Self-Organized TiO2–MnO2 Nanotube Arrays for Efficient Photocatalytic Degradation of Toluene
Abstract
:1. Introduction
2. Results and Discussion
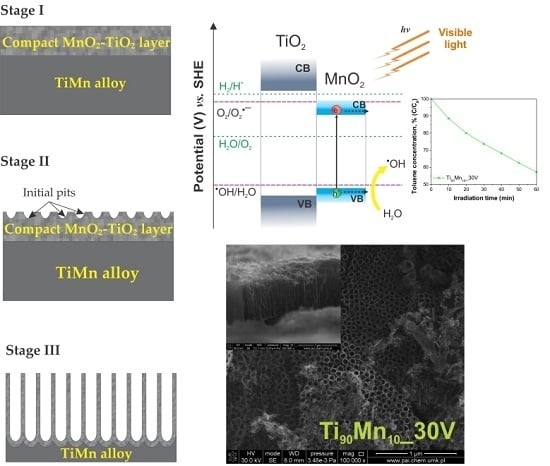
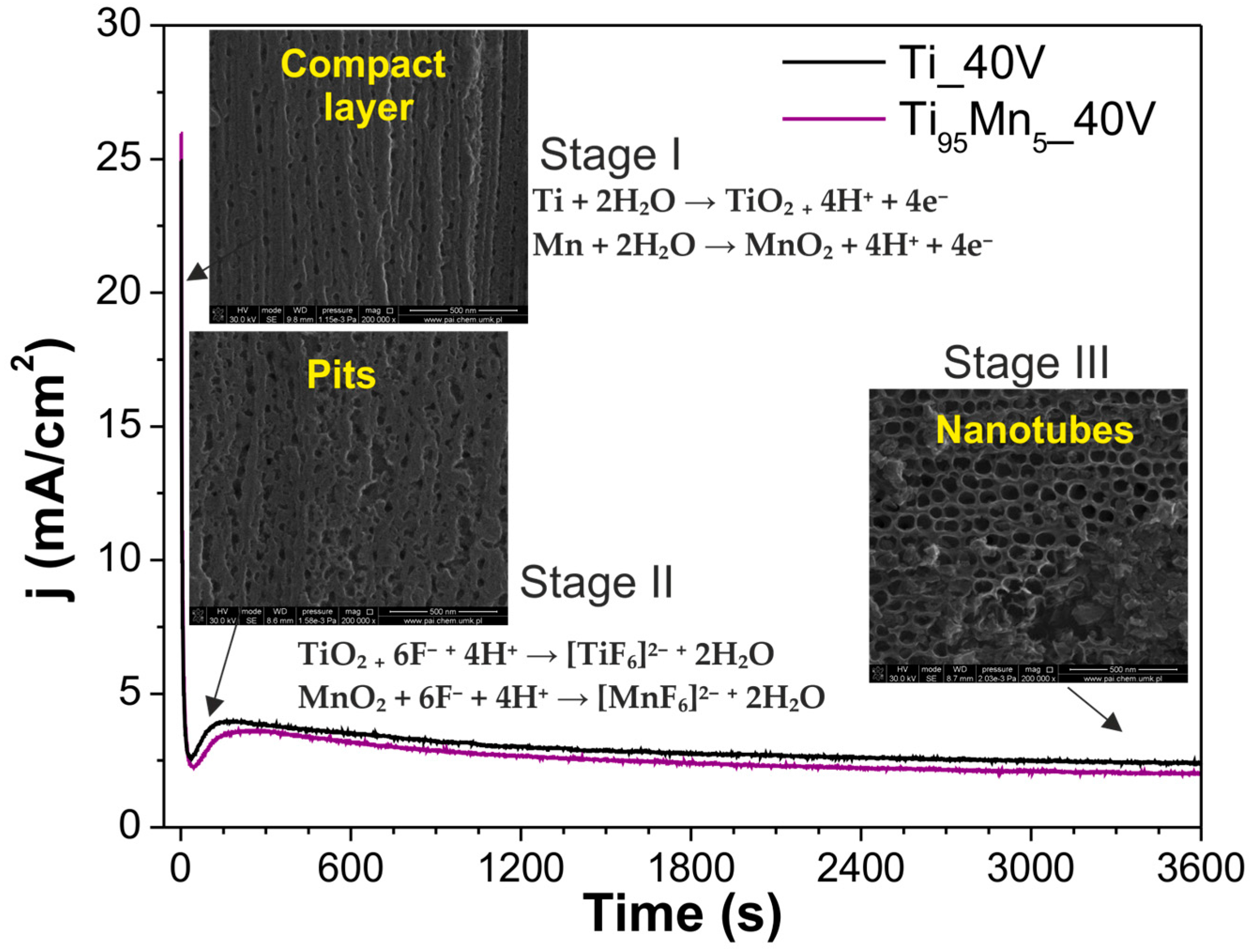
2.1. Morphology and Growth Mechanism
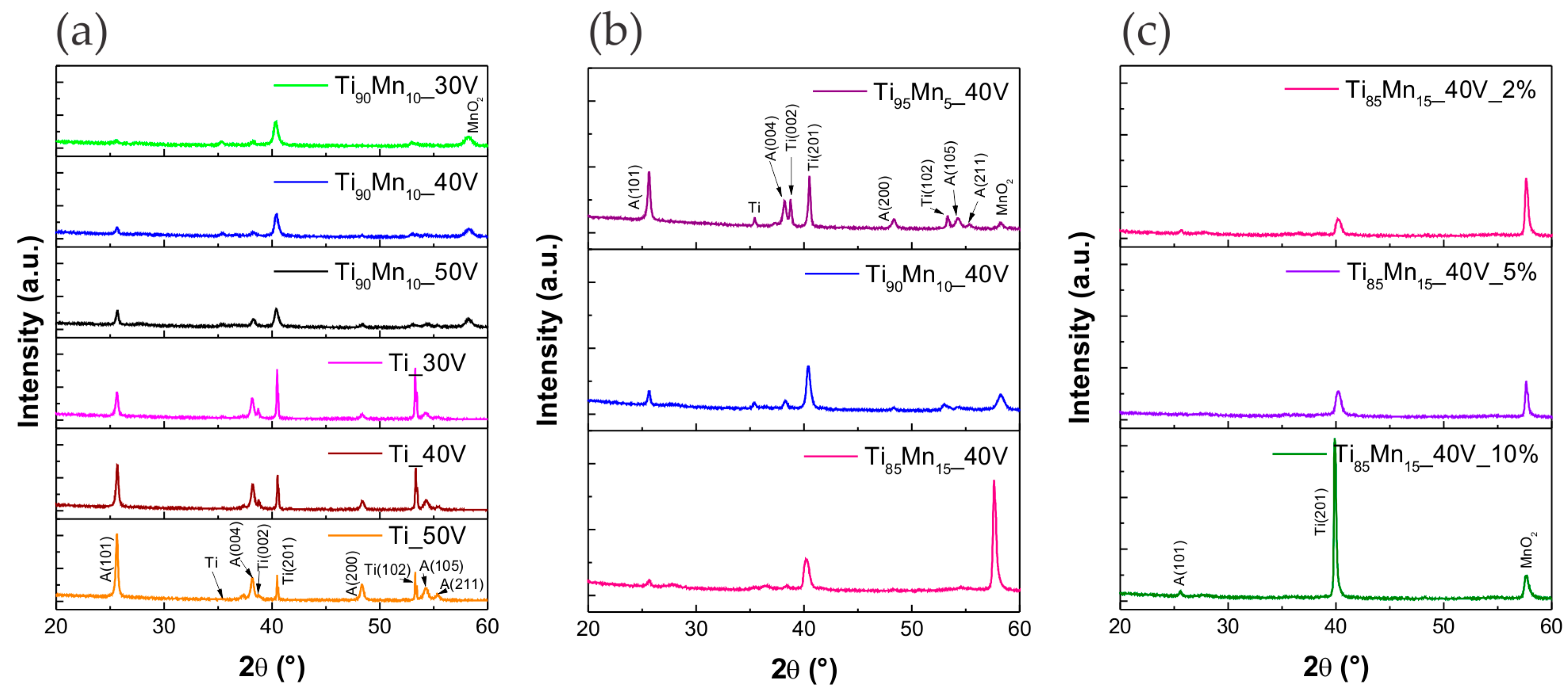
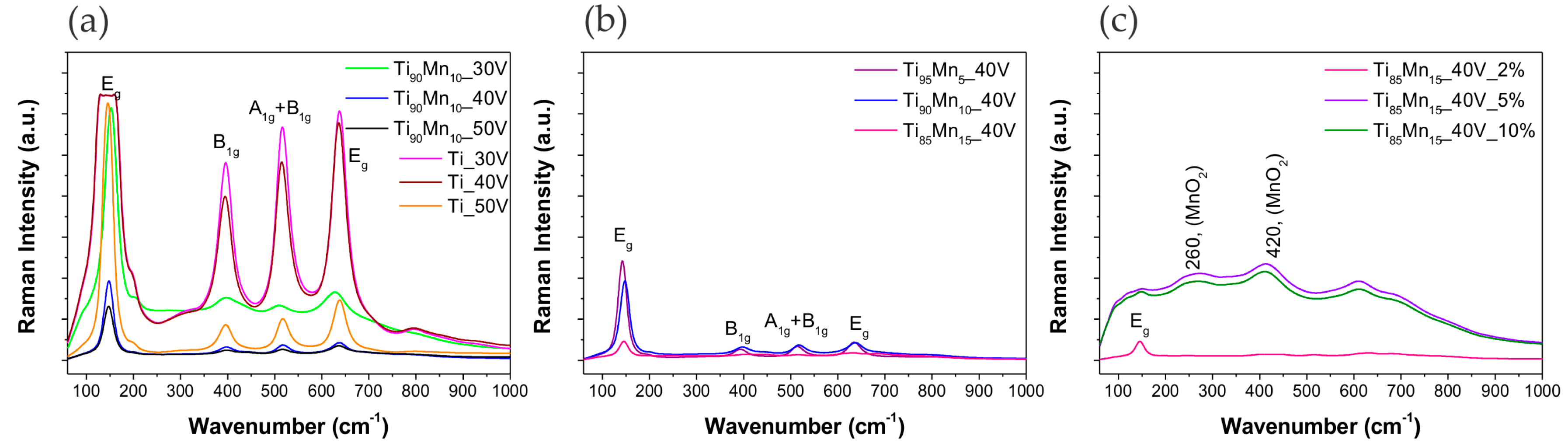
2.2. Structural Properties
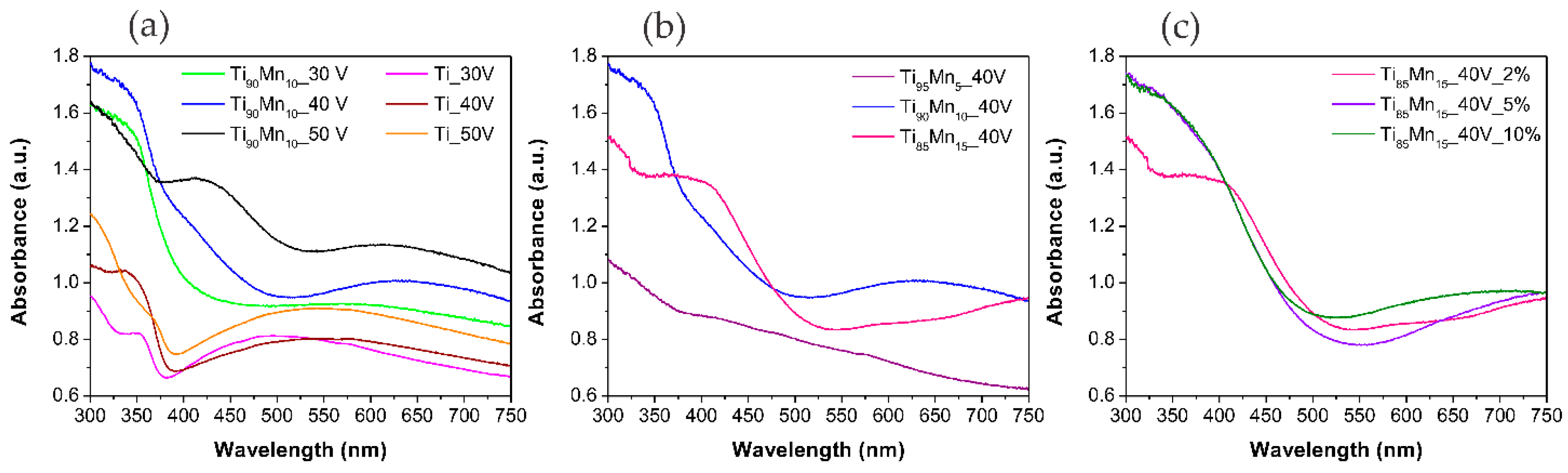
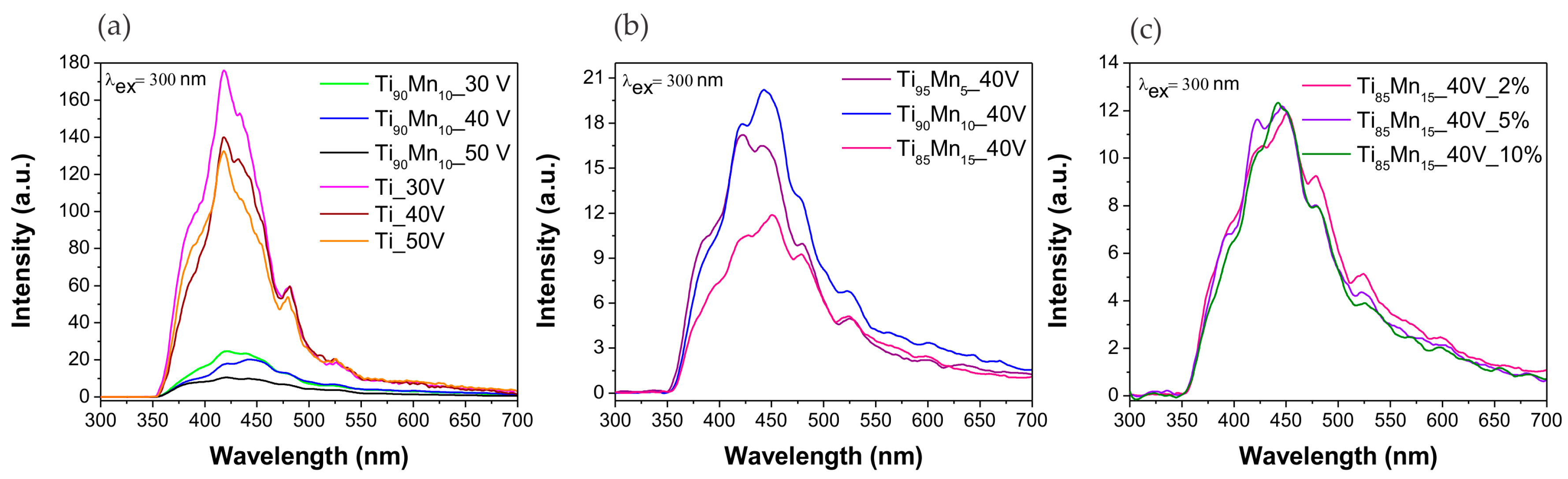
2.3. Optical Properties
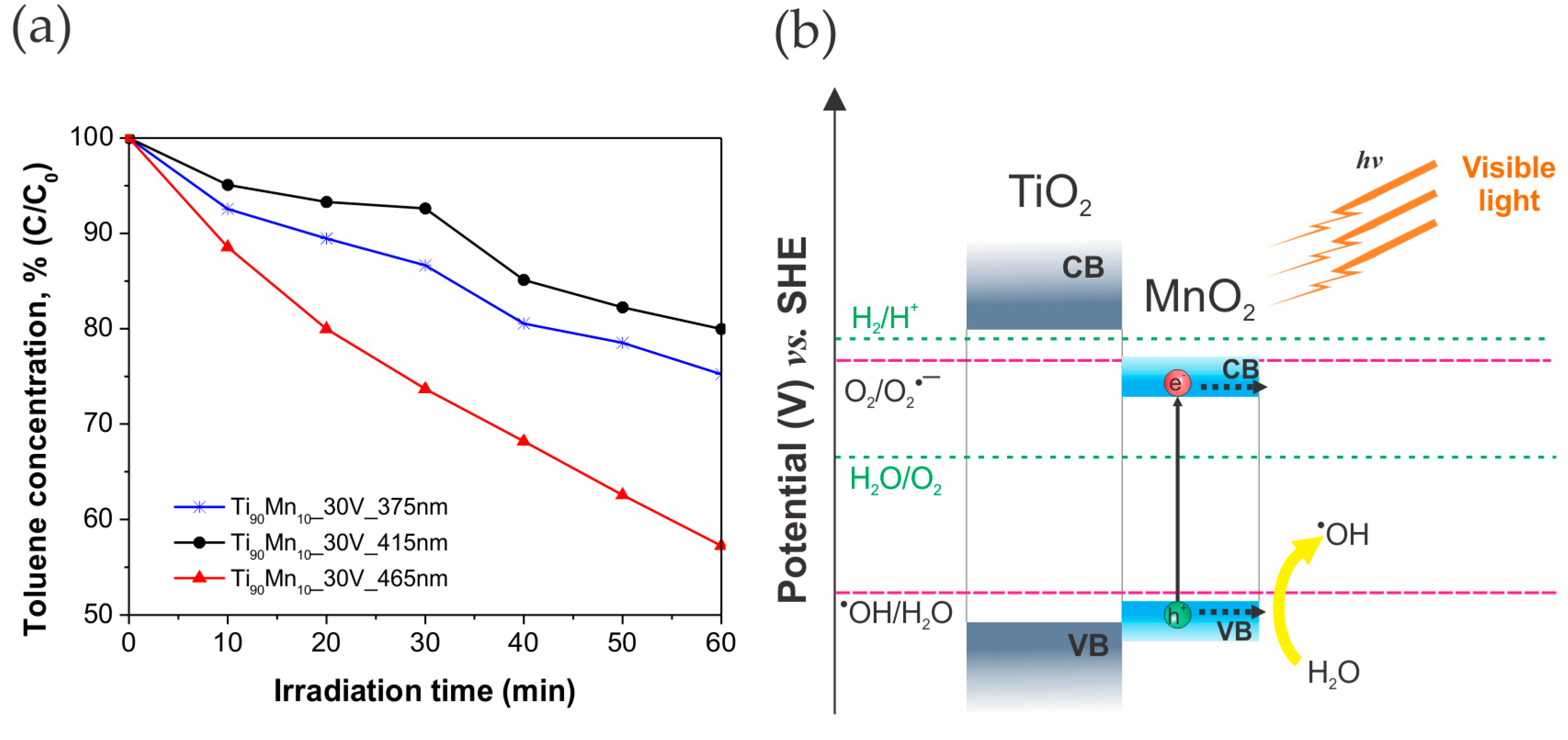
2.4. Photocatalytic Performance
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Pristine TiO2 and TiO2–MnO2 Nanotubes
3.3. Characterization of Pristine TiO2 and TiO2–MnO2 Nanotubes
3.4. Measurement of Photocatalytic Activity
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar]
- Pichat, P. Photocatalysis and Water Purification: From Fundamentals to Recent Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Schneider, J.; Bahnemann, D.; Ye, J.; Puma, G.L.; Dionysiou, D.D. Photocatalysis: Fundamentals and Perspectives; Royal Society of Chemistry: Cambridge, UK, 2016. [Google Scholar]
- Dionysiou, D.D.; Puma, G.L.; Ye, J.; Schneider, J.; Bahnemann, D. Photocatalysis: Applications; Royal Society of Chemistry: Cambridge, UK, 2016. [Google Scholar]
- Pichat, P. Photocatalysis: Fundamentals, Materials and Potential; MDPI: Basel, Switzerland, 2016. [Google Scholar]
- Colmenares Quintero, J.C.; Xu, Y.-J. Heterogeneous Photocatalysis: From Fundamentals to Green Applications; Springer: Berlin/Heidelberg, Germany, 2016; Volume VIII, p. 416. [Google Scholar]
- Mazierski, P.; Nadolna, J.; Lisowski, W.; Winiarski, M.J.; Gazda, M.; Nischk, M.; Klimczuk, T.; Zaleska-Medynska, A. Effect of irradiation intensity and initial pollutant concentration on gas phase photocatalytic activity of TiO2 nanotube arrays. Catal. Today 2017, 284, 19–26. [Google Scholar] [CrossRef]
