Identification of a Quality Marker (Q-Marker) of Danhong Injection by the Zebrafish Thrombosis Model
Abstract
:1. Introduction
2. Results
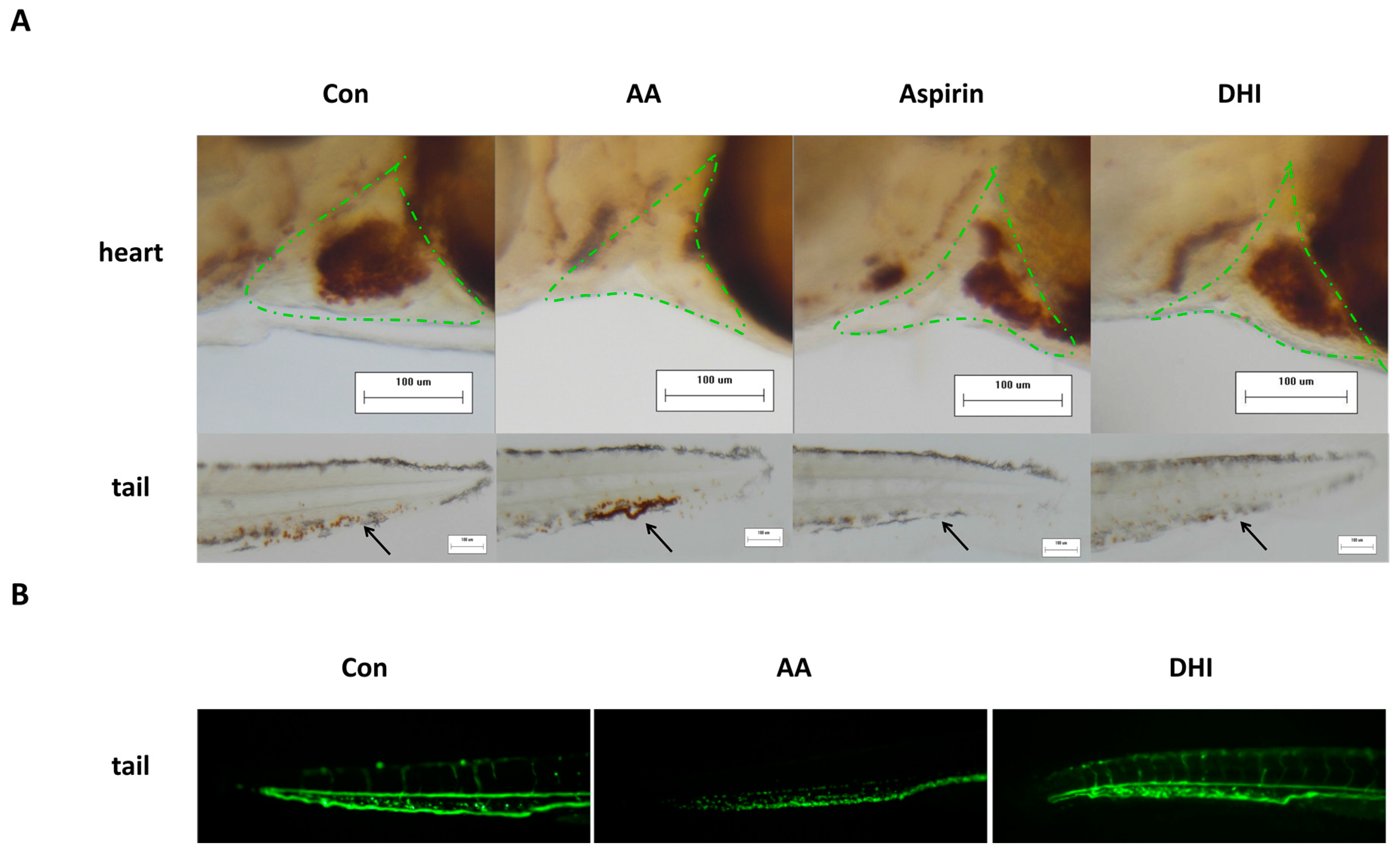
2.1. The Development of the Zebrafish Thrombosis Model
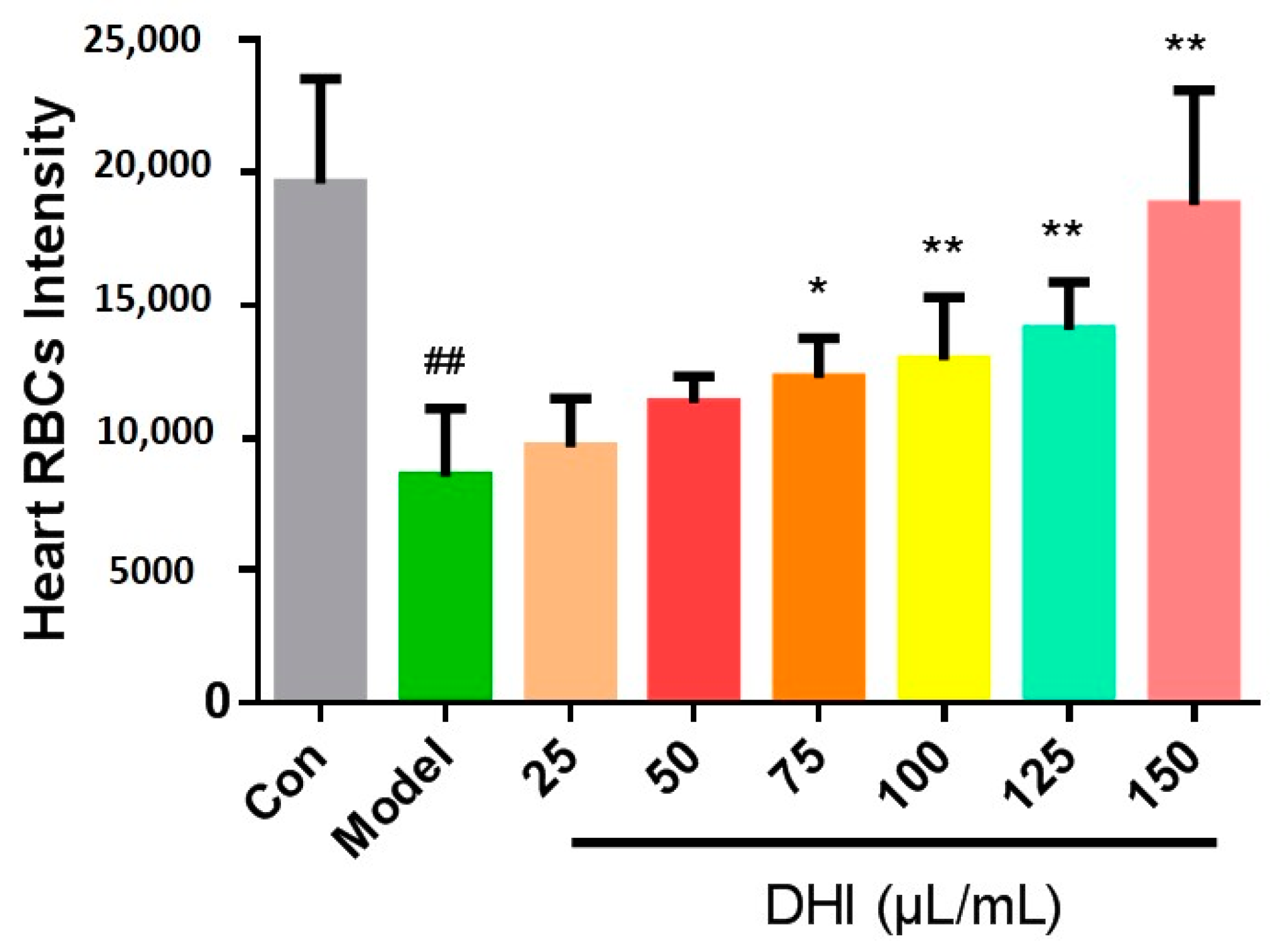
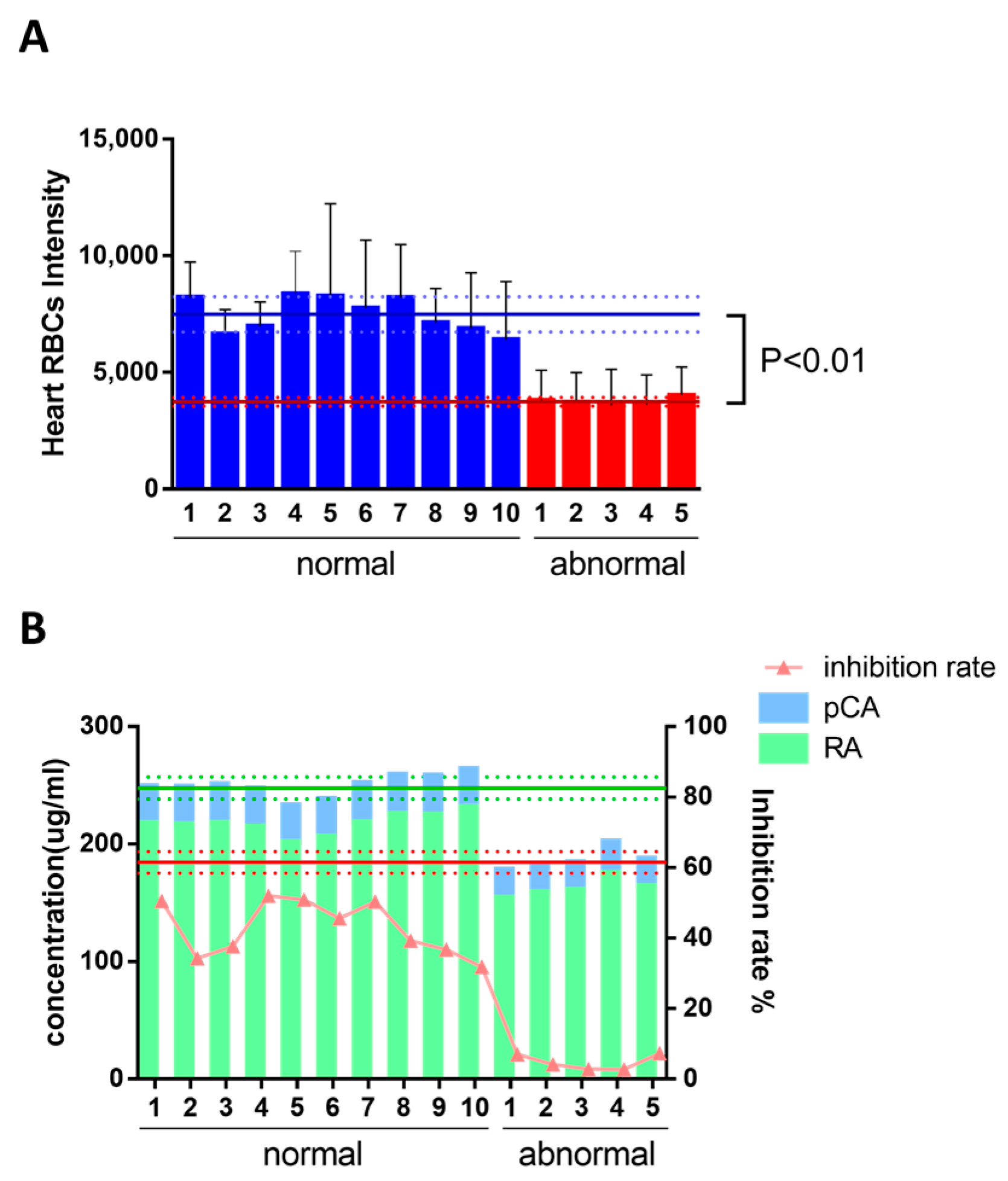
2.2. The Constituents of DHI Improved the Zebrafish Thrombosis
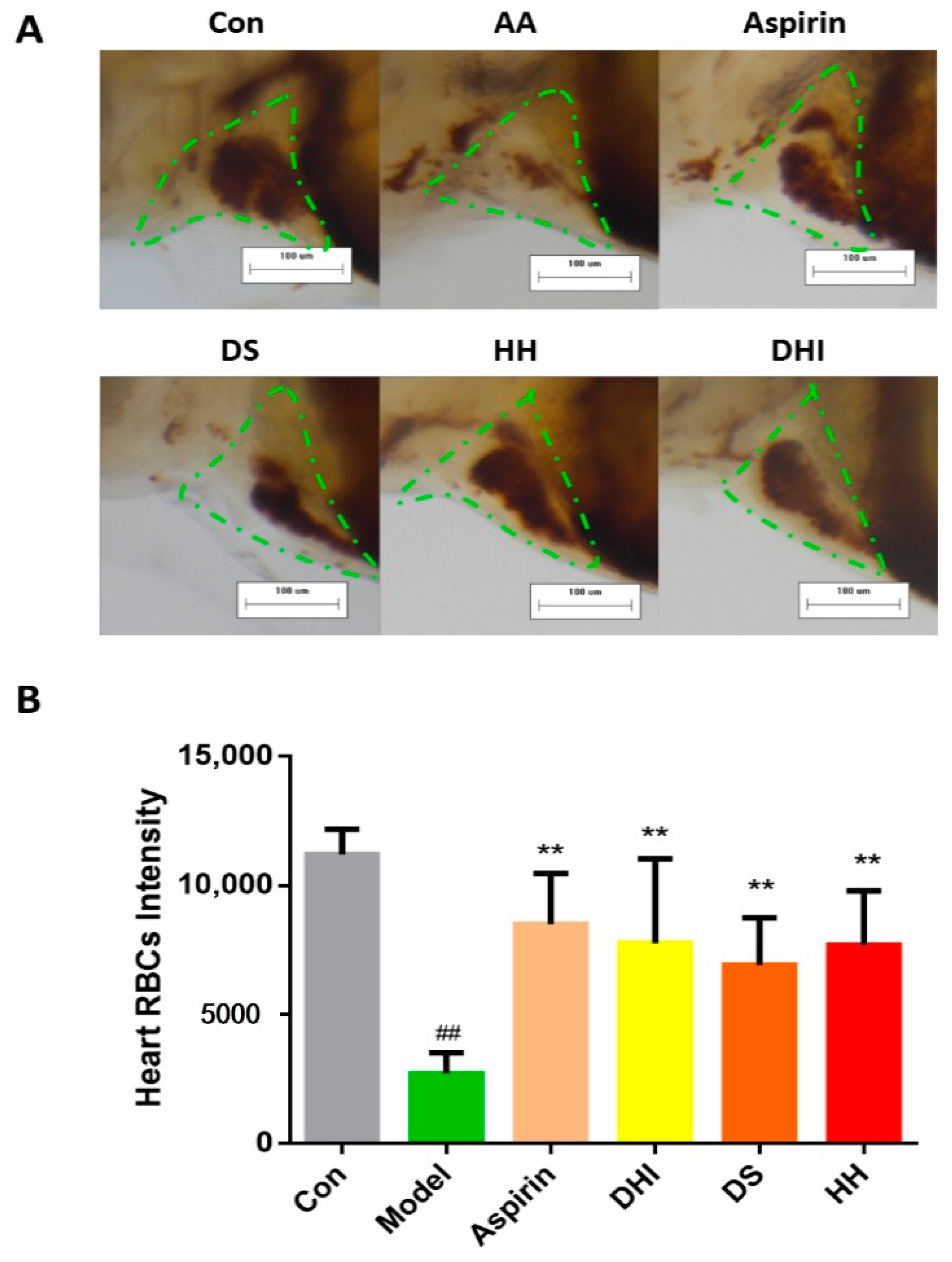
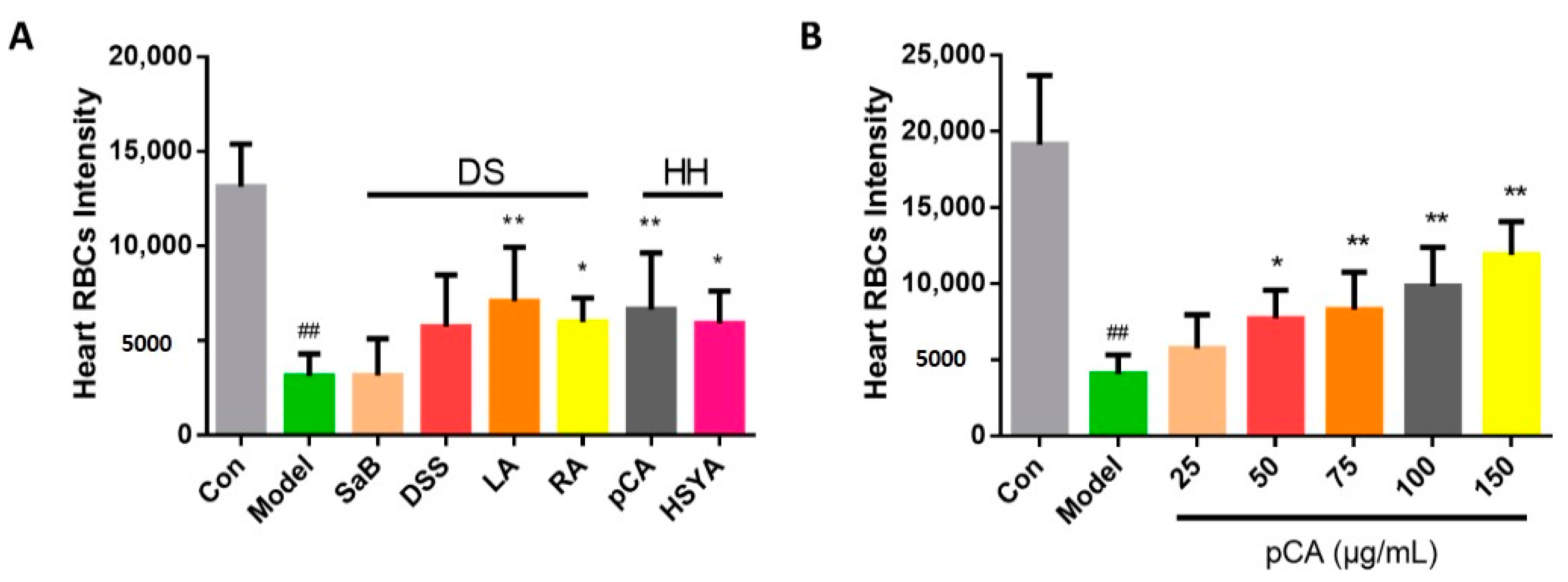
2.3. The Identification of Active Compounds in DHI on Anti-Thrombosis Effects
2.4. Identification of Q-Markers and the Application to Ensure Batch-To-Batch Consistency of DHI
3. Discussion
4. Materials and Methods
4.1. Chemicals, Drugs, and Reagents
4.2. Zebrafish Care and Maintenance
4.3. Experimental Protocol
4.4. Drug Efficiency Assessment
4.5. Staining and Fixing
4.6. Image Capture and Quantitative Analysis of Zebrafish Thrombosis
4.7. Half-Quantitative Content Analysis of DHI
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shu, Y.; Liu, Z.; Zhao, S.; Song, Z.; He, D.; Wang, M.; Zeng, H.; Lu, C.; Lu, A.; Liu, Y. Integrated and global pseudotargeted metabolomics strategy applied to screening for quality control markers of Citrus TCMs. Anal. Bioanal. Chem. 2017, 409, 4849–4865. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, Y.; Shan, L.; Zou, J.; Wu, Y.; Yang, F.; Zhang, Y.; Li, Y.; Zhang, Y. A novel and practical chromatographic “Fingerprint-ROC-SVM” strategy applied to quality analysis of traditional Chinese