Chemical Comparison of Two Drying Methods of Mountain Cultivated Ginseng by UPLC-QTOF-MS/MS and Multivariate Statistical Analysis
Abstract
:1. Introduction
2. Results and Discussions
2.1. UPLC-MS Analysis
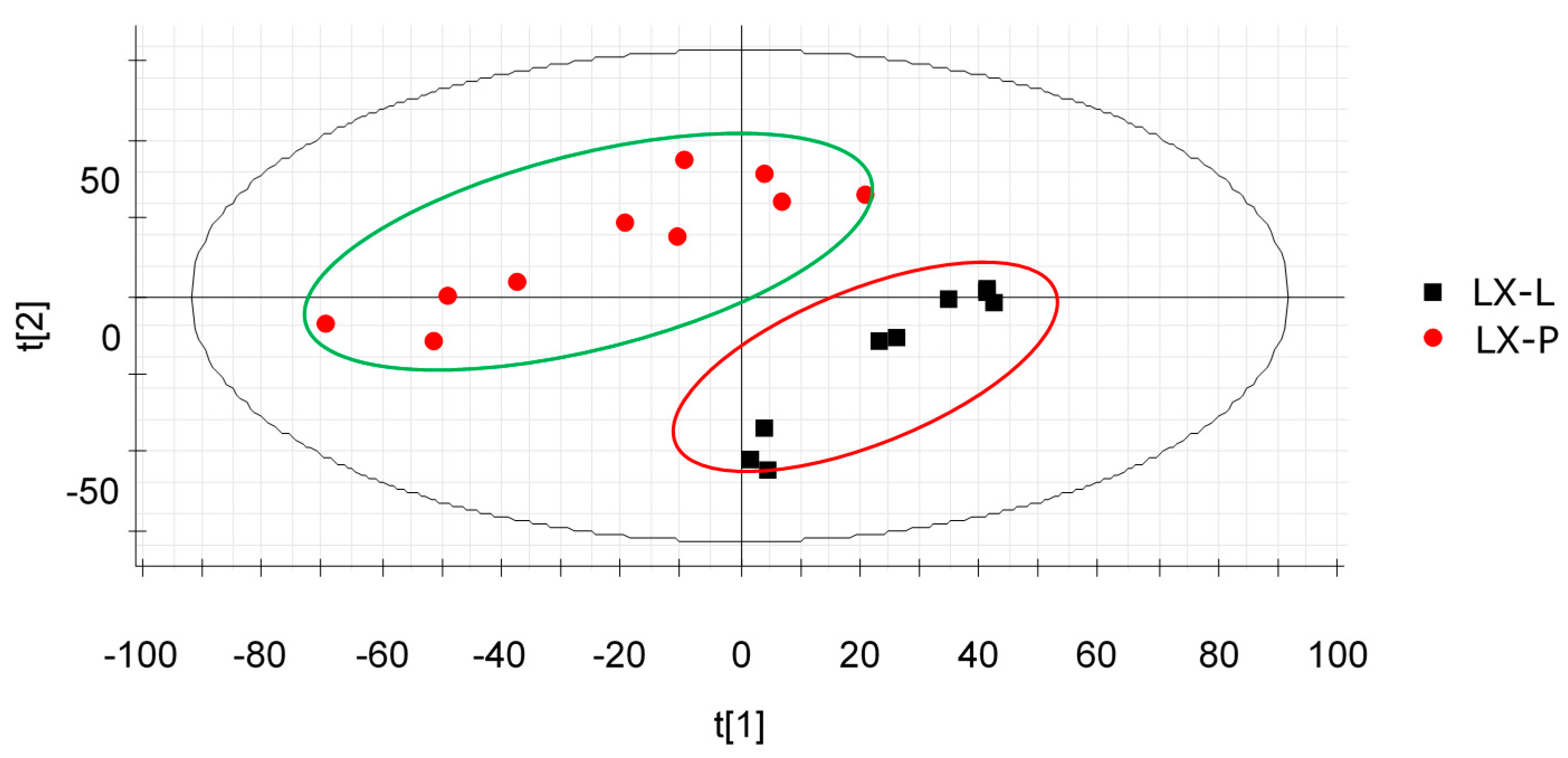
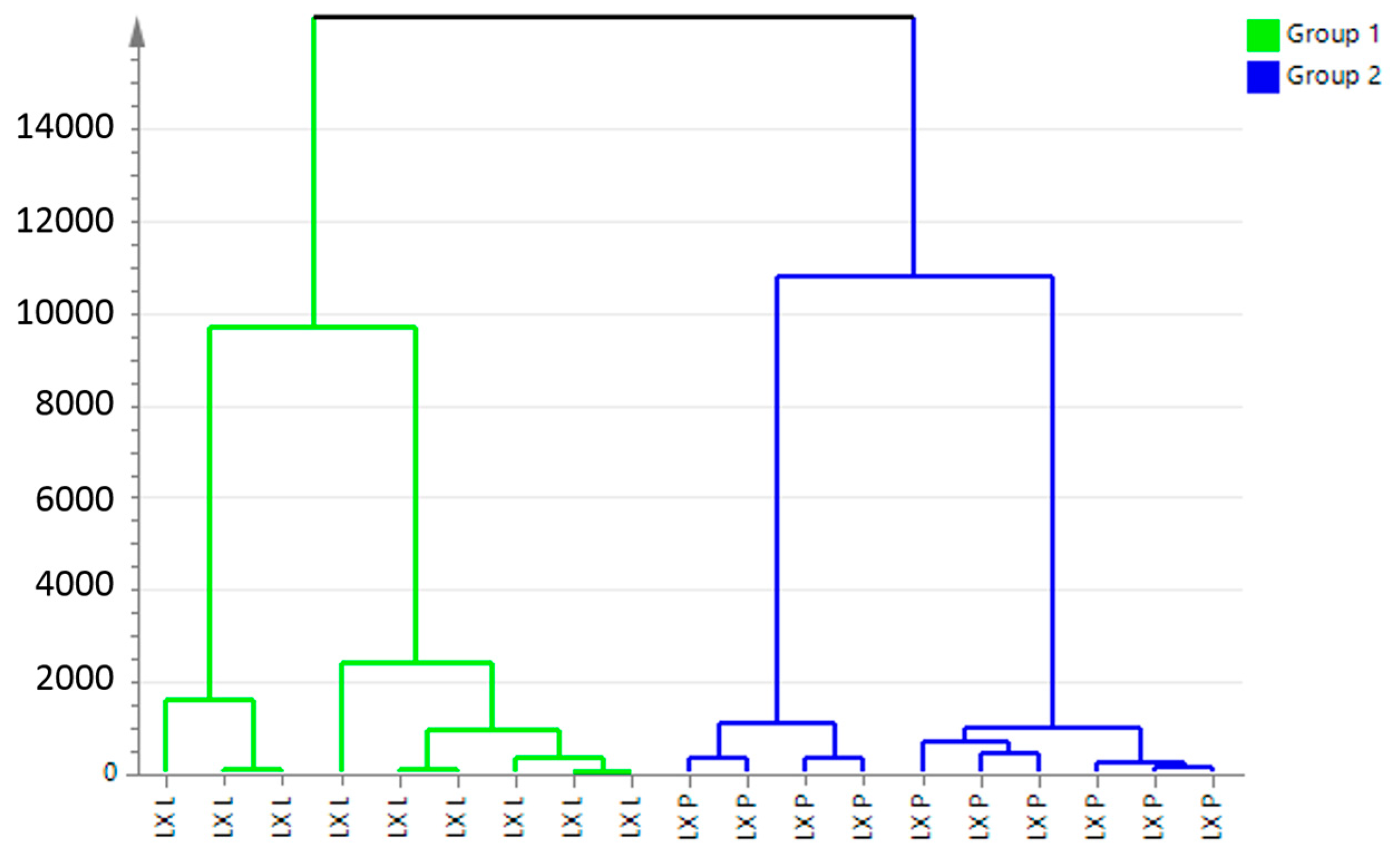
2.2. PCA Analysis
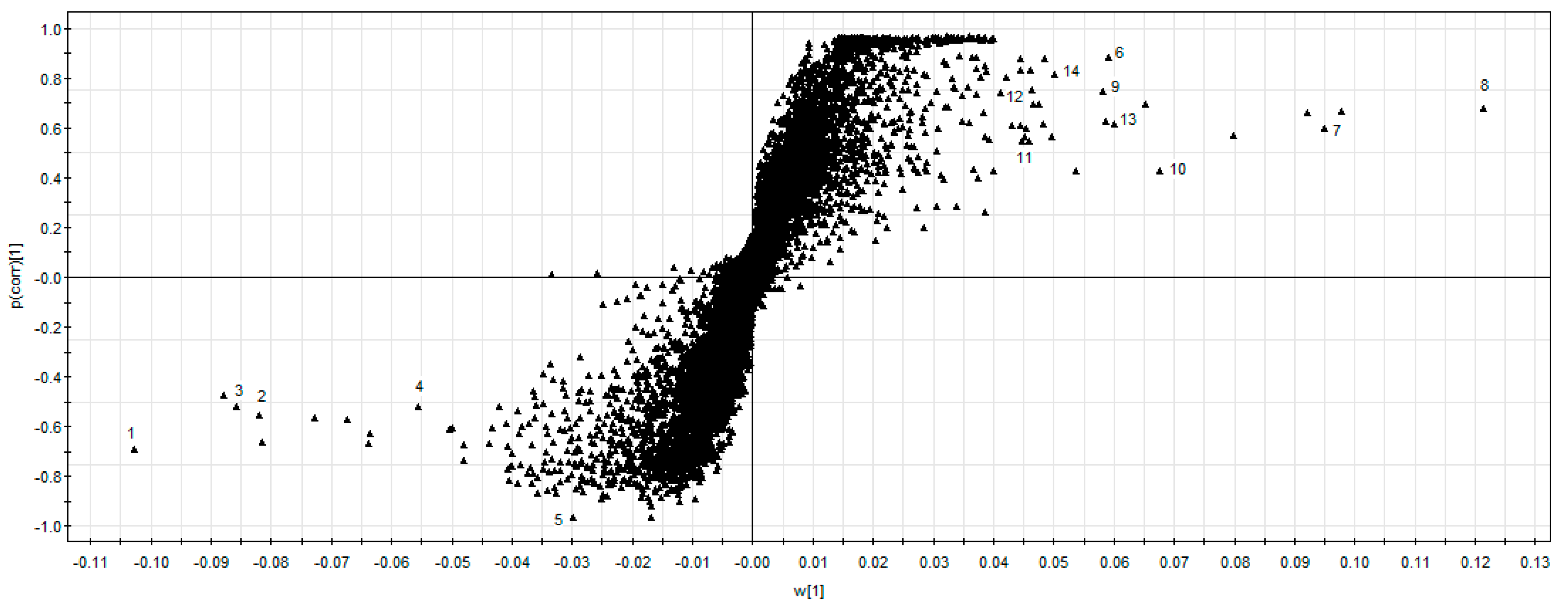
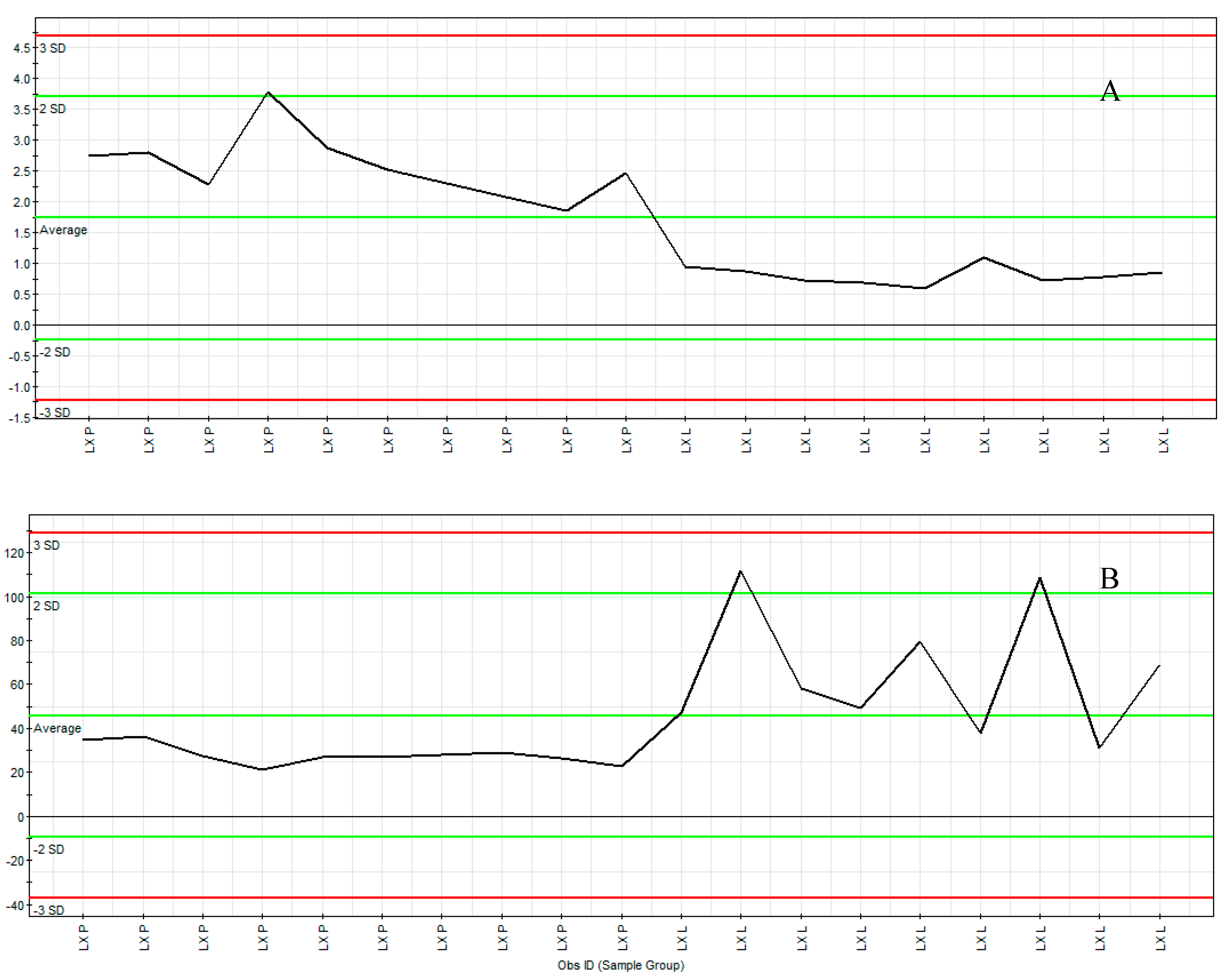
2.3. Marker Ions Analysis
2.4. Maker Ions Assignment
3. Materials and Methods
3.1. Ginseng Samples and Sample Processing
3.2. Sample Preparation
3.3. Reagents
3.4. UPLC-Q-TOF Conditions
3.4.1. Liquid Chromatography Conditions
3.4.2. Mass Spectrometry Conditions
3.5. Data Processing Procedure
3.5.1. The PCA Scores Plot of Samples
3.5.2. The Scatter Plot (S-Plot) from OPLS-DA Analysis
3.5.3. The Elemental Composition Calculation for the Targeting Markers
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hou, G.; Niu, J.; Song, F.; Liu, Z.; Liu, S. Studies on the interactions between ginsenosides and liposome by equilibrium dialysis combined with ultrahigh performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2013, 923–924, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, L.H.; Jia, W.; Liu, X.M.; Dang, H.X.; Mai, W.L.; Ning, W.; Steinmetz, A.; Wang, Y.Q.; Xu, C.J. Comparison of ginsenosides Rg1 and Rb1 for their effects on improving scopolamine-induced learning and memory impairment in mice. Phytother. Res. 2010, 24, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- Schram, G.; Zhang, L.; Derakhchan, K.; Ehrlich, J.R.; Belardinelli, L.; Nattel, S. Ranolazine: Ion-channel-blocking actions and in vivo electrophysiological effects. Br. J. Pharmacol. 