Expression of Adenosine A2B Receptor and Adenosine Deaminase in Rabbit Gastric Mucosa ECL Cells
Abstract
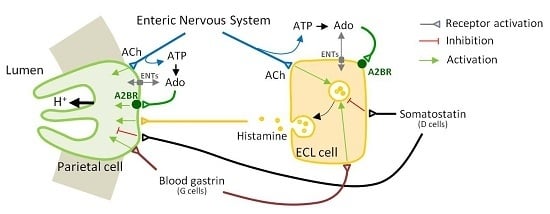
:1. Introduction
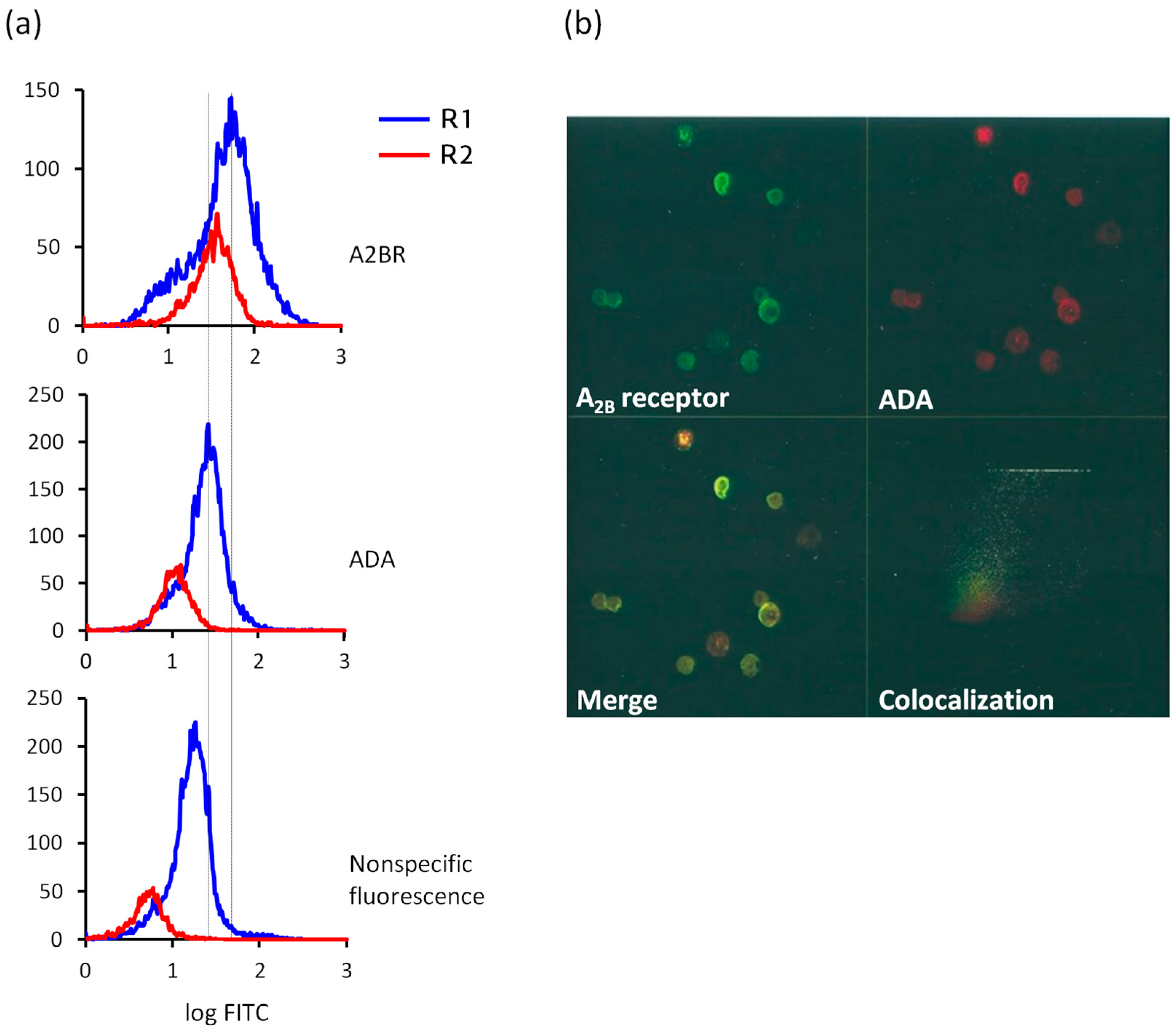
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
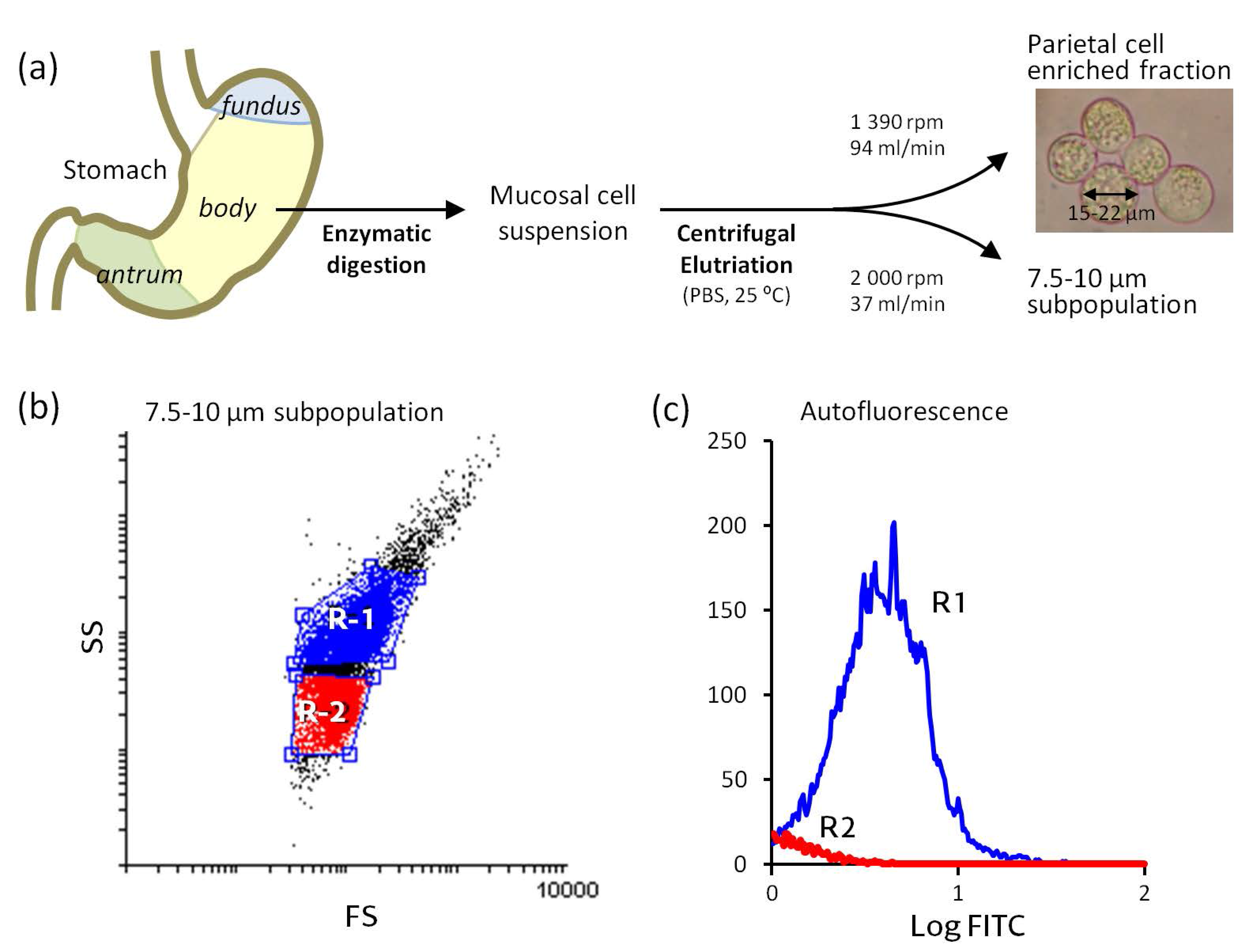
4.2. Isolation and Enrichment of ECL Cells
4.3. Immunostaining Experiments: Flow Cytometry and Confocal Microscopy
4.4. Isolation of ECL Cell Membranes
4.5. Radioligand Binding Experiments
4.6. Determination of Membrane Adenylate Cyclase Activity
4.7. Assessment of Calcium Mobilization in Individual Cells by Microfluorimetry
4.8. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fredholm, B.B. Adenosine—A physiological or pathophysiological agent? J. Mol. Med. 2014, 92, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic signalling in the gastrointestinal tract and related organs in health and disease. Purinergic Signal. 2014, 10, 3–50. [Google Scholar] [CrossRef] [PubMed]
- Ballarin, M.; Fredholm, B.B.; Ambrosio, S.; Mahy, N. Extracellular levels of adenosine and its metabolites in the striatum of awake rats: Inhibition of uptake and metabolism. Acta Physiol. Scand. 1991, 142, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007, 14, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Estevez-Herrera, J.; Dominguez, N.; Pardo, M.R.; Gonzalez-Santana, A.; Westhead, E.W.; Borges, R.; Machado, J.D. ATP: The crucial component of secretory vesicles. Proc. Natl. Acad. Sci. USA 2016, 113, E4098–E4106. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic signalling: Pathophysiology and therapeutic potential. Keio J. Med. 2013, 62, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Harden, T.K.; Sesma, J.I.; Fricks, I.P.; Lazarowski, E.R. Signalling and pharmacological properties of the P2Y receptor. Acta Physiol. 2010, 199, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Linden, J.; Muller, C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—An update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; Irenius, E.; Kull, B.; Schulte, G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem. Pharmacol. 2001, 61, 443–448. [Google Scholar] [CrossRef]
- Feoktistov, I.; Biaggioni, I. Role of adenosine A2B receptors in inflammation. Adv. Pharmacol. 2011, 61, 115–144. [Google Scholar] [PubMed]