medicine injections: Using KuDieZi injection as a case study. Molecules 2017, 22, 1237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Su, J. Application of near infrared spectroscopy to the analysis and fast quality assessment of traditional Chinese medicinal products. Acta Pharm. Sin. B 2014, 4, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.D.; Lu, C.J.; Zhao, R.Z. Identification and quantitative characterization of PSORI-CM01, a Chinese medicine formula for psoriasis therapy, by liquid chromatography coupled with an LTQ Orbitrap mass spectrometer. Molecules 2015, 20, 1594–1609. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Z.; Wang, W.P. Chromatographic fingerprinting coupled with chemometrics for quality control of traditional Chinese medicines. Chimia 2011, 65, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Gong, T.; Tang, M.; Mi, D.; Zhang, X.; Liu, J.; Zhang, Z. An approach based on HPLC-fingerprint and chemometrics to quality consistency evaluation of Liuwei Dihuang Pills produced by different manufacturers. J. Pharm. Biomed. Anal. 2008, 48, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Food, U.; Administration, D. FDA Guidance for Industry-Botanical Drug Products (Draft Guidance); Food and Drug Administration: Rockville, MD, USA, 2000; pp. 18–22. [Google Scholar]
- Xiang, Z.; Wang, X.Q.; Cai, X.J.; Zeng, S. Metabolomics study on quality control and discrimination of three curcuma species based on gas chromatograph-mass spectrometry. Phytochem. Anal. PCA 2011, 22, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Lee, K.F.; Sze, C.W.; Tong, Y.; Tang, S.C.; Ng, T.B.; Zhang, Y.B. Intestinal absorption and bioavailability of traditional Chinese medicines: A review of recent experimental progress and implication for quality control. J. Pharm. Pharmacol. 2013, 65, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-X.; Cheng, Y.-Y.; Guo, D.-A.; Zhang, T.-J.; Li, Y.-Z.; Hou, W.-B.; Huang, L.-Q.; Xu, H.-Y. A new concept on quality marker for quality assessment and process control of Chinese medicines. Chin. Herb. Med. 2017, 9, 3–13. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Y.; Wu, W.; Huang, L.; Guo, D.; Liu, C. Approaches to establish Q-markers for the quality standards of traditional Chinese medicines. Acta Pharm. Sin. B 2017, 7, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ma, L.; Li, S.; Cui, K.; Lei, L.; Ye, Z. Evaluation of the cardiotoxicity of evodiamine in vitro and in vivo. Molecules 2017, 22, 943. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, J.R.; Jobin, C. Think small: Zebrafish as a model system of human pathology. J. Biomed. Biotechnol. 2012, 2012, 817341. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.P.; Herrero-Labrador, R.; Futch, H.S.; Serrano, J.; Romero, A.; Fernandez, A.P.; Samadi, A.; Unzeta, M.; Marco-Contelles, J.; Martinez-Murillo, R. The proof-of-concept of ASS234: Peripherally administered ASS234 enters the central nervous system and reduces pathology in