Optimizing the Readout of Lanthanide-DOTA Complexes for the Detection of Ligand-Bound Copper(I)
Abstract
:1. Introduction
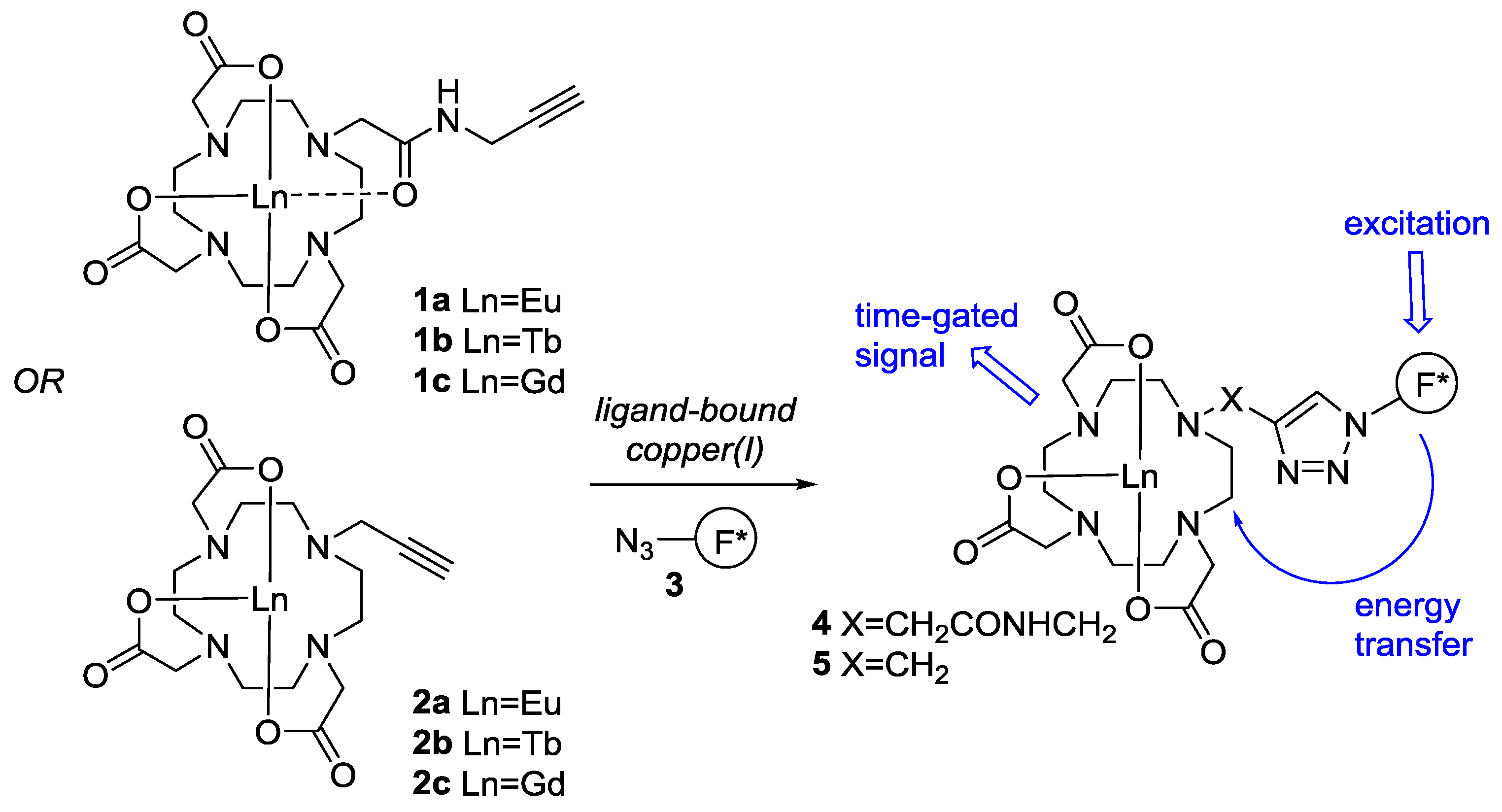
2. Results and Discussion
2.1. Synthesis of Sensor Components
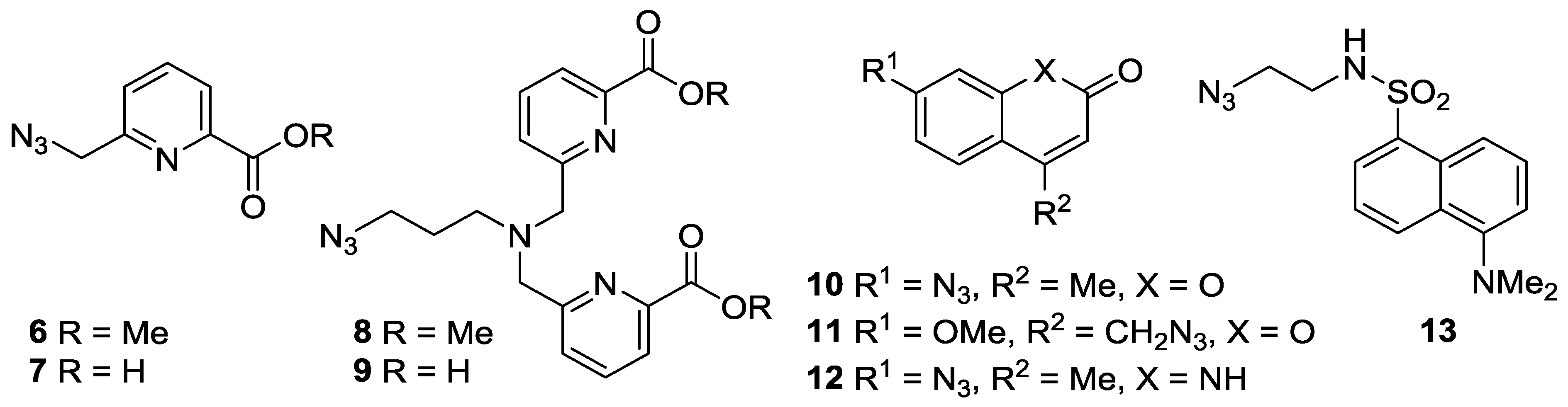
- Picolinate-derivatized ligands have previously been shown to act as sensitizers for europium and terbium ions [32,33,34,35], to undergo cellular entry via diffusion, and to be suitable for two photon excitation studies. Thus picolinate azides 6–9 were prepared from pyridine-2,6-dicarboxylic acid dimethyl ester through ready adaptation of the synthetic route to the 10-coordinate N,N,N′,N′-tetrakis[(6-carboxypyridin-2-yl)methyl]ethylenediamine (tpaen) ligand reported by Mazzanti et al. [33,36,37] (SM Scheme S2; three steps (28% overall), four steps (15% overall), three steps (27% overall), and four steps (22% overall) respectively).
- Lanthanide complexes based on coumarin derivatives were pursued due to the known membrane permeability of coumarin azides [38], and previous reports of strong fluorescence activation of lanthanides by coumarin [39,40,41,42]. Coumarin azides 10 [43] and 11 [44] were both readily prepared in one step from commercially available starting materials (in 72% and 82% yields, respectively).
- A derivative of carbostyril 124, azide 12, was chosen because lanthanides complexed with ligands functionalised with carbostyril 124 have been shown to have long-lifetimes, good water solubility, and measurable brightness [45,46,47]. Diazotization of carbostyril 124, followed by addition of sodium azide, yielded 12 (65%) [48].