- Zavahir, S.; Xiao, Q.; Sarina, S.; Zhao, J.; Bottle, S.; Wellard, M.; Jia, J.; Jing, L.; Huang, Y.; Blinco, J.P.; et al. Selective oxidation of aliphatic alcohols using molecular oxygen at ambient temperature: Mixed-valence vanadium oxide photocatalysts. ACS Catal. 2016, 6, 3580–3588. [Google Scholar] [CrossRef]
- Kang, D.; Kim, T.W.; Kubota, S.R.; Cardiel, A.C.; Cha, H.G.; Choi, K.S. Electrochemical synthesis of photoelectrodes and catalysts for use in solar water splitting. Chem. Rev. 2015, 115, 12839–12887. [Google Scholar] [CrossRef] [PubMed]
- Highfield, J. Advances and recent trends in heterogeneous photo(electro)-catalysis for solar fuels and chemicals. Molecules 2015, 20, 6739–6793. [Google Scholar] [CrossRef] [PubMed]
- Sethi, D.; Jada, N.; Tiwari, A.; Ramasamy, S.; Dash, T.; Pandey, S. Photocatalytic destruction of escherichia coli in water by V2O5/TiO2. J. Photochem. Photobiol. B 2015, 144, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Pichat, P. Self-cleaning materials based on solar photocatalysis. In New and Future Developments in Catalysis: Solar Photocatalysis; Suib, S.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 7 “Solar catalysis”, pp. 167–190. [Google Scholar]
- Ye, M.; Zheng, D.; Wang, M.; Chen, C.; Liao, W.; Lin, C.; Lin, Z. Hierarchically structured microspheres for high-efficiency rutile TiO2-based dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2014, 6, 2893–2901. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, W.; Chen, C.; Zhao, J. Sensitization of titania semiconductor: A promising strategy to utilize visible light. In Photocatalysis and Water Purification; Pichat, P., Ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 199–240. [Google Scholar]
- Zhang, H.; Banfield, J.F. Structural characteristics and mechanical and thermodynamic properties of nanocrystalline TiO2. Chem. Rev. 2014, 114, 9613–9644. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’Shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New insights into the mechanism of visible light photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; White, J.M. Photodecomposition of water over Pt/TiO2 catalysts. Chem. Phys. Lett. 1980, 72, 83–86. [Google Scholar] [CrossRef]