2004, 142, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Shen, L.H.; Zhang, J.T. Anti-amnestic and anti-aging effects of ginsenoside Rgl and Rbl and its mechanism of action. Acta Pharmacol. Sin. 2005, 26, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, T.; Kim, S.W.; Hwang, S.Y.; Sohn, S.H.; Yoo, S.K.; Kim, S.K. Panax ginseng reduces oxidative stress and restores antioxidant capacity in aged rats. Nut. Res. 2012, 32, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Yamabe, N.; Kim, Y.J.; Lee, S.; Cho, E.J.; Park, S.H.; Ham, J.; Kim, H.Y.; Kang, K.S. Increase in antioxidant and anticancer effects of ginsenoside Re-lysine mixture by Maillard reaction. Food Chem. 2013, 138, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Hwang, H.S.; Ko, E.J.; Lee, Y.N.; Kwon, Y.M.; Kim, M.C.; Kang, S.M. Immunomodulatory activity of red ginseng against influenza A virus infection. Nutrients 2014, 6, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.R.; Park, W.P.; Kang, W.M.; Jeon, E.Y.; Jang, J.H. Identification and Analysis of Differentially Expressed Genes in Mountain Cultivated Ginseng and Mountain Wild Ginseng. J. Acupunct. Meridian Stud. 2011, 4, 123–128. [Google Scholar] [CrossRef]
- Liu, D.; Li, Y.-G.; Xu, H.; Sun, S.-Q.; Wang, Z.-T. Differentiation of the root of Cultivated Ginseng, Mountain Cultivated Ginseng and Mountain Wild Ginseng using FT-IR and two-dimensional correlation IR spectroscopy. J. Mol. Struct. 2008, 883–884, 228–235. [Google Scholar] [CrossRef]
- Committee, C.P. Pharmacopeia of People’s Republic of China; Chinese Medicine Science and Technology: Beijing, China, 2005. [Google Scholar]
