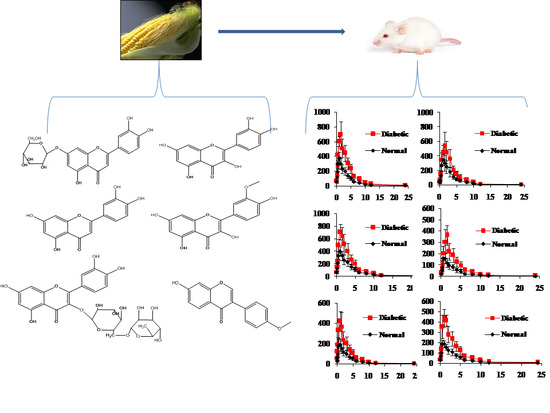
Development of a UPLC-MS/MS Method for Simultaneous Determination of Six Flavonoids in Rat Plasma after Administration of Maydis stigma Extract and Its Application to a Comparative Pharmacokinetic Study in Normal and Diabetic Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of UPLC-MS/MS Conditions
2.2. Optimization of Sample Preparation
2.3. Method Validation
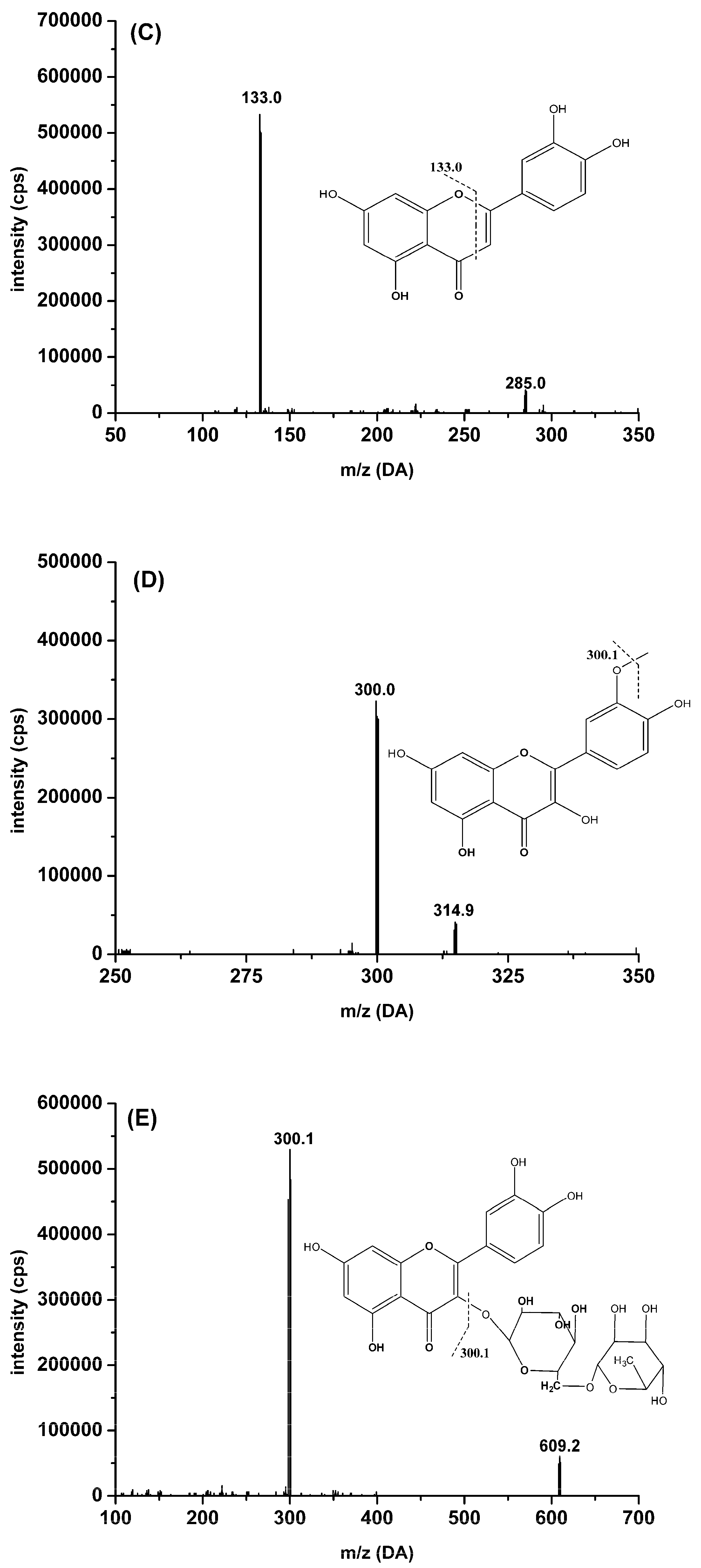
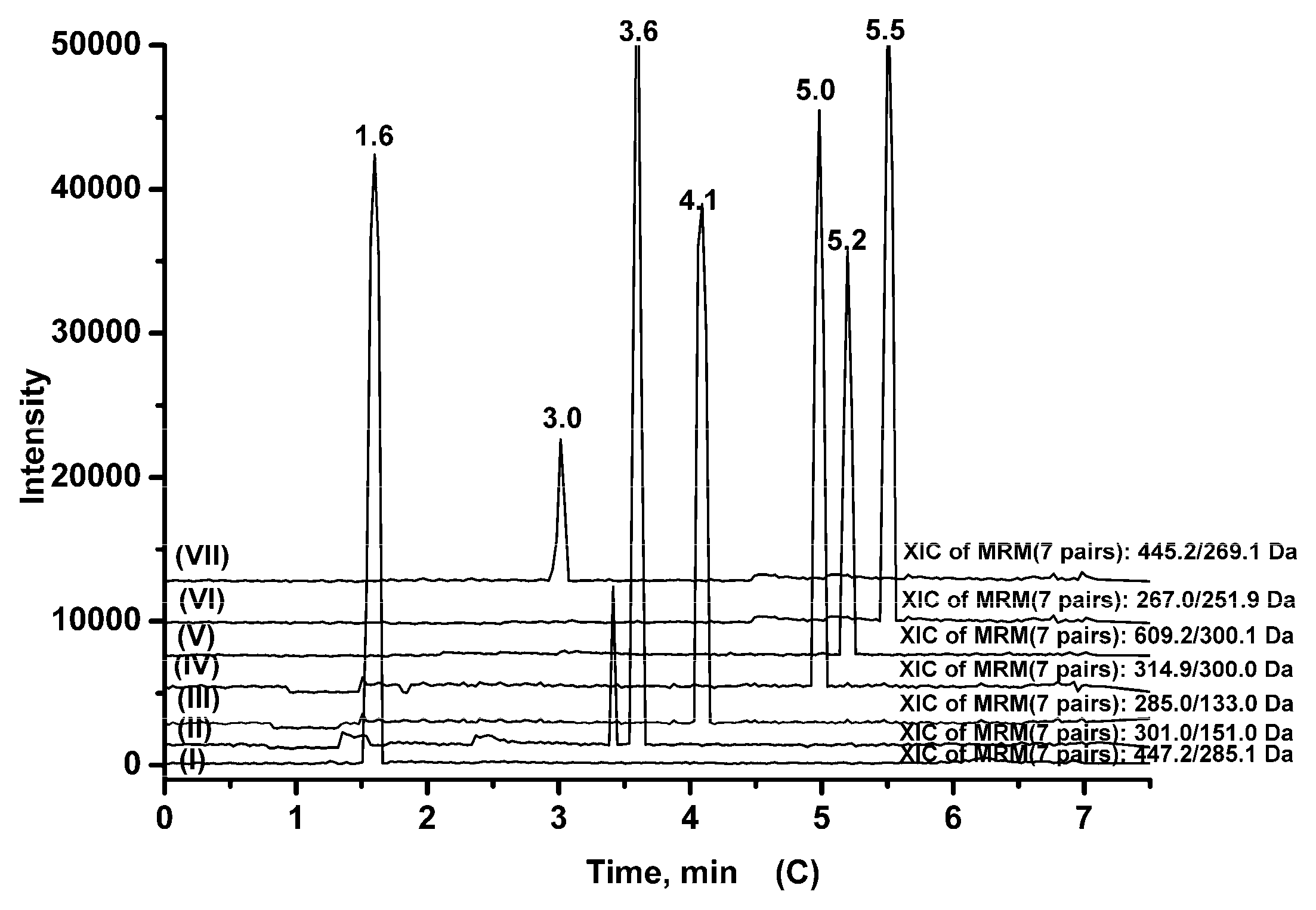
2.3.1. Specificity
2.3.2. Linearity and LLOQ
2.3.3. Precision and Accuracy
2.3.4. Extraction Recovery and Matrix Effect
2.3.5. Stability
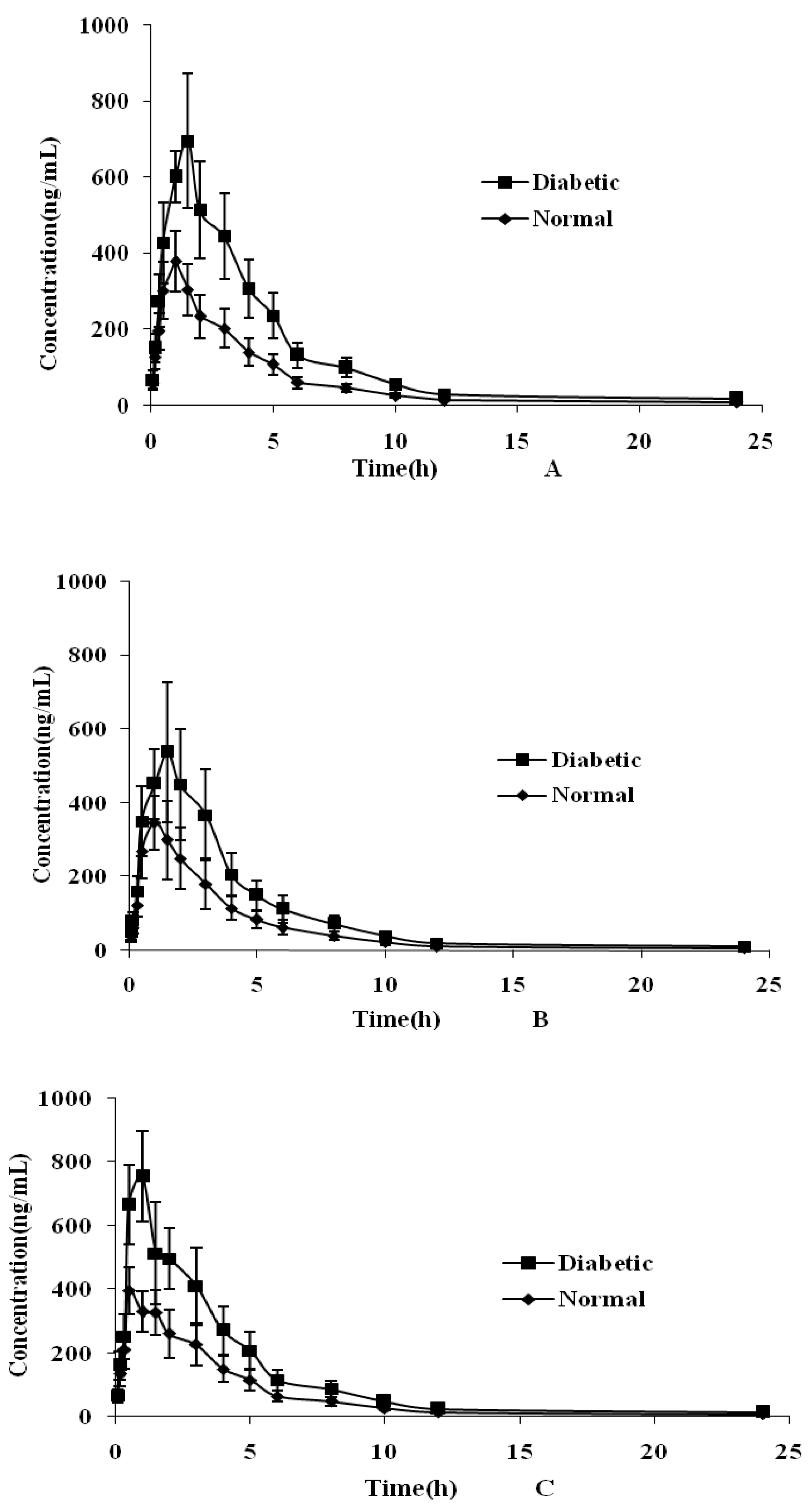
2.4. Plasma Pharmacokinetic Study
3. Experimental
3.1. Materials, Reagents and Animals
3.2. Instruments and UPLC-MS/MS Conditions
3.3. Standard Solution and Quality Control Samples
3.4. Preparation of Maydis stigma Decoction
3.5. Sample Preparation
3.6. Method Validation
3.7. Method Application
3.7.1. Drug Administration and Sampling
3.7.2. Pharmacokinetic Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vasim, K.; Abul-Kalam, N.; Mohd, A.; Mohd, A.; Mohd, M.; Pillai, K.K. A pharmacological appraisal of medicinal plants with antidiabetic potential. J. Pharm. Bioallied. Sci. 2012, 4, 27–42. [Google Scholar]