a male mouse model of Alzheimer disease. J. Psychiatry Neurosci. JPN 2017, 42, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.N.; Xiao, X.; Liu, D.Z.; Ping, N.N.; Zhu, Y.B.; Li, B.; Long, L.H.; Cao, Y.X. Establishing a rat model for the study of vitamin K deficiency. Int. J. Exp. Pathol. 2016, 97, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Valdivieso, D.; Font, R.; Fernandez-Bedmar, Z.; Merinas-Amo, T.; Gomez, P.; Alonso-Moraga, A.; Del Rio-Celestino, M. Role of Zucchini and its distinctive components in the modulation of degenerative processes: Genotoxicity, anti-genotoxicity, cytotoxicity and apoptotic effects. Nutrients 2017, 9, 755. [Google Scholar] [CrossRef] [PubMed]
- Villatoro-Pulido, M.; Font, R.; Obregon-Cano, S.; Moreno-Rojas, R.; Amaro-Lopez, M.A.; Anter, J.; Munoz-Serrano, A.; Bailon, A.D.; Alonso-Moraga, A.; Del Rio-Celestino, M. Cytotoxic and genotoxic effects of metal(oid)s bioactivated in rocket leaves (Eruca vesicaria subsp. sativa Miller). Chemosphere 2013, 93, 2554–2561. [Google Scholar] [CrossRef] [PubMed]
- Villatoro-Pulido, M.; Font, R.; Saha, S.; Obregon-Cano, S.; Anter, J.; Munoz-Serrano, A.; De Haro-Bailon, A.; Alonso-Moraga, A.; Del Rio-Celestino, M. In vivo biological activity of rocket extracts (Eruca vesicaria subsp sativa (Miller) Thell) and sulforaphane. Food Chem. Toxicol. 