- Ohtani, B.; Osaki, H.; Nishimoto, S.; Kagiya, T. A novel photocatalytic process of amine N-alkylation by platinized semiconductor particles suspended in alcohols. J. Am. Chem. Soc. 1986, 108, 308–310. [Google Scholar] [CrossRef]
- Pichat, P. Surface-properties, activity and selectivity of bifunctional powder photocatalysts. New J. Chem. 1987, 11, 135–140. [Google Scholar]
- Choi, W.; Termin, A.; Hoffmann, M.R. The role of metal ion dopants in quantum-sized TiO2: Correlation between photoreactivity and charge carrier recombination dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Mazierski, P.; Lisowski, W.; Grzyb, T.; Winiarski, M.J.; Klimczuk, T.; Mikołajczyk, A.; Flisikowski, J.; Hirsch, A.; Kołakowska, A.; Puzyn, T.; et al. Enhanced photocatalytic properties of lanthanide-TiO2 nanotubes: An experimental and theoretical study. Appl. Catal. B Environ. 2017, 205, 376–385. [Google Scholar] [CrossRef]
- Mazierski, P.; Nischk, M.; Gołkowska, M.; Lisowski, W.; Gazda, M.; Winiarski, M.J.; Klimczuk, T.; Zaleska-Medynska, A. Photocatalytic activity of nitrogen doped TiO2 nanotubes prepared by anodic oxidation: The effect of applied voltage, anodization time and amount of nitrogen dopant. Appl. Catal. B Environ. 2016, 196, 77–88. [Google Scholar] [CrossRef]
- Ouyang, J.; Chang, M.; Li, X. CdS-sensitized ZnO nanorod arrays coated with TiO2 layer for visible light photoelectrocatalysis. J. Mater. Sci. 2012, 47, 4187–4193. [Google Scholar] [CrossRef]
- Diak, M.; Grabowska, E.; Zaleska, A. Synthesis, characterization and photocatalytic activity of noble metal-modified TiO2 nanosheets with exposed {001} facets. Appl. Surf. Sci. 2015, 347, 275–285. [Google Scholar] [CrossRef]