- Umar, A.; Ahmed, Q.U.; Muhammad, B.Y.; Dogarai, B.B.; Soad, S.Z. Anti-hyperglycemic activity of the leaves of Tetracera scandens Linn. Merr. (Dilleniaceae) in alloxan induced diabetic rats. J. Ethnopharmacol. 2010, 131, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Ganiyu, O.; Adedayo, O.A.; Ayodele, J.A.; Thomas-Henleb, J.A.S.; Uwe, S. Inhibitory effect of polyphenol-rich extracts of jute leaf (Corchorus olitorius) on key enzyme linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting) in vitro. J. Funct. Food 2012, 4, 450–458. [Google Scholar]
- Diabetes Altas Seventh Edition Committee. International Diabetes Federation Diabetes Altas. Available online: http://www.diabetesatlas.org (accessed on 7 July 2013).
- Colagiuri, R. Diabetes: A pandemic, a development issue or both? Exp. Rev. Cardiovasc. Ther. 2010, 8, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.T.; Wang, A.B.; Mehendale, S.; Wu, J.; Aung, H.H.; Dey, L.; Qiu, S.X.; Yuan, C.S. Anti-diabetic effects of Gymnema yunnanense extract. Pharmacol. Res. 2003, 47, 323–329. [Google Scholar] [CrossRef]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes, estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J. New approaches to the pharmacotherapy of diabetes. In Textbook of Diabetes, 3rd ed.; Pickup, J.C., William, G.U.K., Eds.; Blackwell Science: Oxford, UK, 2003; Volume 73, pp. 1–73. [Google Scholar]