2.2. Component Coupling by the CuAAC Reaction
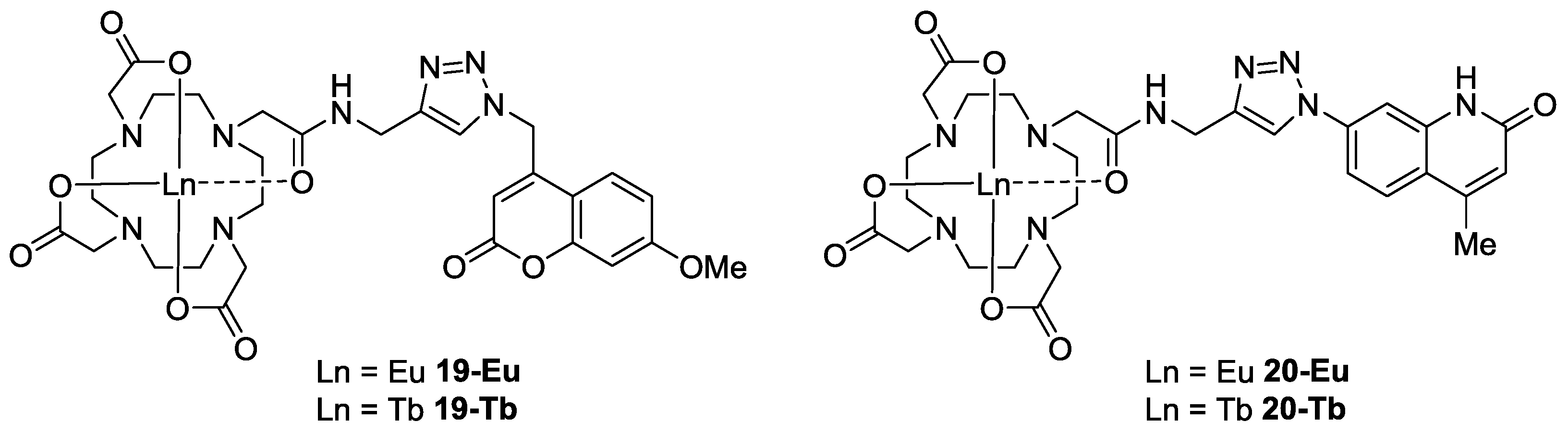
2.3. Initial Analysis of the Sensor Design by Component
2.4. Normalization of Luminescence Output Data by IR
2.5. Metal Ion Specificity for Formation of the Optimum Complex 19-Eu
3. Materials and Methods
3.1. General Procedure for the Synthesis of CuAAC Coupled Complexes 14–21
3.2. Luminescence Measurements on Crude CuAAC Coupled Complexes 14–21
3.3. Normalization of Output of CuAAC Coupled Complexes 19 and 20 by IR
3.4. Data for Purified Lead Complex 19-Eu
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ala, A.; Walker, A.P.; Ashkan, K.; Dooley, J.S.; Schilsky, M.L. Wilson’s disease. Lancet 2007, 369, 397–408. [Google Scholar] [CrossRef]
- Bandmann, O.; Weiss, K.H.; Kaler, S.G. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 2015, 14, 103–113. [Google Scholar] [CrossRef]
- Crisponi, G.; Nurchi, V.M.; Fanni, D.; Gerosa, C.; Nemolatob, S.; Faa, G. Copper-related diseases: From chemistry to molecular pathology. Coord. Chem. Rev. 2010, 254, 876–889. [Google Scholar] [CrossRef]
- Valentine, J.S.; Hart, P.J. Misfolded CuZnSOD and amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 2003, 100, 3617–3622. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, E.; Furukawa, Y. Copper homeostasis as a therapeutic target in amyotrophic lateral sclerosis with SOD1 mutations. Int. J. Mol. Sci. 2016, 17, 636. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Greenough, M.A.; Ramírez Munoz, A.; Bush, A.I.; Opazo, C.M. Metallo-pathways to Alzheimer’s disease: Lessons from genetic disorders of copper trafficking. Metallomics 2016, 8, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Rae, T.D.; Schmidt, P.J.; Pufahl, R.A.; Culotta, V.C.; O’Halloran, T.V. Undetectable intracellular free copper: The requirement of a copper chaperone for superoxide dismutase. Science 1999, 284, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Torres, K.M.; Chang, C.J. Synthetic fluorescent probes for studying copper in biological systems. Chem. Soc. Rev. 2015, 44, 4400–4414. [Google Scholar]
- Aron, A.T.; Ramos-Torres, K.M.; Cotruvo, J.A., Jr.; Chang, C.J. Recognition- and reactivity-based fluorescent probes for studying transition metal signaling in living systems. Acc. Chem. Res. 2015, 48, 2434–2442. [Google Scholar] [CrossRef] [PubMed]
- Viguier, R.F.H.; Hulme, A.N. A sensitized europium complex generated by micromolar concentrations of copper(I): Toward the detection of copper(I) in biology. J. Am. Chem. Soc. 2006, 128, 11370–11371. [Google Scholar] [CrossRef] [PubMed]
- Taki, M.; Iyoshi, S.; Ojida, A.; Hamachi, I.; Yamamoto, Y. Development of highly sensitive fluorescent probes for detection of intracellular copper(I) in living systems. J. Am. Chem. Soc. 2010, 132, 5938–5939. [Google Scholar] [CrossRef] [PubMed]