- Nischk, M.; Mazierski, P.; Wei, Z.; Siuzdak, K.; Kouame, N.A.; Kowalska, E.; Remita, H.; Zaleska-Medynska, A. Enhanced photocatalytic, electrochemical and photoelectrochemical properties of TiO2 nanotubes arrays modified with Cu, AgCu and Bi nanoparticles obtained via radiolytic reduction. Appl. Surf. Sci. 2016, 387, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Mahata, A. Demineralization of organic pollutants on the dye modified TiO2 semiconductor particulate system using visible light. Appl. Catal. B Environ. 2001, 33, 119–125. [Google Scholar] [CrossRef]
- Paramasivam, I.; Nah, Y.C.; Das, C.; Shrestha, N.K.; Schmuki, P. WO3/TiO2 nanotubes with strongly enhanced photocatalytic activity. Chemistry 2010, 16, 8993–8997. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, Y. Electrochemically self-doped TiO2 nanotube arrays for supercapacitors. J. Phys. Chem. C 2014, 118, 5626–5636. [Google Scholar] [CrossRef]
- Ning, X.; Wang, X.; Yu, X.; Li, J.; Zhao, J. Preparation and capacitance properties of Mn-doped TiO2 nanotube arrays by anodisation of Ti–Mn alloy. J. Alloys Compd. 2016, 658, 177–182. [Google Scholar] [CrossRef]
- Islam, A.K.M.F.U.; Islam, R.; Khan, K.A. Studies on the thermoelectric effect in semiconducting MnO2 thin films. J. Mater. Sci. Mater. Electron. 2005, 16, 203–207. [Google Scholar] [CrossRef]
- Zhao, J.; Nan, J.; Zhao, Z.; Li, N.; Liu, J.; Cui, F. Energy-efficient fabrication of a novel multivalence Mn3O4–MnO2 heterojunction for dye degradation under visible light irradiation. Appl. Catal. B Environ. 2017, 202, 509–517. [Google Scholar] [CrossRef]
- Pinaud, B.A.; Chen, Z.; Abram, D.N.; Jaramillo, T.F. Thin films of sodium birnessite-type MnO2: Optical properties, electronic band structure, and solar photoelectrochemistry. J. Phys. Chem. C 2011, 115, 11830–11838. [Google Scholar] [CrossRef]
- Chen, Z.; Jaramillo, T.F.; Deutsch, T.G.; Kleiman-Shwarsctein, A.; Forman, A.J.; Gaillard, N.; Garland, R.; Takanabe, K.; Heske, C.; Sunkara, M.; et al. Accelerating materials development for photoelectrochemical hydrogen production: Standards for methods, definitions, and reporting protocols. J. Mater. Res. 2011, 25, 3–16. [Google Scholar] [CrossRef]