- Li, W.L.; Zheng, H.C.; Bukuru, J.; De Kimpe, N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 2004, 92, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.X.; Chen, Y.S.; Zhang, W.R.; Chen, B.; Qiu, X.M.; He, L.H.; Mu, L.L.; Yang, C.H.; Chen, R. Polysaccharide from Gynura divaricata modulates the activities of intestinal disaccharidases in streptozotocin-induced diabetic rats. Br. J. Nutr. 2011, 106, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Maksimović, Z.; Dobric, S.; Kovacevic, N.; Milovanovic, Z. Diuretic activity of Maydis stigma extract in rats. Die Pharm. Int. J. Pharm. Sci. 2004, 59, 967–971. [Google Scholar]
- Guo, J.Y.; Liu, T.J.; Han, L.N.; Liu, Y.M. The effects of corn silk on glycaemic metabolism. Nutr. Metabol. 2009, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Ghada, M.; Eltohami, M.S.; Adurahman, H.N.; Mahmoud, M.E.; Mudawi, I. In vitro study of the effect of corn silk on glucose uptake by isolated rat hemi-diaphragm. World J. Pharm. Res. 2014, 3, 2190–2195. [Google Scholar]
- Zhao, W.; Yin, Y.; Yu, Z.; Liu, J.; Chen, F. Comparison of anti-diabetic effects of polysaccharides from corn silk on normal and hyperglycemia rats. Int. J. Biol. Macromol. 2012, 50, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Farsi, D.A.; Harris, C.S.; Reid, L.; Bennett, S.A.L.; Haddad, P.S.; Martineau, L.C.; Arnason, J.T. Inhibition of non-enzymatic glycation by silk extracts from a mexican land race and modern inbred lines of maize (Zea mays). Phytother. Res. 2008, 22, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Maksimovic, Z.A.; Kovacevic, N. Preliminary assay on the antioxidative activity of Maydis stigma extracts. Fitoterapia 2003, 74, 144–147. [Google Scholar] [CrossRef]
- Goyal, A.K.; Middha, S.K.; Usha, T.; Sen, A. Analysis of toxic, antidiabetic and antioxidant potential of Bambusa balcooa Roxb. leaf extracts in alloxan-induced diabetic rats. 3 Biotech 2017, 7, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.L.; Zhang, L.J.; Li, Y.N.; Ding, Y.J.; Li, F.L. Purification and anti-fatigue activity of flavonoids from corn silk. Int. J. Phys. Sci. 2010, 5, 321–326. [Google Scholar]
- Ebrahimzadeh, M.A.; Pourmorad, F.; Hafe, S. Antioxidant Activities of Iranian Corn Silk. Turk. J. Biol. 2008, 32, 43–49. [Google Scholar]
- Krishnan, B.; Babu, S.; Walker, J.; Walker, A.B.; Pappachan, J.M. Gastrointestinal complications of diabetes mellitus. World J. Diabetes 2013, 4, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.X.; Shi, Q.Z.; Chen, B.; Zhang, X.J.; Liu, S.Z.; Qiu, X.M. Comparative pharmacokinetics of baicalin in normal and the type 2 diabetic rats after oral administration of the Radix scutellariae extract. Fitoterapia 2012, 83, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Pok, E.H.; Lee, W.J. Gastrointestinal metabolic surgery for the treatment of type 2 diabetes mellitus. World J. Gastroenterol. 2014, 20, 14315–14328. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bharti, S.K.; Kumar, A. Type 2 Diabetes Mellitus: The Concerned Complications and Target Organs. Apollo. Med. 2014, 11, 161–166. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Zhang, J.; Pi, Z.F.; Zheng, Z.; Xing, J.P.; Song, F.R.; Liu, S.; Liu, Z.Q. Application of online microdialysis coupled with liquid chromatography-tandem mass spectrometry method in assessing neuroprotective effect of Rhizoma coptidis on diabetic rats. Anal. Methods 2015, 7, 45–52. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation. Available online: https://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf (accessed on 18 June 2004).
- ICH. ICH Harmonization Tripartite Guideline. In Guidelines for Good Clinical Practice. 2015. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/08/WC500191488.pdf (accessed on 27 July 2017).