- Hwang, J.W.; Oh, J.H.; Yoo, H.S.; Lee, Y.W.; Cho, C.K.; Kwon, K.R.; Yoon, J.H.; Park, J.; Her, S.; Lee, Z.W. Mountain ginseng extract exhibits anti-lung cancer activity by inhibiting the nuclear translocation of NF-κB. Am. J. Chin. Med. 2012, 40, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Chen, F. Determination of moisture content of ginseng by near infra-red reflectance spectroscopy. Food Chem. 1997, 60, 433–436. [Google Scholar] [CrossRef]
- Davidson, V.J.; Martynenko, A.I.; Parhar, N.K.; Sidahmed, M.; Brown, R.B. Forced-Air Drying of Ginseng Root: Pilot-Scale Control System for Three-Stage Process. Dry. Technol. 2009, 27, 451–458. [Google Scholar] [CrossRef]
- Liu, X.; Qiu, Z.; Wang, L.; Chen, Y. Quality evaluation of Panax notoginseng extract dried by different drying methods. Food Bioprod. Process. 2011, 89, 10–14. [Google Scholar] [CrossRef]
- Ren, G.; Feng, C. Drying of American ginseng (Panax quinquefolium roots by microwave-hot air combination. J. Food Eng. 1998, 35, 433–443. [Google Scholar] [CrossRef]
- Popovich, D.G.; Hu, C.; Durance, T.D.; Kitts, D.D. Retention of Ginsenosides in Dried Ginseng Root: Comparison of Drying Methods. J. Food Sci. 2006, 70, s355–s358. [Google Scholar] [CrossRef]
- Ning, X.; Lee, J.; Han, C. Drying characteristics and quality of red ginseng using far-infrared rays. J. Ginseng Res. 2015, 39, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Kasper, J.C.; Winter, G.; Friess, W. Recent advances and further challenges in lyophilization. Eur. J. Pharm. Biopharm. 2013, 85, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Sarraguça, M.C.; Beer, T.D.; Vervaet, C.; Remon, J.P.; Lopes, J.A. A batch modelling approach to monitor a freeze-drying process using in-line Raman spectroscopy. Talanta 2010, 83, 130–138. [Google Scholar]