- Jun, M.E.; Roy, B.; Ahn, K.H. “Turn-on” fluorescent sensing with “reactive” probes. Chem. Soc. Rev. 2011, 47, 7583–7601. [Google Scholar] [CrossRef] [PubMed]
- Butler, S.J.; Lamarque, L.; Pala, R.; Parker, D. EuroTracker dyes: Highly emissive europium complexes as alternative organelle stains for live cell imaging. Chem. Sci. 2014, 5, 1750–1756. [Google Scholar] [CrossRef]
- Germeroth, A.I.; Hanna, J.R.; Karim, R.; Kundel, F.; Lowther, J.; Neate, P.G.N.; Blackburn, E.A.; Wear, M.A.; Campopiano, D.J.; Hulme, A.N. Triazole biotin: A tight-binding biotinidase-resistant conjugate. Org. Biomol. Chem. 2013, 11, 7700–7704. [Google Scholar] [CrossRef] [PubMed]
- Prasuhn, D.E., Jr.; Yeh, R.M.; Obenaus, A.; Manchester, M.; Finn, M.G. Viral MRI contrast agents: Coordination of Gd by native virions and attachment of Gd complexes by azide–alkyne cycloaddition. Chem. Commun. 2007, 1269–1271. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Kohlmeir, E.K.; Meade, T.J. Synthesis of multimeric MR contrast agents for cellular imaging. J. Am. Chem. Soc. 2008, 130, 6662–6663. [Google Scholar] [CrossRef] [PubMed]
- Jauregui, M.; Perry, W.S.; Allain, C.; Vidler, L.R.; Willis, M.C.; Kenwright, A.M.; Snaith, J.S.; Stasiuk, G.J.; Lowe, M.P.; Faulkner, S. Changing the local coordination environment in mono- and bi- nuclear lanthanide complexes through ‘click’ chemistry. Dalton Trans. 2009, 6283–6285. [Google Scholar] [CrossRef] [PubMed]
- Stasiuk, G.J.; Lowe, M.P. Click chemistry with lanthanide complexes: A word of caution. Dalton Trans. 2009, 9725–9727. [Google Scholar] [CrossRef] [PubMed]
- Junker, A.K.R.; Tropiano, M.; Faulkner, S.; Sørensen, T.J. Kinetically inert lanthanide complexes as reporter groups for binding of potassium by 18-crown-6. Inorg. Chem. 2016, 55, 12299–12308. [Google Scholar] [CrossRef] [PubMed]
- Verwilst, P.; Eliseeva, S.V.; Carron, S.; Vander Elst, L.; Burtea, C.; Dehaen, G.; Laurent, S.; Binnemans, K.; Muller, R.N.; Parac-Vogt, T.N.; et al. A modular approach towards the synthesis of target-specific MRI contrast agents. Eur. J. Inorg. Chem. 2011, 3577–3585. [Google Scholar] [CrossRef]
- Mastarone, D.J.; Harrison, V.S.R.; Eckermann, A.L.; Parigi, G.; Luchinat, C.; Meade, T.J. A Modular System for the synthesis of multiplexed magnetic resonance probes. J. Am. Chem. Soc. 2011, 133, 5329–5337. [Google Scholar] [CrossRef] [PubMed]
- Desbois, N.; Pacquelet, S.; Dubois, A.; Michelin, C.; Gros, C.P. Easy access to heterobimetallic complexes for medical imaging applications via microwave-enhanced cycloaddition. Beilstein J. Org. Chem. 2015, 11, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Molloy, J.K.; Kotova, O.; Peacock, R.D.; Gunnlaugsson, T. Synthesis of luminescent homo-dinuclear cationic lanthanide cyclen complexes bearing amide pendant arms through the use of copper catalysed (1,3-Huisgen, CuAAC) click chemistry. Org. Biomol. Chem. 2012, 10, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, O.; Olsson, A. Synthesis of Cyclen Derivatives. Patent WO2006112723 A1, 26 October 2006. [Google Scholar]