Sample Availability: Samples of the compounds of the analytes are available from the authors. |




| Analytes | Linear Range (ng·mL−1) | Regression Equation (×10−3) | Correlation Coefficient (r) |
|---|---|---|---|
| Cynaroside | 1.0–1000 | y = 3.8x + 1.2 | 0.9946 |
| Quercetin | 1.0–1000 | y = 4.7x + 3.1 | 0.9956 |
| Luteolin | 1.0–1000 | y = 1.3x + 2.6 | 0.9968 |
| Isorhamnetin | 1.0–1000 | y = 2.2x + 4.3 | 0.9976 |
| Rutin | 1.0–1000 | y = 1.8x + 6.6 | 0.9988 |
| Formononetin | 1.0–1000 | y = 3.1x + 5.5 | 0.9967 |
| Analytes | Concentration (ng·mL−1) | Intra-day RSD (%) | Inter-day RSD (%) | Accuracy (RE %) | Recovery (%, mean ± SD) | Matrix Effect (%, mean ± SD) |
|---|---|---|---|---|---|---|
| Cynaroside | 5.0 | 8.2 | 8.5 | 8.2 | 91.2 ± 1.9 | 93.2 ± 2.4 |
| 125 | 7.2 | 5.4 | 5.8 | 96.4 ± 4.8 | 96.2 ± 5.3 | |
| 800 | 5.4 | 4.1 | −4.2 | 88.5 ± 5.0 | 94.6 ± 2.8 | |
| Quercetin | 5.0 | 9.2 | 6.7 | 6.8 | 85.7 ± 9.3 | 89.3 ± 7.5 |
| 125 | 5.9 | 8.4 | 5.1 | 96.3 ± 4.7 | 91.8 ± 5.0 | |
| 800 | 7.0 | 4.4 | 3.0 | 85.5 ± 3.2 | 86.2 ± 9.1 | |
| Luteolin | 5.0 | 8.7 | 8.0 | 5.7 | 93.6 ± 3.0 | 92.3 ± 4.6 |
| 125 | 5.3 | 2.2 | 3.1 | 91.5 ± 2.5 | 97.8 ± 0.5 | |
| 800 | 6.7 | 5.3 | −2.3 | 96.9 ± 3.9 | 89.4 ± 6.3 | |
| Isorhamnetin | 5.0 | 9.2 | 9.8 | −5.7 | 87.1 ± 4.6 | 91.3 ± 2.3 |
| 125 | 6.3 | 7.3 | 2.2 | 96.1 ± 8.1 | 88.3 ± 7.2 | |
| 800 | 5.2 | 5.8 | 4.2 | 93.2 ± 6.3 | 94.7 ± 6.1 | |
| Rutin | 5.0 | 8.8 | 7.8 | −4.7 | 85.2 ± 4.1 | 89.1 ± 5.3 |
| 125 | 5.1 | 7.5 | 7.9 | 92.3 ± 4.4 | 91.3 ± 6.2 | |
| 800 | 3.4 | 8.8 | 5.2 | 90.3 ± 3.1 | 94.2 ± 4.3 | |
| Formononetin | 5.0 | 9.0 | 4.9 | −7.7 | 86.2 ± 6.7 | 92.8 ± 5.7 |
| 125 | 7.8 | 5.2 | 3.9 | 94.1 ± 3.5 | 87.0 ± 9.5 | |
| 800 | 6.1 | 5.8 | 2.2 | 96.7 ± 2.7 | 94.2 ± 3.7 |
| Analytes | Concentration (ng·mL−1) | 24 h, Room Temperature | 30 Days, −20 °C | 3 Freeze-Thaw Cycles | 12 h, 4 °C | ||||
|---|---|---|---|---|---|---|---|---|---|
| RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | ||
| Cynaroside | 5.0 | −7.6 | 10.3 | 11.2 | 7.3 | 10.9 | 4.1 | 9.4 | 5.3 |
| 125 | 6.8 | 8.2 | 5.8 | 6.2 | −6.2 | 6.3 | 5.2 | 8.2 | |