- Fang, L.I.; Qiao, L.I.; Song, D.; Liu, P.P.; Chong-Ning, L.V.; Wang, J.; Jia, L.Y.; Jin-Cai, L.U. Effect of different drying methods on ginsenosides in flower of Panax ginseng and Panax quinquefolius. Chin. Tradit. Herb. Dru. 2015, 46, 2937–2942. [Google Scholar]
- Wang, Y.; Huang, M.; Sun, R.; Lei, P. Extraction, characterization of a Ginseng fruits polysaccharide and its immune modulating activities in rats with Lewis lung carcinoma. Carbohydr. Polym. 2015, 127, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Kim, Y.J.; Jeon, J.N.; Wang, C.; Min, J.W.; Noh, H.Y.; Yang, D.C. Effect of White, Red and Black Ginseng on Physicochemical Properties and Ginsenosides. Plant Foods Hum. Nutr. 2015, 70, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Du, B.; Cai, W.; Xu, B. Ginsenosides and amino acids in flavored ginseng chips as affected byfood formulation and processing technology. LWT Food Sci. Technol. 2015, 62, 517–524. [Google Scholar] [CrossRef]
- Qiu, Y.; Lu, X.; Pang, T.; Ma, C.; Li, X.; Xu, G. Determination of radix ginseng volatile oils at different ages by comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry. J. Sep. Sci. 2008, 31, 3451–3457. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the 41 compounds are available from the authors. |

| No. | tR (min) | Precursor Ion and/or Adduct Ions | Exact Mass [M + H]+ | Error (ppm) | Formula | Identification |
|---|---|---|---|---|---|---|
| 1 | 1.99 | 933.5476 [M + H]+ | 933.5423 | 5.6 | C47H80O18 | ginsenoside Re4 |
| 2 | 2.08 | 963.5582 [M + H]+, 980.5865 [M + NH4]+ | 963.5529 | 5.5 | C48H82O19 | notoginsenoside R3 isomer |
| 3 | 2.11 | 933.5474 [M + H]+ | 933.5423 | 5.4 | C47H80O18 | notoginsenoside R1 |