- Feoktistov, I.; Ryzhov, S.; Zhong, H.; Goldstein, A.E.; Matafonov, A.; Zeng, D.; Biaggioni, I. Hypoxia modulates adenosine receptors in human endothelial and smooth muscle cells toward an A2B angiogenic phenotype. Hypertension 2004, 44, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Aherne, C.M.; Kewley, E.M.; Eltzschig, H.K. The resurgence of A2B adenosine receptor signaling. Biochim. Biophys. Acta 2011, 1808, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Huang, P. Adenosine A2B Receptor: From Cell Biology to Human Diseases. Front. Chem. 2016, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Rivera-Nieves, J.; Colgan, S.P. Targeting the A2B adenosine receptor during gastrointestinal ischemia and inflammation. Expert Opin. Ther. Targets 2009, 13, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.; Westerman, K.A.; Faigle, M.; Eltzschig, H.K.; Colgan, S.P. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 2006, 20, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Kopic, S.; Murek, M.; Geibel, J.P. Revisiting the parietal cell. Am. J. Physiol. Cell Physiol. 2010, 298, C1–C10. [Google Scholar] [CrossRef] [PubMed]
- Geibel, J.P.; Wagner, C. An update on acid secretion. Rev. Physiol. Biochem. Pharmacol. 2006, 156, 45–60. [Google Scholar] [PubMed]
- Bengtsson, P.; Lundqvist, G.; Nilsson, G. Inhibition of acid formation and stimulation of somatostatin release by cholecystokinin-related peptides in rabbit gastric glands. J. Physiol. 1989, 419, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.L. Functional anatomy and physiology of gastric secretion. Curr. Opin. Gastroenterol. 2015, 31, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Forte, J.G. Cell biology of acid secretion by the parietal cell. Annu. Rev. Physiol. 2003, 65, 103–131. [Google Scholar] [CrossRef] [PubMed]
- Arin, R.M.; Vallejo, A.I.; Rueda, Y.; Fresnedo, O.; Ochoa, B. Stimulation of gastric acid secretion by rabbit parietal cell A2B adenosine receptor activation. Am. J. Physiol. Cell Physiol. 2015, 309, C823–C834. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Casado, V.; Ciruela, F.; Saura, C.; Mallol, J.; Canela, E.I.; Lluis, C. Cell surface adenosine deaminase: Much more than an ectoenzyme. Prog. Neurobiol. 1997, 52, 283–294. [Google Scholar] [CrossRef]