2012, 50, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zhang, J.; Lin, H.F.; Italiano, J.; Handin, R.I. The identification and characterization of zebrafish hematopoietic stem cells. Blood 2011, 118, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Zon, L.I.; Peterson, R.T. In vivo drug discovery in the zebrafish. Nat. Rev. Drug Discov. 2005, 4, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.P.; Wang, X.Y.; Gao, Y.H.; Chang, Y.X.; Chen, L.; Niu, Z.C.; Ai, J.Q.; Gao, X.M. Danhong injection attenuates isoproterenol-induced cardiac hypertrophy by regulating p38 and NF-kappab pathway. J. Ethnopharmacol. 2016, 186, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.M.; Zuo, Z.; Chow, M.S. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 2005, 45, 1345–1359. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.D.; Tang, L.Y.; Xu, Y.L.; Zhou, G.H.; Wang, Z.J. Towards a better understanding of medicinal uses of Carthamus tinctorius L. in traditional Chinese medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2014, 151, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Liu, C.H.; Zhang, Y.Q.; Mi, S.Q.; Wang, N.S. Pharmacokinetics of ferulic acid and potential interactions with Honghua and clopidogrel in rats. J. Ethnopharmacol. 2011, 137, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.Y.; Xiao, X.; Luo, J.M.; Fu, C.; Wang, Q.W.; Wang, Y.M.; Liang, Q.L.; Luo, G.A. Integrating qualitative and quantitative characterization of traditional Chinese medicine injection by high-performance liquid chromatography with diode array detection and tandem mass spectrometry. J. Sep. Sci. 2014, 37, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Jagadeeswaran, P.; Sheehan, J.P.; Craig, F.E.; Troyer, D. Identification and characterization of zebrafish thrombocytes. Br. J. Haematol. 