| 4 | 2.50 | 947.5628 [M + H]+ | 947.5579 | 5.1 | C48H82O18 | ginsenoside Re |
| 5 | 2.50 | 801.5038 [M + H]+ | 801.5000 | 4.7 | C42H72O14 | ginsenoside Rg1 |
| 6 | 2.77 | 887.5040 [M + H]+, 904.5305 [M + NH4]+ | 887.5004 | 4.0 | C45H74O17 | malonyl-ginsenoside Rg1 |
| 7 | 2.98 | 1033.5633 [M + H]+ | 1033.5583 | 4.8 | C51H84O21 | malonyl-ginsenoside Re |
| 8 | 3.03 | 1033.5630 [M + H]+ | 1033.5583 | 4.5 | C51H84O21 | malonyl-ginsenoside Re isomer |
| 9 | 4.02 | 1241.6609 [M + H]+, 1258.6971 [M + NH4]+ | 1241.6530 | 6.3 | C59H100O27 | ginsenoside Ra3/notoginsenoside R4/notoginsenoside Fa |
| 10 | 4.13 | 1327.6656 [M + H]+, 1327.6980 [M + NH4]+ | 1327.6534 | 9.1 | C62H102O30 | malonyl-ginsenoside Ra3 |
| 11 | 4.25 | 801.5033 [M + H]+ | 801.5000 | 4.1 | C42H72O14 | ginsenoside Rf |
| 12 | 4.37 | 1327.6666 [M + H]+ | 1327.6534 | 9.9 | C62H102O30 | malonyl-notoginsenoside R4 |
| 13 | 4.45 | 1211.6492 [M + H]+, 1228.6871 [M + NH4]+ | 1211.6425 | 5.5 | C58H98O26 | ginsenoside Ra2 |
| 14 | 4.56 | 1109.6176 [M + H]+, 1126.6500 [M + NH4]+ | 1109.6108 | 6.1 | C54H92O23 | ginsenoside Rb1 |
| 15 | 4.60 | 1327.6655[M + H]+ | 1327.6534 | 9.1 | C62H102O30 | malonyl-notoginsenoside Fa |
| 16 | 4.65 | 1195.6194 [M + H]+ | 1195.6112 | 6.8 | C57H94O26 | malonyl-ginsenoside Rb1 |
| 17 | 4.77 | 1079.6058 [M + H]+ | 1079.6002 | 5.1 | C53H90O22 | ginsenoside Rc |
| 18 | 4.77 | 1211.6507 [M + H]+, 1228.6721 [M + NH4]+ | 1211.6425 | 6.7 | C58H98O26 | ginsenoside Ra1 |
| 19 | 4.85 | 1165.6094 [M + H]+ | 1165.6006 | 7.2 | C56H92O25 | malonyl-ginsenoside Rc |
| 20 | 4.85 | 1297.6490 [M + H]+ | 1297.6429 | 4.7 | C61H100O29 | malonyl-ginsenoside Ra2/Ra1 |
| 21 | 4.91 | 1195.6187 [M + H]+, 1212.9451 [M + NH4]+ | 1195.6112 | 6.2 | C57H94O26 | malonyl-ginsenoside Rb1 isomer |