- Sherman, D.M. Electronic structures of iron(III) and manganese(IV) (hydr)oxide minerals: Thermodynamics of photochemical reductive dissolution in aquatic environments. Geochim. Cosmochim. Acta 2005, 69, 3249–3255. [Google Scholar] [CrossRef]
- Sakai, N.; Ebina, Y.; Takada, K.; Sasaki, T. Photocurrent generation from semiconducting manganese oxide nanosheets in response to visible light. J. Phys. Chem. B 2005, 109, 9651–9655. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Huang, L.; Wang, J.Q.; Wang, Y.; Gao, L.; Zhu, J.H.; Zou, Z.G. The direct synthesis of mesoporous structured MnO2/TiO2 nanocomposite: A novel visible-light active photocatalyst with large pore size. Nanotechnology 2008, 19, 185604. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. Engl. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernandez-Garcia, M.; Colon, G. Advanced nanoarchitectures for solar photocatalytic applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef] [PubMed]
- Pichat, P. Are TiO2 nanotubes worth using in photocatalytic purification of air and water? Molecules 2014, 19, 15075–15087. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Mazare, A.; Schmuki, P. One-dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.K.; Raja, K.S.; Misra, M.; Mahajan, V.K.; Ahmadian, M. Synthesis of self-organized mixed oxide nanotubes by sonoelectrochemical anodization of Ti–8Mn alloy. Electrochim. Acta 2007, 53, 590–597. [Google Scholar] [CrossRef]
- Macak, J.M.; Hildebrand, H.; Marten-Jahns, U.; Schmuki, P. Mechanistic aspects and growth of large diameter self-organized TiO2 nanotubes. J. Electroanal. Chem. 2008, 621, 254–266. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Helmholz, L.; Russo, M.E. Spectra of manganese(IV) hexafluoride ion (MnF6=) in environments of Oh and D3d symmetry. J. Chem. Phys. 1973, 59, 5455–5470. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; Pergamon Press: New York, NY, USA, 1966. [Google Scholar]