- Pacheco, R.; Martinez-Navio, J.M.; Lejeune, M.; Climent, N.; Oliva, H.; Gatell, J.M.; Gallart, T.; Mallol, J.; Lluis, C.; Franco, R. CD26, adenosine deaminase, and adenosine receptors mediate costimulatory signals in the immunological synapse. Proc. Natl. Acad. Sci. USA 2005, 102, 9583–9588. [Google Scholar] [CrossRef] [PubMed]
- Gracia, E.; Farre, D.; Cortes, A.; Ferrer-Costa, C.; Orozco, M.; Mallol, J.; Lluis, C.; Canela, E.I.; McCormick, P.J.; Franco, R.; et al. The catalytic site structural gate of adenosine deaminase allosterically modulates ligand binding to adenosine receptors. FASEB J. 2013, 27, 1048–1061. [Google Scholar] [CrossRef] [PubMed]
- Gracia, E.; Cortes, A.; Meana, J.J.; Garcia-Sevilla, J.; Herhsfield, M.S.; Canela, E.I.; Mallol, J.; Lluis, C.; Franco, R.; Casado, V. Human adenosine deaminase as an allosteric modulator of human A1 adenosine receptor: Abolishment of negative cooperativity for [3H](R)-pia binding to the caudate nucleus. J. Neurochem. 2008, 107, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Saura, C.; Ciruela, F.; Casado, V.; Canela, E.I.; Mallol, J.; Lluis, C.; Franco, R. Adenosine deaminase interacts with A1 adenosine receptors in pig brain cortical membranes. J. Neurochem. 1996, 66, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Ciruela, F.; Saura, C.; Canela, E.I.; Mallol, J.; Lluis, C.; Franco, R. Adenosine deaminase affects ligand-induced signalling by interacting with cell surface adenosine receptors. FEBS Lett. 1996, 380, 219–223. [Google Scholar] [CrossRef]
- Mirabet, M.; Herrera, C.; Cordero, O.J.; Mallol, J.; Lluis, C.; Franco, R. Expression of A2B adenosine receptors in human lymphocytes: Their role in T cell activation. J. Cell Sci. 1999, 112 Pt 4, 491–502. [Google Scholar] [PubMed]
- Herrera, C.; Morimoto, C.; Blanco, J.; Mallol, J.; Arenzana, F.; Lluis, C.; Franco, R. Comodulation of CXCR4 and CD26 in human lymphocytes. J. Biol. Chem. 2001, 276, 19532–19539. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.; Casado, V.; Ciruela, F.; Schofield, P.; Mallol, J.; Lluis, C.; Franco, R. Adenosine A2B receptors behave as an alternative anchoring protein for cell surface adenosine deaminase in lymphocytes and cultured cells. Mol. Pharmacol. 2001, 59, 127–134. [Google Scholar] [PubMed]