| 22 | 4.99 | 1079.6069 [M + H]+, 1096.6310 [M + NH4]+ | 1079.6002 | 6.2 | C53H90O22 | ginsenoside Rb2 |
| 23 | 5.10 | 1165.6088 [M + H]+, 1182.641 [M + NH4]+ | 1165.6006 | 7.0 | C56H92O25 | malonyl-ginsenoside Rb2 |
| 24 | 5.23 | 1151.6284 [M + H]+, 1168.6471 [M + NH4]+ | 1151.6213 | 6.1 | C56H94O24 | quinquenoside R1 |
| 25 | 5.24 | 1079.6069 [M + H]+, 1096.6312 [M + NH4]+ | 1079.6002 | 6.2 | C53H90O22 | ginsenoside Rb3 |
| 26 | 5.37 | 1165.6067 [M + H]+, 1182.641 [M + NH4]+ | 1165.6006 | 5.2 | C56H92O25 | malonyl-ginsenoside Rb3 |
| 27 | 5.41 | 1165.6085 [M + H]+, 1182.641 [M + NH4]+ | 1165.6006 | 6.7 | C56H92O25 | malonyl-ginsenoside Rb3 isomer |
| 28 | 5.55 | 947.5621 [M + H]+, 964.5913 [M + NH4]+ | 947.5579 | 4.4 | C48H82O18 | ginsenoside Rd |
| 29 | 5.56 | 767.4960 [M + H]+ | 767.4960 | 1.8 | C42H70O12 | ginsenoside Rg6 |
| 30 | 5.64 | 1033.5644 [M + H]+, 1050.590 [M + NH4]+ | 1033.5583 | 5.9 | C51H84O21 | malonyl-ginsenoside Rd |
| 31 | 5.76 | 1121.6008 [M + H]+ | 1121.6108 | −8.2 | C55H92O23 | ginsenoside Rs1 |
| 32 | 5.92 | 1033.5653 [M + H]+, 1050.590 [M + NH4]+ | 1033.5583 | 6.7 | C51H84O21 | malonyl-ginsenoside Rd isomer |
| 33 | 5.95 | 947.5623 [M + H]+ | 947.5579 | 4.6 | C48H82O18 | gypenoside XVII |
| 34 | 6.01 | 1121.6180 [M + H]+ | 1121.6108 | 5.9 | C55H92O23 | ginsenoside Rs2 |
| 35 | 6.12 | 1147.6347 [M + H]+ | 1147.6264 | 7.2 | C57H94O23 | ginsenoside Ra7 |
| 36 | 6.28 | 917.5440 [M + H]+ | 917.5474 | −3.7 | C47H80O17 | notoginsenoside Fe |
| 37 | 6.36 | 1147.6348 [M + H]+ | 1147.6264 | 7.3 | C57H94O23 | ginsenoside Ra8 |
| 38 | 6.40 | 767.4987 [M + H]+ | 767.4946 | 5.3 | C42H70O12 | ginsenoside F4 |