- Martinelli, J.; Balali-Mood, B.; Panizzo, R.; Lythgoe, M.F.; White, A.J.P.; Ferretti, P.; Steinke, J.H.G.; Vilar, R. Coordination chemistry of amide-functionalised tetraazamacrocycles: Structural, relaxometric and cytotoxicity studies. Dalton Trans. 2010, 39, 10056–10067. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.M.G.; Harte, A.J.; Quinn, S.J.; Gunnlaugsson, T. Recent developments in the field of supramolecular lanthanide luminescent sensors and self-assemblies. Coord. Chem. Rev. 2008, 252, 2512–2527. [Google Scholar] [CrossRef]
- Moore, E.G.; Samuel, A.P.S.; Raymond, K.N. From antenna to assay: Lessons learned in lanthanide luminescence. Acc. Chem. Res. 2009, 42, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, C.P.; Murray, B.S.; New, E.J.; Pal, R.; Parker, D. Cell-penetrating metal complex optical probes: Targeted and responsive systems based on lanthanide luminescence. Acc. Chem. Res. 2009, 42, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Heffern, M.C.; Matosziuk, L.M.; Meade, T.J. Lanthanide probes for bioresponsive imaging. Chem. Rev. 2014, 114, 4496–4539. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chang, H.; Xie, J.; Zhao, B.; Liu, B.; Xu, S.; Pei, W.; Ren, N.; Huang, L.; Huang, W. Recent developments in lanthanide-based luminescent probes. Coord. Chem. Rev. 2014, 273–274, 201–212. [Google Scholar] [CrossRef]
- Sendor, D.; Hilder, M.; Juestel, T.; Junk, P.C.; Kynast, U.H. One dimensional energy transfer in lanthanoid picolinates. Correlation of structure and spectroscopy. New J. Chem. 2003, 27, 1070–1077. [Google Scholar] [CrossRef]
- Chatterton, N.; Bretonnière, Y.; Pécaut, J.; Mazzanti, M. An efficient design for the rigid assembly of four bidentate chromophores in water-stable highly luminescent lanthanide complexes. Angew. Chem. Int. Ed. 2005, 44, 7595–7598. [Google Scholar] [CrossRef] [PubMed]
- Pope, S.J.A.; Laye, R.H. Design, synthesis and photophysical studies of an emissive, europium based, sensor for zinc. Dalton Trans. 2006, 3108. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Morikawa, M.; Kimizuka, N. Conversion of molecular information by luminescent nanointerface self-assembled from amphiphilic Tb(III) complexes. J. Am. Chem. Soc. 2011, 133, 17370–17374. [Google Scholar] [CrossRef] [PubMed]
- Behera, H.; Ramkumar, V.; Madhavan, N. Cation-transporting peptides: Scaffolds for functionalized pores? Chem. Eur. J. 2015, 21, 10179–10184. [Google Scholar] [CrossRef] [PubMed]
- Gracia, S.; Arrachart, G.; Marie, C.; Chapron, S.; Miguirditchian, M.; Pellet-Rostaing, S. Separation of Am(III) by solvent extraction using water-soluble H4tpaen derivatives. Tetrahedron 2015, 71, 5321–5336. [Google Scholar] [CrossRef]
