Synergic Anti-Pruritus Mechanisms of Action for the Radix Sophorae Flavescentis and Fructus Cnidii Herbal Pair
Abstract
:1. Introduction
2. Results
2.1. Putative Target Prediction of RSF and FC
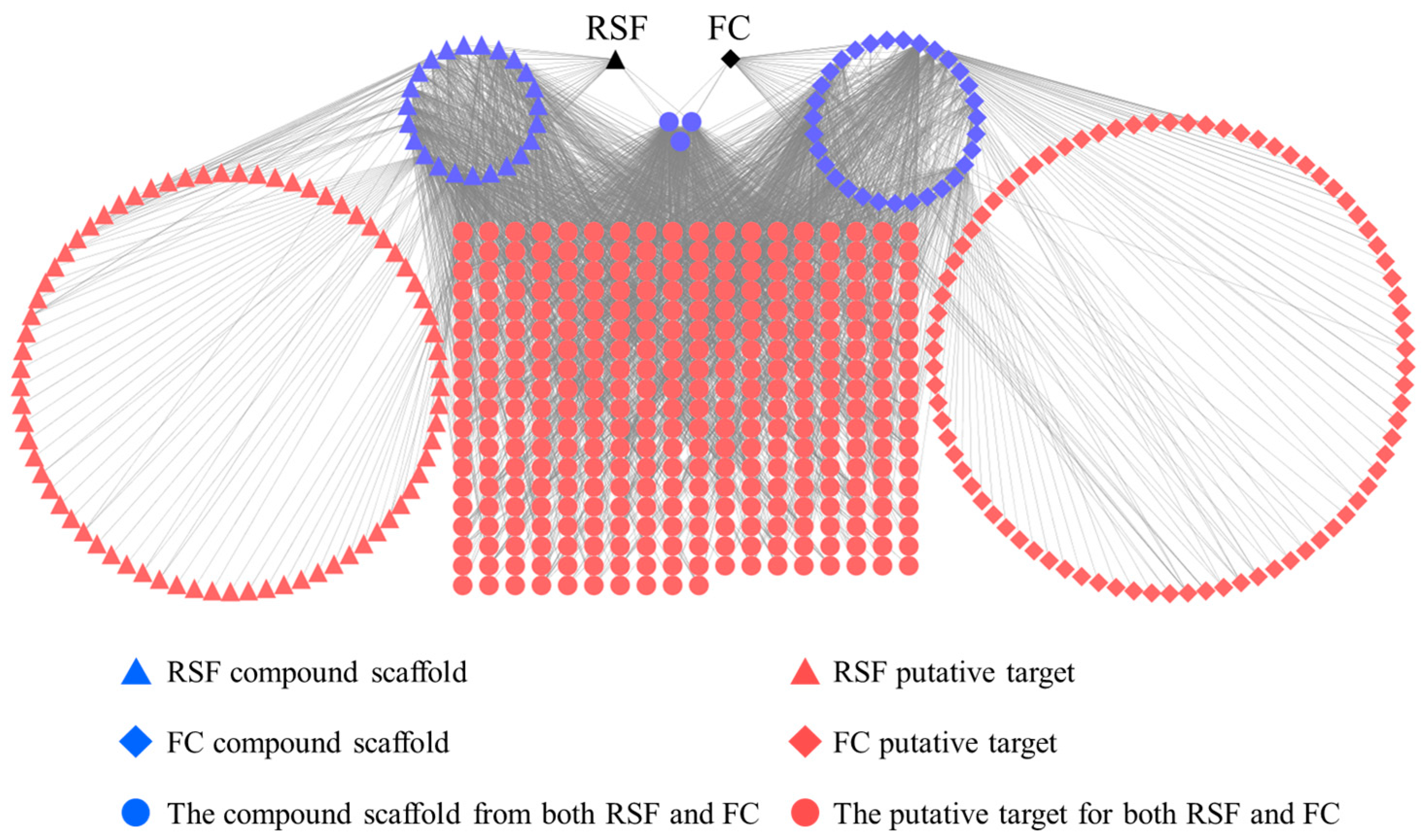
2.2. The Relationship among the Herbs, Ingredients, and Targets of RSF and FC
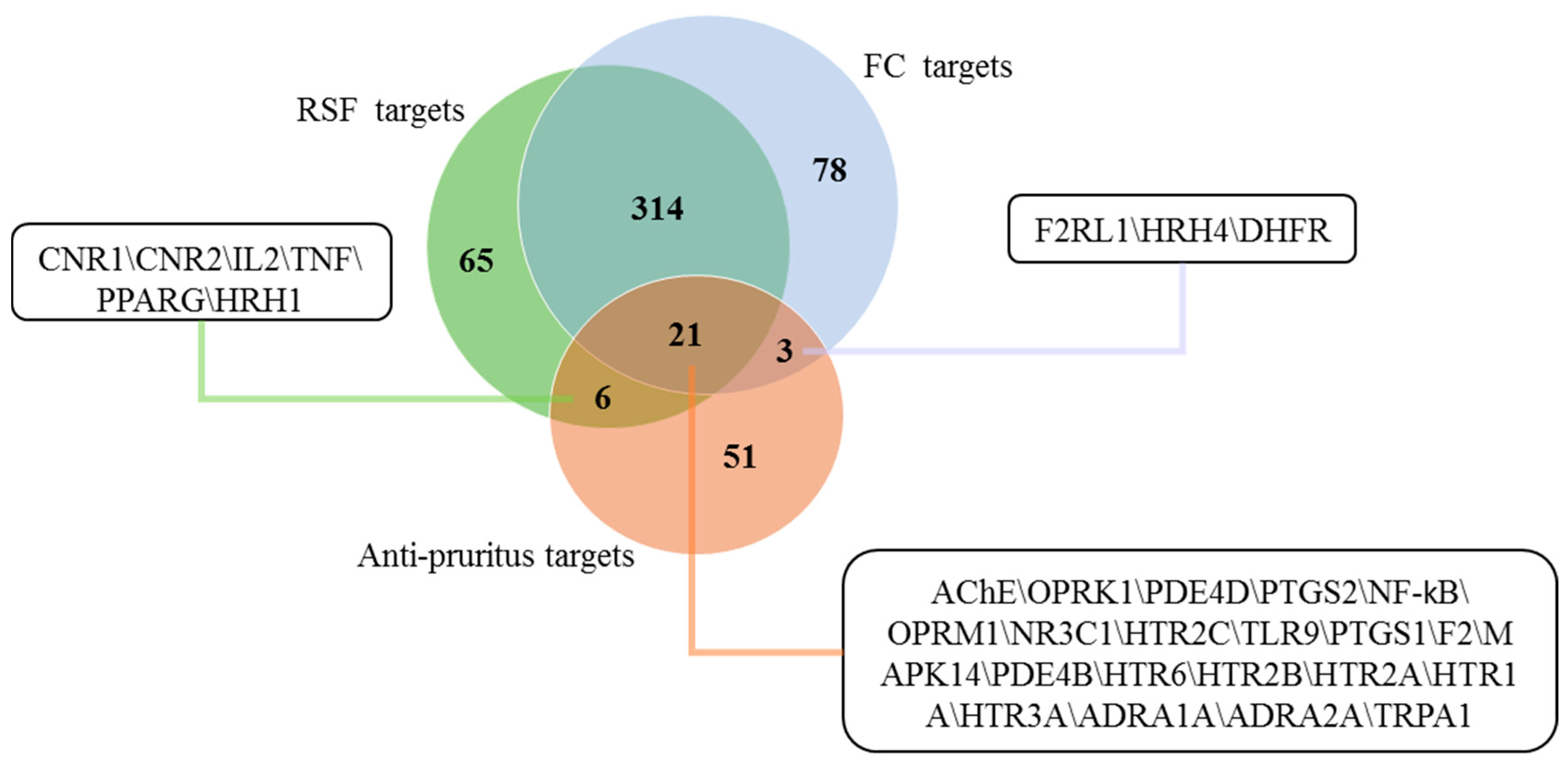
2.3. Putative Anti-Pruritus Targets of RSF and FC
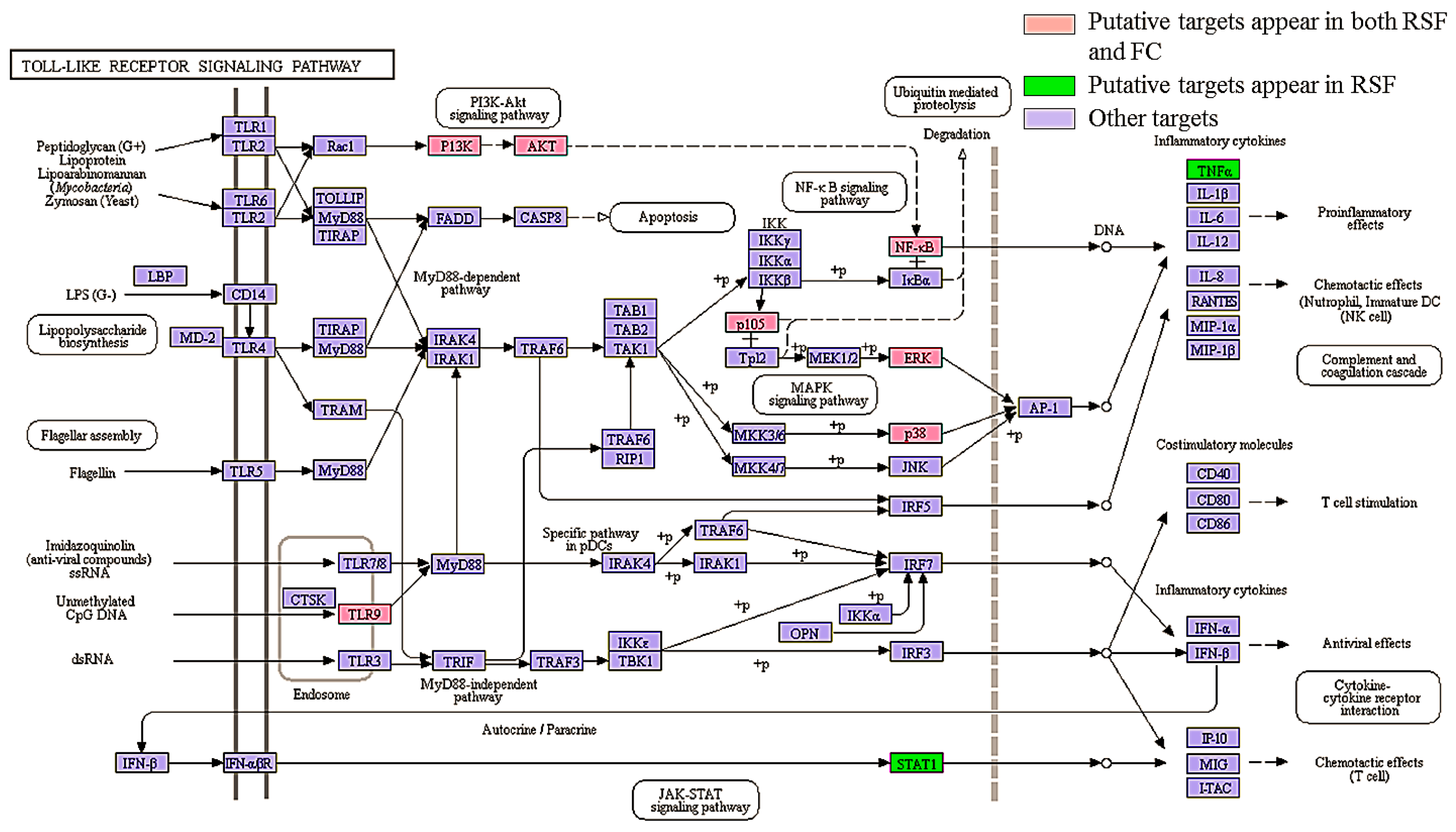
2.4. Interactions between Putative Targets and Anti-Pruritus Targets
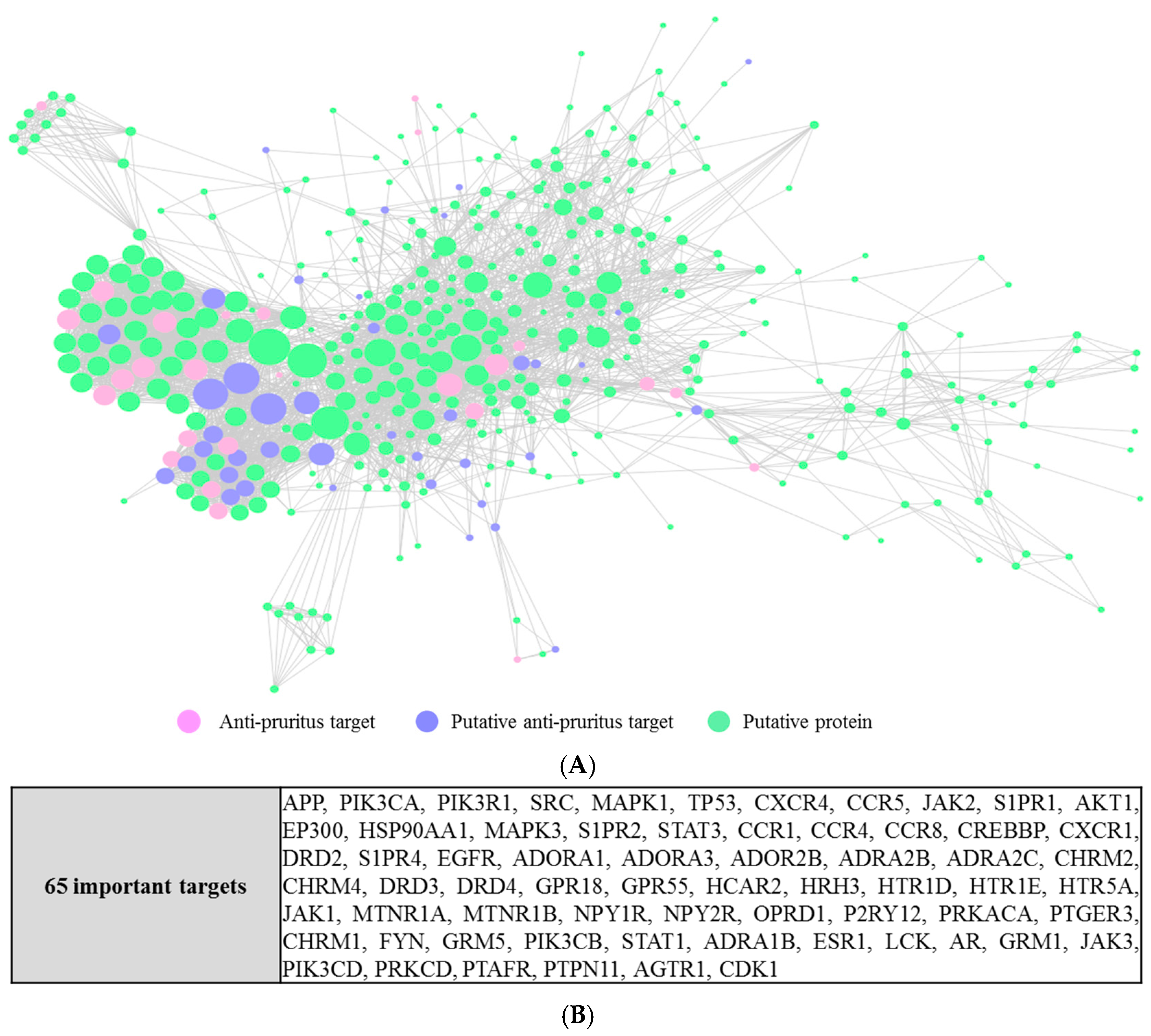
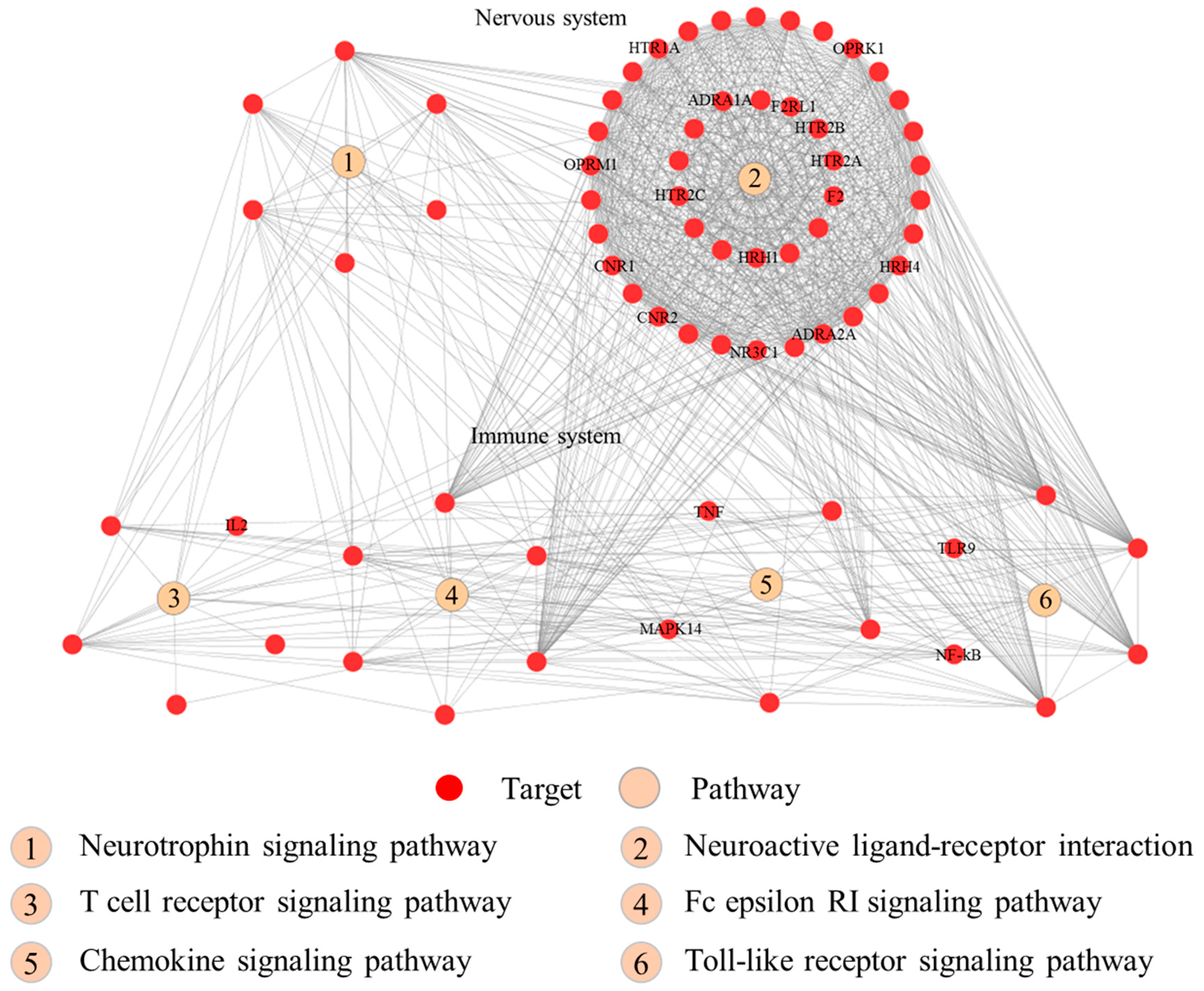
2.5. Functions of the 95 Putative Targets of RSF and FC
3. Discussion
4. Materials and Methods
4.1. Data Preparation
4.2. Drug Targets Prediction for RSF and FC
4.3. Network Construction and Network Analysis
4.4. Gene Ontology and Pathway Enrichment Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Yosipovitch, G.; Greaves, M.W.; Schmelz, M. Itch. Lancet 2003, 361, 690–694. [Google Scholar] [CrossRef]
- Hanifin, J.M.; Ling, M.R.; Langley, R.; Breneman, D.; Rafal, E. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: Part I, efficacy. J. Am. Acad. Dermatol. 2001, 44, S28–S38. [Google Scholar] [CrossRef] [PubMed]
- Jekler, J.; Larkö, O. Combined UVA-UVB versus UVB phototherapy for atopic dermatitis a paired-comparison study. J. Am. Acad. Dermatol. 1990, 22, 19–53. [Google Scholar] [CrossRef]
- Wahlgren, C.; Scheynius, A.; Hägermark, Ö. Antipruritic effect of oral cyclosporin A in atopic dermatitis. Acta Derm. Venereol. 1990, 70, 323–329. [Google Scholar] [PubMed]