| 800 | 5.3 | 6.4 | 4.3 | 2.9 | 3.2 | 7.2 | −7.1 | 6.2 | |
| Quercetin | 5.0 | 7.2 | 4.1 | 6.4 | 8.5 | −9.6 | 6.3 | 8.3 | 7.2 |
| 125 | 3.2 | 3.2 | 1.2 | 3.2 | −7.3 | 9.2 | 5.1 | 4.3 | |
| 800 | 4.5 | 5.2 | −7.2 | 6.3 | 6.4 | 4.2 | 8.2 | 2.9 | |
| Luteolin | 5.0 | −7.4 | 12.3 | 13.7 | 7.8 | −4.7 | 7.0 | −3.9 | 5.9 |
| 125 | 9.9 | 5.0 | −4.9 | 3.1 | 8.8 | 2.9 | 1.8 | 4.9 | |
| 800 | 4.1 | 4.8 | 8.1 | 9.4 | 9.4 | 8.3 | −7.1 | 3.8 | |
| Isorhamnetin | 5.0 | −6.7 | 6.3 | 8.0 | 9.1 | −11.9 | 9.3 | 8.0 | 13.2 |
| 125 | 3.4 | 4.9 | −6.5 | 5.1 | −8.9 | 13.2 | 6.2 | 4.0 | |
| 800 | 6.1 | 5.0 | −5.8 | 8.3 | 4.8 | 1.9 | 7.8 | 2.2 | |
| Rutin | 5.0 | −9.0 | 12.1 | 12.9 | 8.0 | 12.2 | 4.7 | 10.4 | 5.1 |
| 125 | 7.1 | 10.1 | 3.2 | 4.8 | −3.5 | 2.0 | 3.5 | 1.2 | |
| 800 | 4.1 | 7.0 | 5.5 | 2.1 | −4.7 | 5.2 | −3.7 | 9.2 | |
| Formononetin | 5.0 | −8.4 | 13.7 | −9.7 | 8.3 | 1.9 | 2.3 | −9.3 | 8.7 |
| 125 | −2.7 | 2.2 | −4.4 | 2.2 | −2.7 | 2.2 | −4.7 | 3.4 | |
| 800 | 2.9 | 2.2 | −2.4 | 4.2 | 2.9 | 2.2 | 5.0 | 2.2 | |
| Parameters | Cynaroside | Quercetin | Luteolin | Isorhamnetin | Rutin | Formononetin |
|---|---|---|---|---|---|---|
| Normal group | ||||||
| AUC0–t (μgh/L) | 1491 ± 341.1 | 1361 ± 371.3 | 1620 ± 397.1 | 608.5 ± 186.3 | 2688 ± 516.1 | 792.5 ± 185.8 |
| AUC0–∞ (μgh/L) | 1492 ± 341.2 | 1362 ± 371.5 | 1621 ± 397.3 | 608.8 ± 186.5 | 2944 ± 540.7 | 793.0 ± 186.2 |
| MRT (h) | 4.28 ± 0.15 | 3.19 ± 0.24 | 4.01 ± 1.20 | 4.81 ± 0.22 | 6.45 ± 0.53 | 5.23 ± 0.72 |
| T1/2 (h) | 3.16 ± 0.20 | 3.07 ± 0.11 | 5.81 ± 1.16 | 5.87 ± 1.02 | 8.45 ± 1.06 | 5.21 ± 0.74 |
| Tmax (h) | 0.83 ± 0.26 | 1.05 ± 0.31 | 0.45 ± 0.25 | 1.75 ± 0.27 | 1.08 ± 0.20 | 0.81 ± 0.21 |
| Cmax (μg/L) | 397.0 ± 78.27 | 365.8 ± 83.18 | 414.4 ± 56.58 | 183.3 ± 33.25 | 419.5 ± 99.16 | 204.2 ± 24.31 |
| CLZ (L/kg/h) | 17.22 ± 4.251 | 27.35 ± 8.078 | 20.79 ± 4.91 | 29.45 ± 8.090 | 6.013 ± 1.136 | 15.13 ± 3.316 |
| Diabetic group | ||||||
| AUC0–t (μgh/L) | 3072 ± 675.7 * | 2302 ± 641.5 | 2876 ± 681.0 * | 1370 ± 382.9 * | 5017 ± 936.7 * | 1745 ± 391.4 * |
| AUC0–∞ (μgh/L) | 3074 ± 676.2 * | 2303 ± 641.7 * | 2878 ± 681.5 * | 1371 ± 383.3 * | 5498 ± 988.9 * | 1746 ± 391.7 * |