- Beatty, K.E.; Xie, F.; Wang, Q.; Tirrell, D.A. Selective dye-labeling of newly synthesized proteins in bacterial cells. J. Am. Chem. Soc. 2005, 127, 14150–14151. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ubis, J.C.; Alonso, M.T.; Brunet, E. Synthesis and luminiscence properties of europium(III) and terbium(III) complexes of aminopolycarboxylic acid ligands containing 3-aroylcoumarin. Tetrahedron Lett. 1994, 35, 8461–8464. [Google Scholar] [CrossRef]
- Féau, C.; Klein, E.; Kerth, P.; Lebeau, L. Synthesis of a coumarin-based europium complex for bioanalyte labelling. Bioorg. Med. Chem. Lett. 2007, 17, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Borbas, K.E.; Bruce, J.I. Synthesis of asymmetrically substituted cyclen-based ligands for the controlled sensitisation of lanthanides. Org. Biomol. Chem. 2007, 5, 2274–2282. [Google Scholar] [CrossRef] [PubMed]
- Féau, C.; Klein, E.; Kerth, P.; Lebeau, L. Synthesis and properties of europium complexes derived from coumarin-derivatized azamacrocycles. Synth. Met. 2009, 159, 528–536. [Google Scholar] [CrossRef]
- van Kalkeren, H.A.; Bruins, J.J.; Rutjes, F.P.J.T.; van Delft, F.L. Organophosphorus-catalysed Staudinger reduction. Adv. Synth. Catal. 2012, 354, 1417–1421. [Google Scholar] [CrossRef]
- Jawalekar, A.M.; Meeuwenoord, N.; Cremers, J.G.O.; Overkleeft, H.S.; van der Marel, G.A.; Rutjes, F.P.J.T.; van Delft, F.L. Conjugation of nucleosides and oligonucleotides by [3 + 2] cycloaddition. J. Org. Chem. 2008, 73, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.; Halim, M.; Sames, D. Cocktails of Tb3+ and Eu3+ complexes: A general platform for the design of ratiometric optical probes. J. Am. Chem. Soc. 2007, 129, 7570–7577. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.; Selvin, P.R. Carbostyril derivatives as antenna molecules for luminescent lanthanide chelates. Bioconjugate Chem. 2004, 15, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Sammes, P.G.; Yahioglu, G. Modern bioassays using metal chelates as luminescent probes. Nat. Prod. Rep. 1996, 13, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Kamaruddin, M.A.; Hossain, M.I.; Jarasrassamee, B.; Cheng, H.-C.; Ung, P.; O’Malley, W.; Thompson, P.; Graham, B.; Scanlon, D. A facile, click chemistry-based approach to assembling fluorescent chemosensors for protein tyrosine kinases. Bioorg. Med. Chem. Lett. 2011, 21, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Maruani, A.; Alom, S.; Canavelli, P.; Lee, M.T.W.; Morgan, R.E.; Chudasama, V.; Caddick, S. A mild TCEP-based para-azidobenzyl cleavage strategy to transform reversible cysteine thiol labelling reagents into irreversible conjugates. Chem. Commun. 2015, 51, 5279–5282. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Z. Development and applications of the copper-catalyzed azide-alkyne cycloaddition (CuAAC) as a bioorthogonal reaction. Molecules 2016, 21, 1393. [Google Scholar] [CrossRef] [PubMed]