- Arin, R.M.; Vallejo, A.I.; Rueda, Y.; Fresnedo, O.; Ochoa, B. The A2B Adenosine Receptor Colocalizes with Adenosine Deaminase in Resting Parietal Cells from Gastric Mucosa. Biochemistry 2015, 80, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Hakanson, R.; Chen, D.; Andersson, K.; Monstein, H.J.; Zhao, C.M.; Ryberg, B.; Sundler, F.; Mattsson, H. The biology and physiology of the ECL cell. Yale J. Biol. Med. 1994, 67, 123–134. [Google Scholar] [PubMed]
- Zhao, C.M.; Hakanson, R.; Chen, D. Secretory organelles in ECL cells: Effects of pharmacological blockade of the gastrin/CCK2 receptor versus its elimination by gene targeting. Inflammopharmacology 2005, 13, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.J.; Soll, A.H. Cell separation by elutriation: Major and minor cell types from complex tissues. Methods Enzymol. 1989, 171, 482–497. [Google Scholar] [PubMed]
- Modlin, I.M.; Tang, L.H. The gastric enterochromaffin-like cell: An enigmatic cellular link. Gastroenterology 1996, 111, 783–810. [Google Scholar] [CrossRef] [PubMed]
- Geibel, J.; Abraham, R.; Modlin, I.; Sachs, G. Gastrin-stimulated changes in Ca2+ concentration in parietal cells depends on adenosine 3′,5′-cyclic monophosphate levels. Gastroenterology 1995, 109, 1060–1067. [Google Scholar] [CrossRef]
- Yip, L.; Leung, H.C.; Kwok, Y.N. Role of adenosine A1 receptor in the regulation of gastrin release. J. Pharmacol. Exp. Ther. 2004, 310, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Yip, L.; Kwok, Y.N. Role of adenosine A2A receptor in the regulation of gastric somatostatin release. J. Pharmacol. Exp. Ther. 2004, 309, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.K.; Chen, J.F.; Kieffer, T.J.; Kwok, Y.N. Regulation of somatostatin release by adenosine in the mouse stomach. J. Pharmacol. Exp. Ther. 2009, 329, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Aran, J.M.; Colomer, D.; Matutes, E.; Vives-Corrons, J.L.; Franco, R. Presence of adenosine deaminase on the surface of mononuclear blood cells: Immunochemical localization using light and electron microscopy. J. Histochem. Cytochem. 1991, 39, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Casado, V.; Casillas, T.; Mallol, J.; Canela, E.I.; Lluis, C.; Franco, R. The adenosine receptors present on the plasma membrane of chromaffin cells are of the A2b subtype. J. Neurochem. 1992, 59, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Feoktistov, I.; Biaggioni, I. Adenosine A2B receptors. Pharmacol. Rev. 1997, 49, 381–402. [Google Scholar] [PubMed]