| MRT (h) | 4.53 ± 0.15 | 3.41 ± 0.25 | 3.82 ± 0.90 | 5.01 ± 0.42 | 6.49 ± 0.48 | 5.53 ± 0.57 |
| T1/2 (h) | 3.26 ± 0.22 | 3.21 ± 0.68 | 5.42 ± 1.03 | 5.37 ± 0.32 | 8.65 ± 1.15 | 5.88 ± 0.54 |
| Tmax (h) | 1.25 ± 0.27 * | 1.42 ± 0.20 * | 0.63 ± 0.41 * | 2.25 ± 0.57 * | 1.41 ± 0.49 * | 1.25 ± 0.27 * |
| Cmax (μg/L) | 769.3 ± 111.2 * | 551.9 ± 168.3 * | 746.2 ± 104.4 * | 430.3 ± 40.81 * | 744.2 ± 137.1 * | 477.1 ± 52.21 * |
| CLZ (L/kg/h) | 8.323 ± 1.966 * | 16.21 ± 4.912 * | 11.68 ± 2.721 * | 12.95 ± 3.402 * | 3.216 ± 0.591 * | 6.849 ± 1.439 * |
| Analytes | Q1 (amu) | Q3 (amu) | DP (V) | CE (eV) | tR (min) |
|---|---|---|---|---|---|
| Cynaroside | 447.2 | 285.1 | −70 | −35 | 1.6 |
| Quercetin | 301.0 | 151.0 | −70 | −33 | 3.6 |
| Luteolin | 285.0 | 133.0 | −70 | −40 | 4.1 |
| Isorhamnetin | 314.9 | 300.0 | −70 | −33 | 5.0 |
| Rutin | 609.2 | 300.1 | −70 | −45 | 5.2 |
| Formononetin | 267.0 | 251.9 | −100 | −30 | 5.5 |
| Baicalin (IS) | 445.2 | 269.1 | −70 | −35 | 3.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, B.-B.; Chen, Z.-X.; Liu, M.-Y.; Wei, M.-J. Development of a UPLC-MS/MS Method for Simultaneous Determination of Six Flavonoids in Rat Plasma after Administration of Maydis stigma Extract and Its Application to a Comparative Pharmacokinetic Study in Normal and Diabetic Rats. Molecules 2017, 22, 1267. https://doi.org/10.3390/molecules22081267
Wei B-B, Chen Z-X, Liu M-Y, Wei M-J. Development of a UPLC-MS/MS Method for Simultaneous Determination of Six Flavonoids in Rat Plasma after Administration of Maydis stigma Extract and Its Application to a Comparative Pharmacokinetic Study in Normal and Diabetic Rats. Molecules. 2017; 22(8):1267. https://doi.org/10.3390/molecules22081267
Chicago/Turabian StyleWei, Bin-Bin, Zai-Xing Chen, Ming-Yan Liu, and Min-Jie Wei. 2017. "Development of a UPLC-MS/MS Method for Simultaneous Determination of Six Flavonoids in Rat Plasma after Administration of Maydis stigma Extract and Its Application to a Comparative Pharmacokinetic Study in Normal and Diabetic Rats" Molecules 22, no. 8: 1267. https://doi.org/10.3390/molecules22081267