- Haldón, E.; Nicasio, M.C.; Pérez, P.J. Copper-catalysed azide–alkyne cycloadditions (CuAAC): An update. Org. Biomol. Chem. 2015, 13, 9528–9550. [Google Scholar] [CrossRef] [PubMed]
- Berg, R.; Straub, B.F. Advancements in the mechanistic understanding of the copper-catalyzed azide–alkyne cycloaddition. Beilstein J. Org. Chem. 2013, 9, 2715–2750. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.R.; Hilgraf, R.; Sharpless, K.B.; Fokin, V.V. Polytriazoles as copper(I)-stabilizing ligands in catalysis. Org. Lett. 2004, 6, 2853–2855. [Google Scholar] [CrossRef] [PubMed]
- Sum, S.; Wu, P. Mechanistic insights into Cu(I)-catalyzed azide-alkyne ‘click’ cycloaddition monitored by real time infrared spectroscopy. J. Phys. Chem. A 2010, 114, 8331–8336. [Google Scholar]
- Lau, Y.H.; Wu, Y.; de Andrade, P.; Galloway, W.R.J.D.; Spring, D.R. A two-component ‘double-click’ approach to peptide stapling. Nat. Protoc. 2015, 10, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Albuszis, M.; Roth, P.J.; Pauer, W.; Moritz, H.-U. Macroporous uniform azide- and alkyne-functional polymer microspheres with tuneable surface area: synthesis, in-depth characterization and click-modification. Polym. Chem. 2014, 5, 5689–5699. [Google Scholar] [CrossRef]
- Michinobu, T.; Hiraki, K.; Inazawa, Y.; Katayama, Y.; Masai, E.; Nakamura, M.; Ohara, S.; Shigehara, K. Click synthesis and adhesive properties of novel biomass-based polymers from lignin-derived stable metabolic intermediate. Polymer J. 2011, 43, 648–653. [Google Scholar] [CrossRef]
- Gokmen, M.T.; Camp, W.V.; Colver, P.J.; Bon, S.A.F.; Prez, F.E. Fabrication of porous “clickable” polymer beads and rods through generation of high internal phase emulsion (HIPE) droplets in a simple microfluidic device. Macromolecules 2009, 42, 9289–9292. [Google Scholar] [CrossRef]
- Misaka, H.; Kakuchi, R.; Zhang, C.; Sakai, R.; Satoh, T.; Kakuchi, T. Synthesis of well-defined macrocyclic poly(δ-valerolactone) by “click cyclization”. Macromolecules 2009, 42, 5091–5096. [Google Scholar] [CrossRef]
- Hasegawa, T.; Umeda, M.; Numata, M.; Li, C.; Bae, A.H.; Fujisawa, T.; Haraguchi, S.; Sakurai, K.; Shinkai, S. “Click chemistry” on polysaccharides: a convenient, general, and monitorable approach to develop (1→3)-β-d-glucans with various functional appendages. Carbohydr. Res. 2006, 341, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.H.; Rutledge, P.J.; Watkinson, M.; Todd, M.H. Chemical sensors that incorporate click-derived triazoles. Chem. Soc. Rev. 2011, 40, 2848–2866. [Google Scholar] [CrossRef] [PubMed]