- Devaraj, S.; Munichandraiah, N. Effect of crystallographic structure of MnO2 on its electrochemical capacitance properties. J. Phys. Chem. C 2008, 112, 4406–4417. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Ke, R.; Luo, C.; Hao, J. Effects of precursors on the surface Mn species and the activities for NO reduction over MnOx/TiO2 catalysts. Catal. Commun. 2007, 8, 1896–1900. [Google Scholar] [CrossRef]
- Liao, J.-Y.; Higgins, D.; Lui, G.; Chabot, V.; Xiao, X.; Chen, Z. Multifunctional TiO2–C/MnO2 core–double-shell nanowire arrays as high-performance 3D electrodes for lithium ion batteries. Nano Lett. 2013, 13, 5467–5473. [Google Scholar] [CrossRef] [PubMed]
- Borbón-Nuñez, H.A.; Dominguez, D.; Muñoz-Muñoz, F.; Lopez, J.; Romo-Herrera, J.; Soto, G.; Tiznado, H. Fabrication of hollow TiO2 nanotubes through atomic layer deposition and MWCNT templates. Powder Technol. 2017, 308, 249–257. [Google Scholar] [CrossRef]
- Jana, S.; Basu, S.; Pande, S.; Ghosh, S.K.; Pal, T. Shape-selective synthesis, magnetic properties, and catalytic activity of single crystalline β-MnO2 nanoparticles. J. Phys. Chem. C 2007, 111, 16272–16277. [Google Scholar] [CrossRef]
- Wei, M.; Konishi, Y.; Zhou, H.; Sugihara, H.; Arakawa, H. Synthesis of single-crystal manganese dioxide nanowires by a soft chemical process. Nanotechnology 2005, 16, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Pande, S.; Sinha, A.K.; Sarkar, S.; Pradhan, M.; Basu, M.; Saha, S.; Pal, T. A green chemistry approach for the synthesis of flower-like Ag-doped MnO2 nanostructures probed by surface-enhanced raman spectroscopy. J. Phys. Chem. C 2009, 113, 1386–1392. [Google Scholar] [CrossRef]
- Luo, J.; Zhu, H.T.; Fan, H.M.; Liang, J.K.; Shi, H.L.; Rao, G.H.; Li, J.B.; Du, Z.M.; Shen, Z.X. Synthesis of single-crystal tetragonal α-MnO2 nanotubes. J. Phys. Chem. C 2008, 112, 12594–12598. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, X.; Li, X.; Yang, F.; Jin, B.; Xu, T.; Li, G.; Li, M. Electrodeposition synthesis of MnO2/TiO2 nanotube arrays nanocomposites and their visible light photocatalytic activity. Mater. Res. Bull. 2014, 59, 32–36. [Google Scholar] [CrossRef]