1999, 107, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Ganis, J.J.; Hsia, N.; Trompouki, E.; de Jong, J.L.; DiBiase, A.; Lambert, J.S.; Jia, Z.; Sabo, P.J.; Weaver, M.; Sandstrom, R.; et al. Zebrafish globin switching occurs in two developmental stages and is controlled by the LCR. Dev. Biol. 2012, 366, 185–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.T.; Wang, Y.F.; Olaleye, O.; Zhu, Y.; Gao, X.M.; Kang, L.Y.; Zhao, T. Characterization of in vivo antioxidant constituents and dual-standard quality assessment of Danhong injection. Biomed. Chromatogr. BMC 2013, 27, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Streisinger, G.; Walker, C.; Dower, N.; Knauber, D.; Singer, F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature 1981, 291, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J.P. The zebrafish: A new model organism for integrative physiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R3–R9. [Google Scholar] [CrossRef] [PubMed]
- Langheinrich, U. Zebrafish: A new model on the pharmaceutical catwalk. BioEssays News Rev. Mol. Cell. Dev. Biol. 2003, 25, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. Off. J. Soc. Toxicol. 2005, 86, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Fu, C.; Nie, S.; You, Y. The index and improvement effect of using Danhong injection to patients with atherosclerosis symptoms of coronary heart disease (CHD). Pak. J. Pharm. Sci. 2014, 27 (Suppl. S5), 1699–1704. [Google Scholar] [PubMed]
