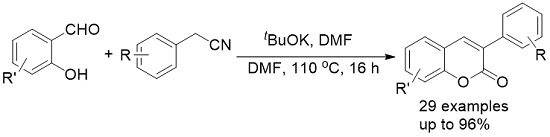
Synthesis of 2H-Chromenones from Salicylaldehydes and Arylacetonitriles
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials and General Procedures
3.1.1. Materials
3.1.2. General Procedures
3.2. Synthesis of Adducts (Specific Spectral Reference Supplementary Materials)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Musa, M.A.; Cooperwood, J.S.; Khan, M.O.F. A Review of Coumarin Derivatives in Pharmacotherapy of Breast Cancer. Curr. Med. Chem. 2008, 15, 2664–2679. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Singla, R.; Jaitak, V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2015, 101, 476–495. [Google Scholar] [CrossRef] [PubMed]
- Swarnakar, N.K.; Jain, A.K.; Singh, R.P. Oral bioavailability, therapeutic efficacy and reactive oxygen species scavenging properties of coenzyme Q10-loaded polymeric nanoparticles. Biomaterials 2011, 32, 6860–6874. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Vazquez-Rodriguez, S.; Santana, L.; Uriarte, E.; Fuentes-Edfuf, C.; Santos, Y.; Munoz-Crego, A. Looking for new targets: Simple coumarins as antibacterial agents. Med. Chem. 2012, 8, 1140–1145. [Google Scholar] [PubMed]
- Shi, Y.; Zhou, C.H. Synthesis and evaluation of a class of new coumarin triazole derivatives as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2011, 42, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Choochuay, K.; Chunhacha, P.; Pongrakhananon, V. Imperatorin sensitizes anoikis and inhibits anchorage-independent growth of lung cancer cells. J. Nat. Med. 2013, 67, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Basanagouda, M.; Vishwanath, B.; Barigidad, N.; Laxmeshwar, S.; Devaru, S.; Venkatesh, N. Synthesis, structure–activity relationship of iodinated-4-aryloxymethyl-coumarins as potential anti-cancer and anti-mycobacterial agents. Eur. J. Med. Chem. 2014, 74, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I.; Raleva, S.; Genova, P. Structure-Activity Relationships of Synthetic Coumarins as HIV-1 Inhibitors. Bioinorg. Chem. Appl. 2006, 2006, 68274. [Google Scholar] [CrossRef] [PubMed]
- Sugino, T.; Tanaka, K. Solvent-Free Coumarin Synthesis. Chem. Lett. 2001, 30, 110–111. [Google Scholar] [CrossRef]
- Fringuelli, F.; Pani, G.; Piermatti, O. ChemInform Abstract: Low-Polluting Chemical Processes-Aldol and Allylation Reactions in Water. Cheminform 1996, 27. [Google Scholar] [CrossRef]
- Johnson, J.R. The Perkin Reaction and Related Reactions. Org. React. 1942, 1, 210. [Google Scholar]
- Shriner, R.L. The reformatsky reaction. Org. React. 1942, 1, 1. [Google Scholar]
- Yavari, I.; Hekmat-Shoar, R.; Zonouzi, A. ChemInform Abstract: A New and Efficient Route to 4-Carboxymethylcoumarins Mediated by Vinyltriphenylphosphonium Salt. Tetrahedron Lett. 1998, 29, 2391–2392. [Google Scholar] [CrossRef]
- Song, C.E.; Jung, D.U.; Choung, S.Y. Dramatic enhancement of catalytic activity in an ionic liquid: Highly practical Friedel-Crafts alkenylation of arenes with alkynes catalyzed by metal triflates. Angew. Chem. 2004, 43, 6183–6185. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.; Thirupathi, N.; Babu, M.H. ChemInform Abstract: Synthesis of Substituted 3-Iodocoumarins and 3-Iodobutenolides via Electrophilic Iodocyclization of Ethoxyalkyne Diols. J. Org. Chem. 2013, 78, 5878–5888. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Wang, C.; Huang, M. ChemInform Abstract: Preparation of 3-Acyl-4-arylcoumarins via Metal-Free Tandem Oxidative Acylation/Cyclization between Alkynoates with Aldehydes. J. Org. Chem. 2015, 80, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.; Thirupathi, N.; Haribabu, M. Tandem aldehyde–alkyne–amine coupling/cycloisomerization: A new synthesis of coumarins. Beilstein. J. Org. Chem. 2013, 9, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Feng, X.; Zhang, Y. An anion-exchange strategy for 3D hierarchical (BiO)2CO3/amorphous Bi2S3 heterostructures with increased solar absorption and enhanced visible light photocatalysis. RSC Adv. 2015, 5, 11714–11723. [Google Scholar] [CrossRef]
- Feng, J.B.; Wu, X.F. Oxidative Synthesis of Quinazolinones under Metal-free Conditio. J. Heterocycl. Chem. 2016. [Google Scholar] [CrossRef]
- Choi, Y.L.; Lim, H.S.; Lim, H.J.; Heo, J.-N. One-Pot Transition-Metal-Free Synthesis of Dibenzo[b,f]oxepins from 2-Halobenzaldehydes. Org. Lett. 2012, 14, 5102–5105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Meng, T.; Fan, R.; Wu, J. General and efficient route for the synthesis of 3,4-disubstituted coumarins via Pd-catalyzed site-selective cross-coupling reactions. J. Org. Chem. 2007, 72, 7279–7286. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |






| Entry | Base | Solvent | T (°C) | Yield (%) [b] |
|---|---|---|---|---|
| 1 | tBuOK | DMF [c] | 110 | 77 |
| 2 | tBuOK [d] | DMF [c] | 110 | 75 |
| 3 | tBuOK | DMF | 110 | 81 |
| 4 | tBuOK | DMF | 110 | 48 |
| 80 | ||||
| 5 | K2CO3 | DMF | 110 | 34 |
| 6 | K3PO4 | DMF | 110 | 55 |
| 7 | KOH | DMF | 110 | 27 |
| 8 | tBuOLi | DMF | 110 | 75 |
| 9 | NaOMe | DMF | 110 | 55 |
| 10 | tBuONa | DMF | 110 | 74 |
| 11 | tBuOK | DMF | 90 | 73 |
| 12 | tBuOK | DMF | 130 | 41 |
| 13 | tBuOK | DMAc | 110 | 54 |
| 14 | tBuOK | DMSO | 110 | 30 |
| 15 | tBuOK | Toluene | 110 | 9 |
| 16 | tBuOK | o-xylene | 110 | 12 |
| 17 | tBuOK | 1,4-dioxane | 110 | 15 |

| Entry | Substrate | Product | Yield (%) [b] |
|---|---|---|---|
| 1 |  |  | 81 |
| 2 |  |  | 77 |
| 3 |  |  | 65 |
| 4 |  |  | 93 |
| 5 |  |  | 66 |
| 6 |  |  | 96 |
| 7 |  |  | 52 |
| 8 |  |  | 40 |
| 9 |  |  | 90 |

| Entry | Substrate | Product | Yield (%) [b] |
|---|---|---|---|
| 1 |  |  | 81 |
| 2 |  |  | 77 |
| 3 |  |  | 65 |
| 4 |  |  | 70 |
| 5 |  |  | 70 |
| 6 |  |  | 86 |
| 7 |  |  | 78 |
| 8 |  |  | 93 |
| 9 |  |  | 68 |
| 10 |  |  | 66 |
| 11 |  |  | 70 |
| 12 |  |  | 40 |
| 13 |  |  | 51 |
| 14 |  |  | 0 |
| 15 |  |  | 0 |
| 16 |  |  | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Zhu, H.; Zhang, H.; Yang, Y.; Wang, F. Synthesis of 2H-Chromenones from Salicylaldehydes and Arylacetonitriles. Molecules 2017, 22, 1197. https://doi.org/10.3390/molecules22071197
Li C, Zhu H, Zhang H, Yang Y, Wang F. Synthesis of 2H-Chromenones from Salicylaldehydes and Arylacetonitriles. Molecules. 2017; 22(7):1197. https://doi.org/10.3390/molecules22071197
Chicago/Turabian StyleLi, Chengcai, Hailin Zhu, Hang Zhang, Yongfeng Yang, and Feng Wang. 2017. "Synthesis of 2H-Chromenones from Salicylaldehydes and Arylacetonitriles" Molecules 22, no. 7: 1197. https://doi.org/10.3390/molecules22071197