- Ding, S.; Liyong, W.; Shaoyan, Z.; Qiuxiang, Z.; Yu, D.; Shujuan, L.; Yanchao, L.; Quanying, K. Hydrothermal synthesis, structure and photocatalytic property of nano-TiO2–MnO2. Sci. China Ser. B 2003, 46, 542. [Google Scholar] [CrossRef]
- Tang, H.; Berger, H.; Schmid, P.E.; Lévy, F. Optical properties of anatase (TiO2). Solid State Commun. 1994, 92, 267–271. [Google Scholar] [CrossRef]
- Knorr, F.J.; Mercado, C.C.; McHale, J.L. Trap-state distributions and carrier transport in pure and mixed-phase TiO2: Influence of contacting solvent and interphasial electron transfer. J. Phys. Chem. C 2008, 112, 12786–12794. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
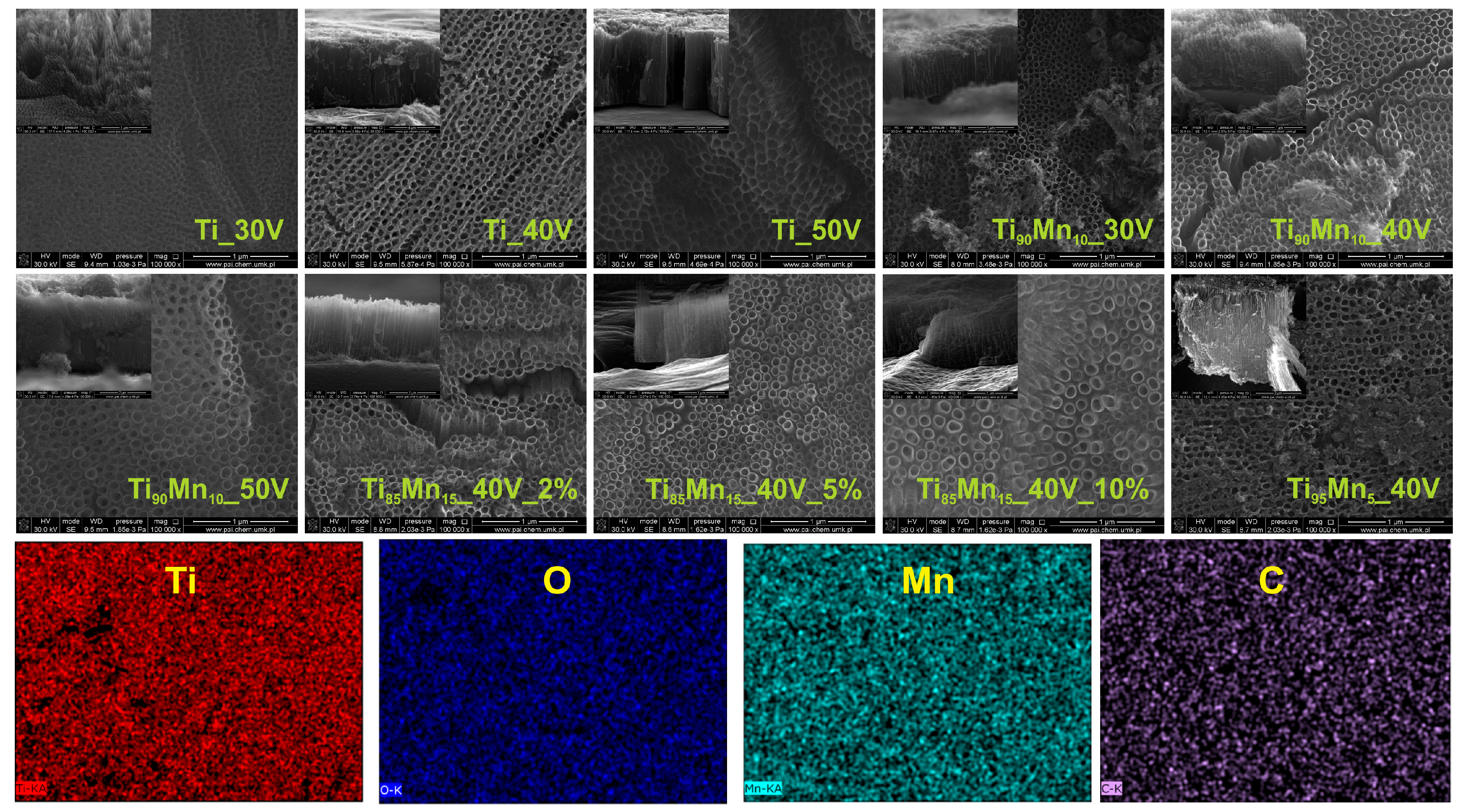

| Sample Label | Preparation Parameters | External Diameter (nm) | Tube Length (μm) | Wall Thickness (nm) | Average Crystallite Size (nm) | EDX Analysis | |||
|---|---|---|---|---|---|---|---|---|---|
| Electrolyte, Applied Voltage | Ti (wt. %) | Mn (wt. %) | C (wt. %) | O (wt. %) | |||||
| Ti_30V | EG 98% (v/v), H2O 2% (v/v), NH4F 0.09 M, 30 V | 81 ± 9 | 1.5 ± 0.1 | 10 ± 2 | 33 | 71.47 | 0 | 0.19 | 28.34 |
| Ti_40V | EG 98% (v/v), H2O 2% (v/v), NH4F 0.09 M, 40 V | 100 ± 7 | 5 ± 0.4 | 13 ± 2 | 34 | 66.73 | 0 | 0.03 | 33.24 |
| Ti_50V | EG 98% (v/v), H2O 2% (v/v), NH4F 0.09 M, 50 V | 120 ± 12 | 16.2 ± 0.2 | 18 ± 3 | 38 | 67.69 | 0 | 0.03 | 32.28 |
| Ti90Mn10_30V | EG 98% (v/v), H2O 2% (v/v), NH4F 0.09 M, 30 V | 76 ± 9 | 1 ± 0.1 | 8 ± 3 | 31 | 76.15 | 8.91 | 0.01 | 14.83 |
| Ti90Mn10_40V | EG 98% (v/v), H2O 2% (v/v), NH4F 0.09 M, 40 V | 92 ± 8 | 1.5 ± 0.1 | 9 ± 3 | 32 | 82.73 | 7.77 | 0.01 | 9.51 |
| Ti90Mn10_50V | EG 98% (v/v), H2O 2% (v/v), NH4F 0.09 M, 50 V | 118 ± 4 | 2.8 ± 0.1 | 9 ± 2 | 34 | 68.79 | 6.46 | 0.03 | 24.72 |
| Ti85Mn15_40V_2% | EG 98% (v/v), H2O 2% (v/v), NH4F 0.09 M, 40 V | 94 ± 11 | 1.3 ± 0.1 | 9 ± 2 | 31 | 77.20 | 11.14 | 0.01 | 11.67 |