- Zhu, X.Y.; Liu, H.C.; Guo, S.Y.; Xia, B.; Song, R.S.; Lao, Q.C.; Xuan, Y.X.; Li, C.Q. A zebrafish thrombosis model for assessing antithrombotic drugs. Zebrafish 2016, 13, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; von Hofsten, J.; Olsson, P.E. Generating transparent zebrafish: A refined method to improve detection of gene expression during embryonic development. Mar. Biotechnol. 2001, 3, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Jouve, R.; Rolland, P.-H.; Delboy, C.; Mercier, C. Thromboxane B2, 6-keto-PGF1α, PGE2, PGF2α, and PGA1 plasma levels in arteriosclerosis obliterans: Relationship to clinical manifestations, risk factors, and arterial pathoanatomy. Am. Heart J. 1984, 107, 45–52. [Google Scholar] [CrossRef]
- Tantry, U.S.; Bliden, K.P.; Gurbel, P.A. Overestimation of platelet aspirin resistance detection by thrombelastograph platelet mapping and validation by conventional aggregometry using arachidonic acid stimulation. J. Am. Coll. Cardiol. 2005, 46, 1705–1709. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.T.; Qu, H.B. Characterisation of the degradation of salvianolic acid B using an on-line spectroscopic analysis system and multivariate curve resolution. Phytochem. Anal. 2012, 23, 103–109. [Google Scholar]
