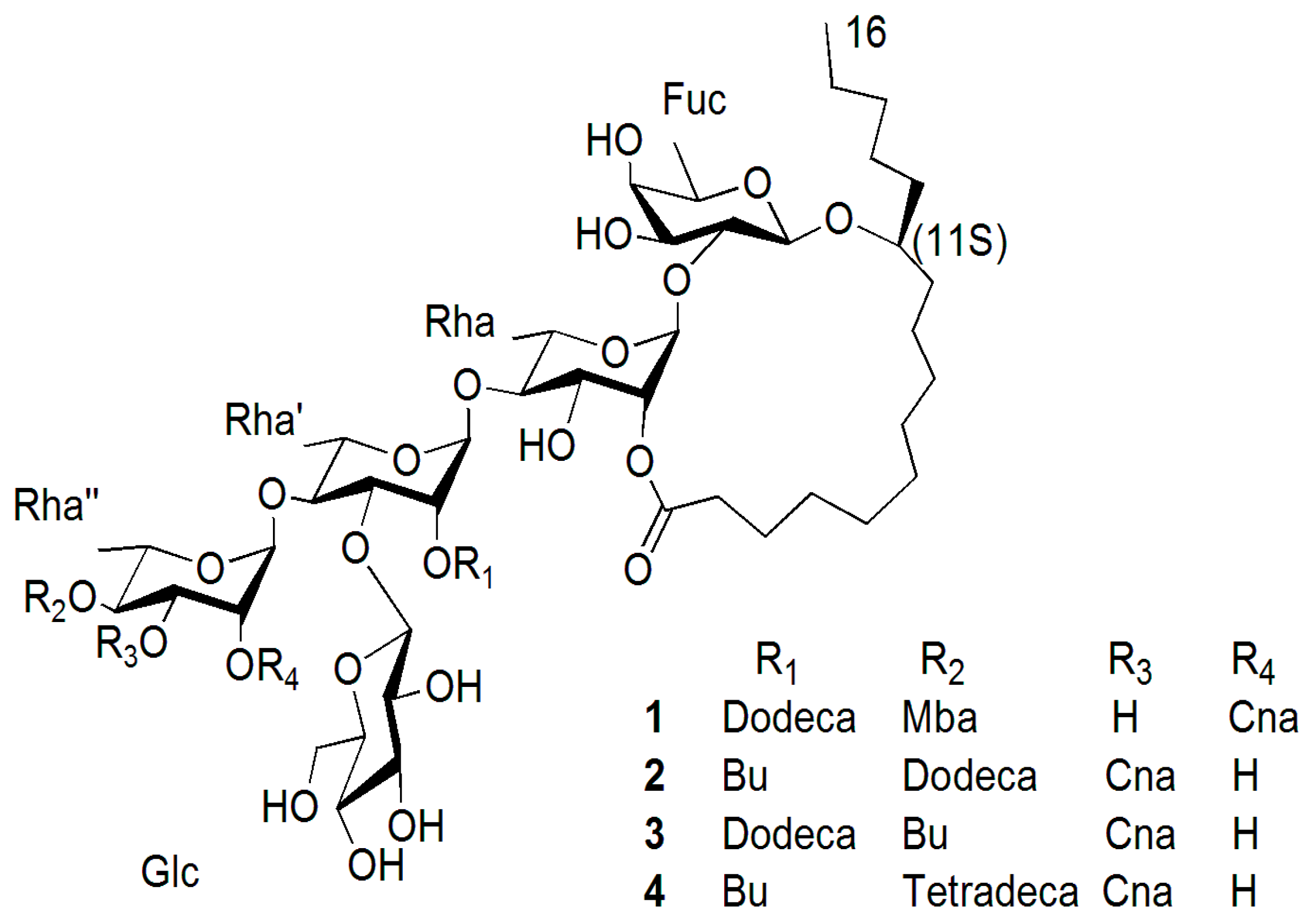
| Position | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| 13C | 1H | 13C | 1H | 13C | 1H | 13C | 1H | |
| Fuc-1 | 104.6 | 4.78 d (7.0) | 104.4 | 4.73 d (7.5) | 104.6 | 4.72 d (7.2) | 104.0 | 4.72 d (7.5) |
| 2 | 80.2 | 4.19 dd (7.0, 9.5) | 79.7 | 4.15 dd (7.5, 9.5) | 80.2 | 4.17 dd (7.2, 9.4) | 79.7 | 4.16 dd (7.5, 9.5) |
| 3 | 73.6 | 4.15 dd (9.5, 3.0) | 73.2 | 4.03 * | 73.7 | 4.14 dd (9.4, 3.0) | 72.8 | 4.04 * |
| 4 | 73.0 | 3.98 d (3.0) | 72.1 | 3.90 * | 73.2 | 3.96 d (3.0) | 72.7 | 3.90 * |
| 5 | 70.8 | 3.77 br q (6.5) | 71.1 | 3.73 br q (6.5) | 71.1 | 3.74 br q (6.6) | 70.6 | 3.73 br q (6.5) |
| 6 | 17.4 | 1.52 d (6.0) | 16.7 | 1.48 d (6.5) | 17.7 | 1.50 d (6.0) | 16.7 | 1.49 d (6.5) |
| Rha-1 | 98.6 | 5.53 br s | 98.3 | 5.50 br s | 98.8 | 5.51 br s | 98.3 | 5.52 br s |
| 2 | 73.4 | 5.95 br s | 73.2 | 5.92 br s | 73.7 | 5.93 br s | 73.2 | 5.93 br s |
| 3 | 73.2 | 5.03 dd (3.0, 9.0) | 68.7 | 5.02 dd (3.0, 9.0) | 69.3 | 5.03 dd (3.3, 9.3) | 68.7 | 5.01 dd (3.0, 9.0) |
| 4 | 82.0 | 4.19 * | 82.0 | 4.16 dd (9.0, 9.0) | 82.5 | 4.18 * | 82.1 | 4.16 dd (9.0, 9.0) |
| 5 | 69.2 | 4.48 * | 68.3 | 4.47 dd (9.0, 5.0) | 68.5 | 4.37 * | 68.3 | 4.47 dd (9.0, 5.0) |
| 6 | 19.0 | 1.58 d (5.4) | 18.9 | 1.63 d (5.0) | 19.5 | 1.63 d (5.4) | 18.9 | 1.63 d (5.0) |
| Rha′-1 | 99.3 | 5.80 br s | 100.1 | 5.82 br s | 100.6 | 5.84 br s | 100.1 | 5.82 br s |
| 2 | 73.2 | 6.32 br s | 73.4 | 6.31 br s | 73.9 | 6.33 br s | 73.4 | 6.30 br s |
| 3 | 79.1 | 4.79 * | 78.8 | 4.78 * | 79.3 | 4.79 dd (2.9, 9.2) | 78.7 | 4.78 * |
| 4 | 79.9 | 4.36 * | 79.6 | 4.35 * | 80.1 | 4.36 dd (9.2, 9.2) | 79.7 | 4.35 * |