| 39 | 6.51 | 917.5518 [M + H]+ | 917.5474 | 4.7 | C47H80O17 | vinaginsenoside R16 |
| 40 | 7.29 | 785.5082 [M + H]+ | 785.5051 | 3.9 | C42H72O13 | ginsenoside Rg3 |
| 41 | 16.68 | 663.4530 [M + H]+, 685.4382 [M + Na]+ | 663.4472 | 8.7 | C38H62O9 | ginsenosde Rs6/Rs7 |
| No. | Identification | tR (min) | Molecular Formula | Ion | Mean Measured Mass | Theoretical Exact Mass | Mass Accuracy (ppm) | Fragment Ions | Classification |
|---|---|---|---|---|---|---|---|---|---|
| 1 | ginsenoside Rb1 | 4.56 | C54H92O23 | [M + H]+ | 1109.6176 | 1109.6180 | −0.4 | 929, 767, 605, 425 | LX-P |
| 2 | ginsenosede Rc | 4.76 | C53H90O22 | [M + H]+ | 1079.6058 | 1079.6002 | 5.1 | 929, 767, 605 | LX-P |
| 3 | ginsenoside Rb2 | 4.98 | C53H90O22 | [M + H]+ | 1079.6069 | 1079.6002 | 6.2 | 929, 767, 605, 425 | LX-P |
| 4 | ginsenoside Rg6 | 5.56 | C56H94O24 | [M + H]+ | 767.4960 | 767.4946 | 1.8 | 621, 459 | LX-P |
| 5 | dendrolasin | 10.00 | C15H22O | [M + H]+ | 219.1748 | 219.1749 | −0.5 | 203, 149 | LX-P |
| 6 | mal-ginsenoside Re | 2.98 | C51H84O21 | [M + H]+ | 1033.5633 | 1033.5583 | 4.8 | 1015, 853, 767, 605 | LX-L |
| 7 | mal-ginsenoside Rb1 | 4.65 | C57H94O26 | [M + H]+ | 1195.6194 | 1195.6112 | 6.8 | 1109, 1015, 853, 835, 785, 605, 425 | LX-L |
| 8 | mal-ginsenoside Rc | 4.85 | C56H92O25 | [M + H]+ | 1165.6094 | 1165.6006 | 7.5 | 1187, 1079, 1015, 853, 835,605, 425, 411 | LX-L |
| 9 | mal-ginsenoside Rb1 isomer | 4.91 | C57H94O26 | [M + H]+ | 1195.6187 | 1195.6112 | 6.2 | 1109, 1015, 853, 785 | LX-L |
| 10 | mal-ginsenoside Rb2 | 5.10 | C56H92O25 | [M + H]+ | 1165.6088 | 1165.6006 | 7.0 | 1079, 871, 853, 411 | LX-L |
| 11 | mal-ginsenoside Rb3 | 5.37 | C56H92O25 | [M + H]+ | 1165.6067 | 1165.6006 | 5.2 | 1079, 871, 853, 411 | LX-L |