- Klotz, K.N.; Hessling, J.; Hegler, J.; Owman, C.; Kull, B.; Fredholm, B.B.; Lohse, M.J. Comparative pharmacology of human adenosine receptor subtypes—Characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch. Pharmacol. 1998, 357, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Klotz, K.N.; Linden, J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001, 53, 527–552. [Google Scholar] [PubMed]
- Linden, J.; Thai, T.; Figler, H.; Jin, X.; Robeva, A.S. Characterization of human A2B adenosine receptors: Radioligand binding, western blotting, and coupling to Gqin human embryonic kidney 293 cells and HMC-1 mast cells. Mol. Pharmacol. 1999, 56, 705–713. [Google Scholar] [PubMed]
- Zeng, N.; Walsh, J.H.; Kang, T.; Helander, K.G.; Helander, H.F.; Sachs, G. Selective ligand-induced intracellular calcium changes in a population of rat isolated gastric endocrine cells. Gastroenterology 1996, 110, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hocker, M.; Koh, T.J.; Wang, T.C. The human histidine decarboxylase promoter is regulated by gastrin and phorbol 12-myristate 13-acetate through a downstream cis-acting element. J. Biol. Chem. 1996, 271, 14188–14197. [Google Scholar] [PubMed]
- Prinz, C.; Kajimura, M.; Scott, D.R.; Mercier, F.; Helander, H.F.; Sachs, G. Histamine secretion from rat enterochromaffinlike cells. Gastroenterology 1993, 105, 449–461. [Google Scholar] [CrossRef]
- Sandor, A.; Kidd, M.; Lawton, G.P.; Miu, K.; Tang, L.H.; Modlin, I.M. Neurohormonal modulation of rat enterochromaffin-like cell histamine secretion. Gastroenterology 1996, 110, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Sachs, G.; Zeng, N.; Prinz, C. Physiology of isolated gastric endocrine cells. Annu. Rev. Physiol. 1997, 59, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.A.; Quesada, A.R.; Nunez, D.C.I.; Sanchez-Jimenez, F. Histamine, polyamines, and cancer. Biochem. Pharmacol. 1999, 57, 1341–1344. [Google Scholar] [CrossRef]