- Draelos, Z.D. Use of topical corticosteroids and topical calcineurin inhibitors for the treatment of atopic dermatitis in thin and sensitive skin areas. Curr. Med. Res. Opin. 2008, 24, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jiang, P.; Zhang, W. Molecular networks for the study of TCM pharmacology. Brief. Bioinform. 2010, 11, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, Y.; Tan, W.; Wu, X.; Chen, R.; Cao, J.; Chen, M.; Wang, Y. Compatibility art of traditional Chinese medicine: From the perspective of herb pairs. J. Ethnopharmacol. 2012, 143, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Ung, C.Y.; Li, H.; Cao, Z.W.; Li, Y.X.; Chen, Y.Z. Are herb-pairs of traditional Chinese medicine distinguishable from others? Pattern analysis and artificial intelligence classification study of traditionally defined herbal properties. J. Ethnopharmacol. 2007, 111, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, M.; Shi, R.; Yang, M. Radix Sophorae flavescentis for Chronic Hepatitis B A Systematic Review of Randomized Trials. Am. J. Chin. Med. 2003, 31, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cai, W.; Yang, Q.; Lu, Z.; Li, J.; Yu, J. Compound Radix Sophorae Flavescentis exerts antitumor effects by inhibiting the proliferation and inducing the apoptosis of esophageal carcinoma TE-8 cells. Oncol. Lett. 2015, 10, 2209–2213. [Google Scholar] [PubMed]
- Drew, A.K.; Bensoussan, A.; Whyte, I.M.; Dawson, A.H.; Zhu, X.; Myers, S.P. Chinese herbal medicine toxicology database monograph on Radix Sophorae Flavescentis, ku shen. Clin. Toxicol. 2002, 40, 173–176. [Google Scholar] [CrossRef]