| 5 | 69.0 | 4.52 * | 68.0 | 4.50 * | 68.4 | 4.50 dd (9.2, 6.5) | 67.7 | 4.50 * |
| 6 | 19.1 | 1.63 d (6.0) | 19.1 | 1.64 d (6.5) | 19.4 | 1.65 d (6.0) | 18.8 | 1.64 d (6.5) |
| Rha″-1 | 100.3 | 6.58 br s | 103.2 | 6.27 br s | 103.7 | 6.27 br s | 103.2 | 6.26 br s |
| 2 | 70.8 | 6.37 br s | 69.1 | 5.25 br s | 69.5 | 5.26 br s | 69.1 | 5.26 br s |
| 3 | 68.2 | 6.00 dd (3.1, 10.0) | 71.5 | 6.00 dd (3.0, 10.0) | 72.0 | 6.01 dd (3.1, 10.0) | 71.5 | 6.00 dd (3.0, 10.0) |
| 4 | 73.0 | 4.09 * | 71.3 | 6.08 dd (10.0, 10.0) | 71.8 | 6.09 dd (10.0, 10.0) | 71.3 | 6.08 dd (10.0, 10.0) |
| 5 | 68.4 | 4.37 * | 69.7 | 4.44 * | 70.2 | 4.48 dd (10.0, 6.2) | 69.7 | 4.47 * |
| 6 | 18.4 | 1.77 d (6.3) | 17.7 | 1.42 d (6.5) | 18.2 | 1.43 d (6.2) | 17.7 | 1.42 d (6.5) |
| Glc-1 | 105.6 | 5.01 d (7.8) | 105.0 | 5.07 d (7.5) | 105.8 | 5.09 d (7.8) | 105.3 | 5.08 d (7.5) |
| 2 | 75.0 | 3.90 dd (7.8, 9.0) | 74.9 | 3.97 * | 75.5 | 3.95 dd (7.8, 9.0) | 74.9 | 3.97 * |
| 3 | 78.3 | 4.07 * | 78.2 | 4.10 * | 78.7 | 4.08 dd * | 78.2 | 4.10 * |
| 4 | 71.5 | 3.92 * | 68.3 | 3.93 * | 68.7 | 3.94 * | 68.0 | 3.93 * |
| 5 | 78.2 | 3.85 * | 77.9 | 3.83 m | 78.4 | 3.81 * | 77.5 | 3.85 m |
| 6 | 63.2 | 4.05 * | 62.5 | 4.09 * | 63.2 | 4.09 * | 62.5 | 4.09 * |
| 4.32 * | 4.40 * | 4.43 * | 4.40 * | |||||
| Ag-1 | 173.5 | 173.3 | 173.4 | 173.3 | ||||
| 2 | 34.7 | 2.29 m | 34.3 | 2.27 m | 33.5 | 2.23 m | 34.3 | 2.29 m |
| 2.46 m | 2.44 m | 2.40 m | 2.45 m | |||||
| 11 | 82.4 | 3.86 m | 82.2 | 3.80 m | 82.7 | 3.83 m | 82.2 | 3.82 m |
| 16 | 14.7 | 0.86 * | 14.1 | 0.83 t (7.0) | 14.6 | 0.86 * | 14.1 | 0.84 t (7.0) |
| Cna-1 | 166.5 | 166.3 | 166.8 | 166.3 | ||||
| 2 | 118.9 | 6.66 d (16.0) | 118.5 | 6.58 d (16.0) | 118.9 | 6.66 d (16.0) | 118.3 | 6.58 d (16.0) |