| 12 | mal-ginsenoside Rd iosmer | 5.93 | C51H84O21 | [M + H]+ | 1033.5653 | 1033.5583 | 6.7 | 947, 871, 785, 605 | LX-L |
| 13 | gypenoside XVII | 5.95 | C48H82O18 | [M + H]+ | 947.5623 | 947.5579 | 4.6 | 785, 767, 605, 443 | LX-L |
| 14 | notoginsenoside Fe | 6.28 | C47H80O17 | [M + H]+ | 917.5517 | 917.5474 | −3.7 | 899, 785, 737, 605 | LX-L |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.-f.; Xu, S.-y.; Zhang, Y.; Zhang, H.; Liu, M.-n.; Liu, H.; Gao, Y.; Xue, X.; Xiong, H.; Lin, R.-c.; et al. Chemical Comparison of Two Drying Methods of Mountain Cultivated Ginseng by UPLC-QTOF-MS/MS and Multivariate Statistical Analysis. Molecules 2017, 22, 717. https://doi.org/10.3390/molecules22050717
Xu X-f, Xu S-y, Zhang Y, Zhang H, Liu M-n, Liu H, Gao Y, Xue X, Xiong H, Lin R-c, et al. Chemical Comparison of Two Drying Methods of Mountain Cultivated Ginseng by UPLC-QTOF-MS/MS and Multivariate Statistical Analysis. Molecules. 2017; 22(5):717. https://doi.org/10.3390/molecules22050717
Chicago/Turabian StyleXu, Xin-fang, Shu-ya Xu, Ying Zhang, Hui Zhang, Meng-nan Liu, Huan Liu, Yan Gao, Xue Xue, Hui Xiong, Rui-chao Lin, and et al. 2017. "Chemical Comparison of Two Drying Methods of Mountain Cultivated Ginseng by UPLC-QTOF-MS/MS and Multivariate Statistical Analysis" Molecules 22, no. 5: 717. https://doi.org/10.3390/molecules22050717
APA StyleXu, X.-f., Xu, S.-y., Zhang, Y., Zhang, H., Liu, M.-n., Liu, H., Gao, Y., Xue, X., Xiong, H., Lin, R.-c., & Li, X.-r. (2017). Chemical Comparison of Two Drying Methods of Mountain Cultivated Ginseng by UPLC-QTOF-MS/MS and Multivariate Statistical Analysis. Molecules, 22(5), 717. https://doi.org/10.3390/molecules22050717