- Hwang, G.B.; Lee, J.E.; Nho, C.W.; Lee, B.U.; Lee, S.J.; Jung, J.H.; Bae, G.N. Short-term effect of humid airflow on antimicrobial air filters using Sophora flavescens nanoparticles. Sci. Total Environ. 2012, 421–422, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.H.; Kim, J.S.; Kang, S.S.; Son, K.H.; Chang, H.W.; Kim, H.P. Anti-inflammatory and anti-arthritic activity of total flavonoids of the roots of Sophora flavescens. J. Ethnopharmacol. 2010, 127, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.M.; Wang, A.M.; Wang, X.Y.; Shen, S.R. Effects of ethanol extract of Radix Sophorae Flavescentis on activity of colon cancer HT29 cells. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lin, H.; Zhang, Y.; Chen, X.; Hua, B.; Hou, W.; Qi, X.; Pei, Y.; Zhu, X.; Zhao, Z.; et al. Compound Kushen Injection suppresses human breast cancer stem-like cells by down-regulating the canonical Wnt/β-catenin pathway. J. Exp. Clin. Cancer Res. 2011, 28, 103. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Bauer, R.; Melchart, D.; Xiao, P.G.; Staudinger, A. Chromatographic Fingerprint Analysis of Herbal Medicines; Springer International Publishing: Berlin, Germany, 2011; pp. 499–508. [Google Scholar]
- Mastuda, H.; Tomohiro, N.; Ido, Y.; Kubo, M. Anti-allergic effects of cnidii monnieri fructus (dried fruits of Cnidium monnieri) and its major component, osthol. Biol. Pharm. Bull. 2002, 25, 809–812. [Google Scholar]