| 3 | 146.7 | 7.83 d (16.0) | 145.2 | 7.85 d (16.0) | 145.7 | 7.86 d (16.0) | 145.3 | 7.85 d (16.0) |
| 1′ | 134.7 | 135.3 | 135.0 | 135.3 | ||||
| 2′ and 6′ | 128.5 | 7.36 m | 128.6 | 7.43 m | 128.8 | 7.42 m | 128.4 | 7.43 m |
| 3′ and 5′ | 129.3 | 7.30 m | 128.9 | 7.32 m | 129.6 | 7.33 m | 129.1 | 7.33 m |
| 4′ | 130.3 | 7.30 m | 130.8 | 7.32 m | 131.1 | 7.33 m | 131.0 | 7.33 m |
| Dodeca-1 | 174.0 | 173.4 | 173.4 | |||||
| 2 | 34.4 | 2.32 * | 34.2 | 2.48 m | 34.9 | 2.34 * | ||
| 12 | 14.7 | 0.87 * | 14.1 | 0.83 t (7.0) | 14.6 | 0.86 * | ||
| Mba-1 | 176.6 | |||||||
| 2 | 41.7 | 2.46 m | ||||||
| 2-CH3 | 16.7 | 1.23 d (7.0) | ||||||
| 4 | 12.1 | 0.86 t (7.0) | ||||||
| Bu-1 | 175.8 | 7.32 m | 174.8 | 175.8 | ||||
| 2 | 34.0 | 2.30 m | 34.8 | 2.38 t (7.8) | 34.0 | 2.26 m | ||
| 4 | 14.1 | 0.83 t (7.0) | 14.6 | 0.86 * | 14.1 | 0.84 t (7.0) | ||
| Tetradeca-1 | 173.4 | |||||||
| 2 | 34.2 | 2.53 | ||||||
| 14 | 14.1 | 0.84 t (7.0) | ||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, B.-W.; Sun, J.-J.; Pan, J.-T.; Wu, X.-H.; Yin, Y.-Q.; Yan, Y.-S.; Hu, J.-Y. Four Pentasaccharide Resin Glycosides from Argyreia acuta. Molecules 2017, 22, 440. https://doi.org/10.3390/molecules22030440
Yu B-W, Sun J-J, Pan J-T, Wu X-H, Yin Y-Q, Yan Y-S, Hu J-Y. Four Pentasaccharide Resin Glycosides from Argyreia acuta. Molecules. 2017; 22(3):440. https://doi.org/10.3390/molecules22030440
Chicago/Turabian StyleYu, Bang-Wei, Jing-Jing Sun, Jie-Tao Pan, Xiu-Hong Wu, Yong-Qin Yin, You-Shao Yan, and Jia-Yan Hu. 2017. "Four Pentasaccharide Resin Glycosides from Argyreia acuta" Molecules 22, no. 3: 440. https://doi.org/10.3390/molecules22030440