- Zhou, X.L.; Chan, S.W.; Tseng, H.L.; Deng, Y.; Hoi, P.M.; Choi, P.S.; Or, P.M.Y.; Yang, J.M.; Lam, F.F.Y.; Lee, S.M.Y.; et al. Danshensu is the major marker for the antioxidant and vasorelaxation effects of Danshen (Salvia miltiorrhiza) water-extracts produced by different heat water-extractions. Phytomedicine 2012, 19, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.Q.; Qu, H.; Liu, Y. Multiple fingerprints and multi-comonent quantitative analysis of Danhong injection. Chin. Tradit. Herb. Drugs 2014, 45, 490–497. [Google Scholar]
Sample Availability: Not available. |

| Correlation Coefficient | |
|---|---|
| HMF * | 0.9291 |
| DSS | −0.7388 |
| PA | 0.8960 |
| pCA | 0.9150 |
| SaD * | 0.7826 |
| RA | 0.8564 |
| SaB | 0.7876 |
| SaA * | 0.9021 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Zhao, X.; Liu, H.; Wang, Y.; Zhao, C.; Zhao, T.; Zhao, B.; Wang, Y. Identification of a Quality Marker (Q-Marker) of Danhong Injection by the Zebrafish Thrombosis Model. Molecules 2017, 22, 1443. https://doi.org/10.3390/molecules22091443
Qi Y, Zhao X, Liu H, Wang Y, Zhao C, Zhao T, Zhao B, Wang Y. Identification of a Quality Marker (Q-Marker) of Danhong Injection by the Zebrafish Thrombosis Model. Molecules. 2017; 22(9):1443. https://doi.org/10.3390/molecules22091443
Chicago/Turabian StyleQi, Yuqing, Xiaoping Zhao, Hao Liu, Yimin Wang, Chao Zhao, Tao Zhao, Buchang Zhao, and Yi Wang. 2017. "Identification of a Quality Marker (Q-Marker) of Danhong Injection by the Zebrafish Thrombosis Model" Molecules 22, no. 9: 1443. https://doi.org/10.3390/molecules22091443