- Zeng, N.; Athmann, C.; Kang, T.; Lyu, R.M.; Walsh, J.H.; Ohning, G.V.; Sachs, G.; Pisegna, J.R. PACAP type I receptor activation regulates ECL cells and gastric acid secretion. J. Clin. Investig. 1999, 104, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.T.; Ai, W.; Sinclair, N.F.; Colucci, R.; Raychowdhury, R.; Koh, T.J.; Wang, T.C. PACAP and gastrin regulate the histidine decarboxylase promoter via distinct mechanisms. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 286, G51–G59. [Google Scholar] [CrossRef] [PubMed]
- Arin, R.M.; Rueda, Y.; Casis, O.; Gallego, M.; Vallejo, A.I.; Ochoa, B. Basolateral expression of GRP94 in parietal cells of gastric mucosa. Biochemistry 2014, 79, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Casado, V.; Franco, R.; Mallol, J.; Lluis, C.; Canela, E.I. Optimal association-saturation procedure for estimating association and dissociation rate parameters in receptor studies. Application to solubilized A1 adenosine receptors. Biochem. J. 1992, 281 Pt 2, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Nordstedt, C.; Fredholm, B.B. A modification of a protein-binding method for rapid quantification of cAMP in cell-culture supernatants and body fluid. Anal. Biochem. 1990, 189, 231–234. [Google Scholar] [CrossRef]
- Jimenez, A.I.; Castro, E.; Mirabet, M.; Franco, R.; Delicado, E.G.; Miras-Portugal, M.T. Potentiation of ATP calcium responses by A2B receptor stimulation and other signals coupled to Gs proteins in type-1 cerebellar astrocytes. GLIA 1999, 26, 119–128. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arin, R.M.; Vallejo, A.I.; Rueda, Y.; Fresnedo, O.; Ochoa, B. Expression of Adenosine A2B Receptor and Adenosine Deaminase in Rabbit Gastric Mucosa ECL Cells. Molecules 2017, 22, 625. https://doi.org/10.3390/molecules22040625
Arin RM, Vallejo AI, Rueda Y, Fresnedo O, Ochoa B. Expression of Adenosine A2B Receptor and Adenosine Deaminase in Rabbit Gastric Mucosa ECL Cells. Molecules. 2017; 22(4):625. https://doi.org/10.3390/molecules22040625
Chicago/Turabian StyleArin, Rosa María, Ana Isabel Vallejo, Yuri Rueda, Olatz Fresnedo, and Begoña Ochoa. 2017. "Expression of Adenosine A2B Receptor and Adenosine Deaminase in Rabbit Gastric Mucosa ECL Cells" Molecules 22, no. 4: 625. https://doi.org/10.3390/molecules22040625
APA StyleArin, R. M., Vallejo, A. I., Rueda, Y., Fresnedo, O., & Ochoa, B. (2017). Expression of Adenosine A2B Receptor and Adenosine Deaminase in Rabbit Gastric Mucosa ECL Cells. Molecules, 22(4), 625. https://doi.org/10.3390/molecules22040625