- Matsuda, H.; Ido, Y.; Hirata, A.; Ino, Y.; Naruto, S.; Amamiya, T.; Kuboa, M. Antipruritic effect of Cnidii Monnieri Fructus (fruits of Cnidium monnieri CUSSON). Biol. Pharm. Bull. 2002, 25, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Mouduo, L.; Cuixia, Q.; Liping, Q.; Junyong, Z.; Changquan, L. Application of traditional Chinese medicine injection in treatment of primary liver cancer: A review. J. Tradit. Chin. Med. 2012, 32, 299–307. [Google Scholar]
- Hopkins, A.L. Network pharmacology. Nat. Biotechnol. 2007, 25, 1110–1111. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Wang, Z.; Wang, S.; Ravula, R.; Yang, L.; Xu, J.; Wang, C.; Zuo, Z.; Chow, M.S.; Shi, L.; et al. Discovery of molecular mechanisms of traditional Chinese medicinal formula Si-Wu-Tang using gene expression microarray and connectivity map. PLoS ONE 2011, 6, e18278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, X.; Wang, D.; Li, R.; Li, X.; Xu, Y.; Liu, Z.; Song, Z.; Lin, Y.; Li, Z.; et al. A systems biology-based investigation into the therapeutic effects of Gansui Banxia Tang on reversing the imbalanced network of hepatocellular carcinoma. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, M.; Zhang, B.; Liu, C.; Guo, Q.; Sun, Y.; Wang, D.; Wang, C.; Jiang, Y.; Lin, N.; et al. Uncovering pharmacological mechanisms of Wu-tou decoction acting on rheumatoid arthritis through systems approaches: Drug-target prediction, network analysis and experimental validation. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, P.; Li, Y.; Tian, Y.; Wang, Y. Systems pharmacology-based dissection of mechanisms of Chinese medicinal formula Bufei Yishen as an effective treatment for chronic obstructive pulmonary disease. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ding, P.; Yan, X.; Zheng, M.; Zhou, H.; Xu, Y.; Du, Y.; Gu, Q.; Xu, J. ASDB: A resource for probing protein functions with small molecules. Bioinformatics 2016, 32, 1752–1754. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liang, H.; Yin, T.; Wang, B.; Zhao, Y.Y. Main flavonoids from Sophora flavescenes. Yao Xue Xue Bao 2008, 43, 833–837. [Google Scholar] [PubMed]
- Li, S.; Wang, S. Determination of alkaloids in Radix Sophorae Flavescentis by high performance capillary electrophoresis. Zhongguo Zhong Yao Za Zhi 1999, 24, 100–102. [Google Scholar] [PubMed]
- Davalos, D.; Baeten, K.M.; Whitney, M.A.; Mullins, E.S.; Friedman, B.; Olson, E.S.; Ryu, J.K.; Smirnoff, D.S.; Petersen, M.A.; Bedard, C.; et al. Early detection of thrombin activity in neuroinflammatory disease. Ann. Neurol. 2014, 75, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Doh, S.T.; Chan, J.Y.; Kong, A.N.; Cai, L. Regulatory potential for concerted modulation of Nrf2- and Nfkb1-mediated gene expression in inflammation and carcinogenesis. Br. J. Cancer 2008, 99, 2070–2082. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.A.; Moore, A.R.; Colville-Nash, P.R. COX-1, COX-2, and COX-3 and the future treatment of chronic inflammatory disease. Lancet 2000, 355, 646–648. [Google Scholar] [CrossRef]
- Chen, X.; Ji, Z.L.; Chen, Y.Z. TTD Therapeutic Target Database. Nucleic Acids Res. 2002, 30, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Potenzieri, C.; Undem, B.J. Basic mechanisms of itch. Clin. Exp. Allergy 2012, 42, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.Y. Emerging Drugs for atopic dermatitis. Expert Opin. Emerg. Drugs 2009, 14, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Benecke, H.; Lotts, T.; Ständer, S. Investigational drugs for pruritus. Expert Opin. Investig. Drugs 2013, 22, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Ständer, S.; Luger, T.A. Itch in Atopic Dermatitis—Pathophysiology and Treatment. Acta Dermatovenerol. Croat. 2010, 18, 289–296. [Google Scholar] [PubMed]
- Davidson, S.; Giesler, G.J. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010, 33, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ji, R.R. New insights into the mechanisms of itch: Are pain and itch controlled by distinct mechanisms? Pflugers Arch. 2013, 465, 1671–1685. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Kuhn, M.; Simonovic, M.; Roth, A.; Minguez, P.; Doerks, T.; Stark, M.; Muller, J.; Bork, P.; et al. The STRING database in 2011: Functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 2011, 39, D561–D568. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Z.Q.; Wu, L.J.; Zhang, X.G.; Wang, Y.Y.; Li, Y.D. Understanding ZHENG in traditional Chinese medicine in the context of neuro-endocrine-immune network. IET Syst. Biol. 2007, 1, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Lidstone, V.; Thorns, A. Pruritus in cancer patients. Cancer Treat. Rev. 2001, 27, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.J.; Savides, T.J. Endoscopic ultrasound staging and novel therapeutics for pancreatic cancer. Surg. Oncol. Clin. N. Am. 2010, 19, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Dasanu, C.; Dutcher, J. Eosinophilia and Pruritus With Interleukin-2 Treatment in Patients With Metastatic Renal Cell Carcinoma. J. Immunother. 2006, 29, 639. [Google Scholar]