| Ti85Mn15_40V_5% | EG 95% (v/v), H2O 5% (v/v), NH4F 0.09 M, 40 V | 90 ± 7 | 1.3 ± 0.1 | 9 ± 2 | 35 | 79.94 | 12.40 | 0.01 | 7.66 |
| Ti85Mn15_40V_10% | EG 90% (v/v), H2O 10% (v/v), NH4F 0.09 M, 40 V | 115 ± 8 | 1.1 ± 0.1 | 11 ± 2 | 34 | 61.76 | 9.11 | 1.18 | 27.95 |
| Ti95Mn5_40V | EG 98% (v/v), H2O 2% (v/v), NH4F 0.09 M, 40 V | 94 ± 8 | 3.4 ± 0.3 | 9 ± 1 | 32 | 70.89 | 2.10 | 0.03 | 27.00 |
| Sample Label | Photocatalytic Toluene Degradation | |
|---|---|---|
| Initial Reaction Rate × 102 (μmol·dm−3·min−1) | Reaction Rate Constant × 103 (min−1) | |
| Ti_30V | 0.37 ± 0.09 | 0.42 ± 0.10 |
| Ti_40V | 0.43 ± 0.09 | 0.49 ± 0.10 |
| Ti_50V | 0.64 ± 0.04 | 0.72 ± 0.04 |
| Ti90Mn10_30V | 8.54 ± 0.53 | 9.57 ± 0.59 |
| Ti90Mn10_40V | 4.97 ± 0.30 | 5.57 ± 0.33 |
| Ti90Mn10_50V | 6.04 ± 0.08 | 6.77 ± 0.09 |
| Ti85Mn15_40V_2% | 4.18 ± 0.77 | 4.69 ± 0.87 |
| Ti85Mn15_40V_5% | 3.79 ± 0.43 | 4.24 ± 0.48 |
| Ti85Mn15_40V_10% | 5.84 ± 1.61 | 6.54 ± 1.81 |
| Ti95Mn5_40V | 5.76 ± 0.12 | 6.45 ± 0.14 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nevárez-Martínez, M.C.; Kobylański, M.P.; Mazierski, P.; Wółkiewicz, J.; Trykowski, G.; Malankowska, A.; Kozak, M.; Espinoza-Montero, P.J.; Zaleska-Medynska, A. Self-Organized TiO2–MnO2 Nanotube Arrays for Efficient Photocatalytic Degradation of Toluene. Molecules 2017, 22, 564. https://doi.org/10.3390/molecules22040564
Nevárez-Martínez MC, Kobylański MP, Mazierski P, Wółkiewicz J, Trykowski G, Malankowska A, Kozak M, Espinoza-Montero PJ, Zaleska-Medynska A. Self-Organized TiO2–MnO2 Nanotube Arrays for Efficient Photocatalytic Degradation of Toluene. Molecules. 2017; 22(4):564. https://doi.org/10.3390/molecules22040564
Chicago/Turabian StyleNevárez-Martínez, María C., Marek P. Kobylański, Paweł Mazierski, Jolanta Wółkiewicz, Grzegorz Trykowski, Anna Malankowska, Magda Kozak, Patricio J. Espinoza-Montero, and Adriana Zaleska-Medynska. 2017. "Self-Organized TiO2–MnO2 Nanotube Arrays for Efficient Photocatalytic Degradation of Toluene" Molecules 22, no. 4: 564. https://doi.org/10.3390/molecules22040564