- Uttamapinant, C.; Tangpeerachaikul, A.; Grecian, S.; Clarke, S.; Singh, U.; Slade, P.; Gee, K.R.; Ting, A.T. Fast, cell-compatible click chemistry with copper-chelating azides for biomolecular labeling. Angew. Chem. Int. Ed. 2012, 51, 5852–5856. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Coquiere, D.; Alenda, A.; Garrier, E.; Prange, T.; Li, Y.; Reinaud, O.; Jabin, I. Efficient synthesis of calix[6]tmpa: A new calix[6]azacryptand with unique conformational and host-guest properties. Chem. Eur. J. 2006, 12, 6393–6402. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.G.; El-Gamel, N.E. Structural, spectroscopic and thermal characterization of 2-tert-butylaminomethylpyridine-6-carboxylic acid methylester and its Fe(III), Co(II), Ni(II), Cu(II), Zn(II) and UO(2)(II) complexes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 61, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not Available. |
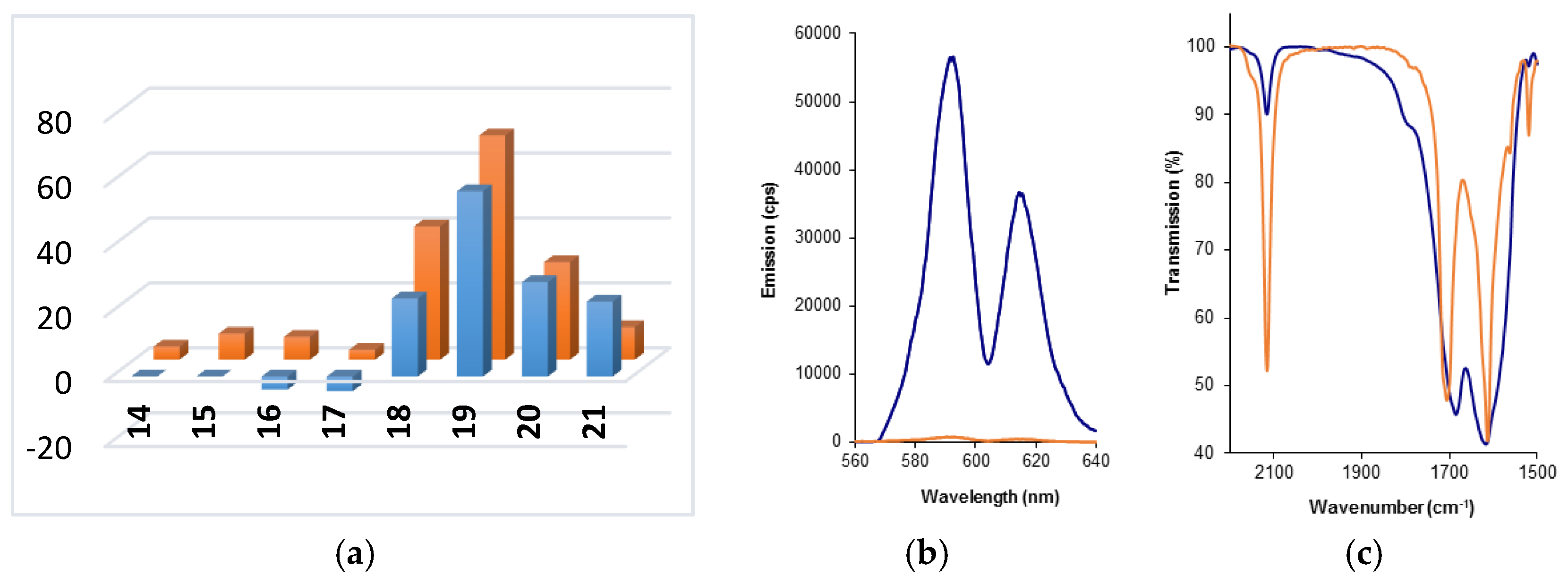
| Entry | Alkyne | Azide | Sensor | λex/nm | Emission/cps b (wavelength/nm) | |
|---|---|---|---|---|---|---|
| 1 | 1a | 6 | 14-Eu | 325 | 100 | (615) |
| 2 | 1b | 6 | 14-Tb | 325 | 240 | (545) |
| 3 | 1a | 7 | 15-Eu | 325 | 800 | (615) |
| 4 | 1b | 7 | 15-Tb | 325 | 338 | (545) |
| 5 | 1a | 8 | 16-Eu | 300 | 610 | (615) |
| 6 | 1b | 8 | 16-Tb | 300 | 845 | (545) c |
| 7 | 1a | 9 | 17-Eu | 300 | 1300 | (615) |
| 8 | 1b | 9 | 17-Tb | 300 | 791 | (545) c |
| 9 | 1a | 10 | 18-Eu | 345 | 5371 | (593) |
| 10 | 1b | 10 | 18-Tb | 345 | 6078 | (545) |
| 11 | 1a | 11 | 19-Eu | 325 | 56,592 | (593) |
| 12 | 1b | 11 | 19-Tb | 325 | 10,000 | (545) |
| 13 | 1a | 12 | 20-Eu | 345 | 16,665 | (593) |
| 14 | 1b | 12 | 20-Tb | 345 | 25,000 | (545) |
| 15 | 1b | 13 | 21-Tb | 350 | 4530 | (545) |
| Entry | Sensor | Output a (fold) | Wavenumber b (cm−1) | Conversion (%) | Normalized Output c (fold) |
|---|---|---|---|---|---|
| 1 | 19-Eu | 69 | 1612 (1612) | 84 | 82 |
| 2 | 19-Tb | 57 | 1614 (1615) | 85 | 67 |
| 3 | 20-Eu | 30 | 1628 (1624) | 83 | 36 |
| 4 | 20-Tb | 29 | 1614 (1626) | 68 | 43 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanna, J.R.; Allan, C.; Lawrence, C.; Meyer, O.; Wilson, N.D.; Hulme, A.N. Optimizing the Readout of Lanthanide-DOTA Complexes for the Detection of Ligand-Bound Copper(I). Molecules 2017, 22, 802. https://doi.org/10.3390/molecules22050802
Hanna JR, Allan C, Lawrence C, Meyer O, Wilson ND, Hulme AN. Optimizing the Readout of Lanthanide-DOTA Complexes for the Detection of Ligand-Bound Copper(I). Molecules. 2017; 22(5):802. https://doi.org/10.3390/molecules22050802
Chicago/Turabian StyleHanna, Jill R., Christopher Allan, Charlotte Lawrence, Odile Meyer, Neil D. Wilson, and Alison N. Hulme. 2017. "Optimizing the Readout of Lanthanide-DOTA Complexes for the Detection of Ligand-Bound Copper(I)" Molecules 22, no. 5: 802. https://doi.org/10.3390/molecules22050802