- Bosonnet, L. Pruritus: Scratching the surface. Eur. J. Cancer Care 2003, 12, 162–165. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. TLR signaling. Semin. Immunol. 2007, 19, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Yong-Jing, G.; Rong, J.R. Emerging role of toll-like receptors in the control of pain and itch. Neurosci. Bull. 2012, 28, 131–144. [Google Scholar]
- Liu, Y.; Xu, Y.; Ji, W.; Li, X.; Sun, B.; Gao, Q.; Su, C. Anti-tumor activities of matrine and oxymatrine: Literature review. Tumour Biol. 2014, 35, 5111–5119. [Google Scholar] [CrossRef] [PubMed]
- Simons, F.E. Advances in H1-antihistamine. N. Engl. J. Med. 2004, 351, 2203–2217. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Gerhold, K.A.; Bifolck-Fisher, A.; Liu, Q.; Patel, K.N.; Dong, X.; Bautista, D.M. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat. Neurosci. 2011, 14, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, M.; Watkinson, A.; McGlone, F.; Rukwied, R. Histamine induced responses are attenuated by a cannabinoid receptor agonist in human skin. Inflamm. Res. 2003, 52, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; Neisius, U.; Ikoma, A.; Fartasch, M.; Heyer, G.; Skov, P.S.; Luger, T.A.; Schmelz, M. Proteinase-activated receptor-2 mediates itch: A novel pathway for pruritus in human skin. Exp. Dermatol. 2008, 13, 591. [Google Scholar] [CrossRef]
- Tenda, Y.; Yamashita, M.; Kimura, M.Y.; Hasegawa, A.; Shimizu, C.; Kitajima, M.; Onodera, A.; Suzuki, A.; Seki, N.; Nakayama, T. Hyperresponsive TH2 cells with enhanced nuclear factor-kappa B activation induce atopic dermatitis-like skin lesions in Nishiki-nezumi Cinnamon/Nagoya mice. J. Allergy Clin. Immunol. 2006, 118, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Vabulas, R.M.; Ahmad-Nejad, P.; da Costa, C.; Miethke, T.; Kirschning, C.J.; Hacker, H.; Wagner, H. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J. Biol. Chem. 2001, 276, 31332–31339. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.M.; Fish, E.N. Chemokines attractive mediators of the immune response. Semin. Immunol. 2003, 15, 5–14. [Google Scholar] [CrossRef]
- Uchida, T.; Suto, H.; Ra, C.; Ogawa, H.; Kobata, T.; Okumura, K. Preferential expression of Th2-type chemokine and its receptor in atopic dermatitis. Int. Immunol. 2002, 14, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Veraldi, S. Rapid relief of intertrigo-associated pruritus due to Candida albicans with isoconazole nitrate and diflucortolone valerate combination therapy. Mycoses 2013, 56, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Mônica Mourthé de Alvim, A.; José Roberto Monteiro, C.; Daniel Martins Barbosa, M.G.; Flávia Fontes, F.; Rodrigo Guimarães, O.; Renata Magali, R.; Silluzio, F.; Geraldo Magela Gomes da, C. Hidradenitis suppurativa literature review and case report. J. Coloproctol. 2012, 32, 196–201. [Google Scholar]
- Tkaczyk, C.; Gilfillan, A.M. Fc(epsilon)Ri-dependent signaling pathways in human mast cells. Clin. Immunol. 2001, 99, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Novak, N.; Allam, J.P.; Hagemann, T.; Jenneck, C.; Laffer, S.; Valenta, R.; Kochan, J.; Bieber, T. Characterization of FcepsilonRI-bearing CD123 blood dendritic cell antigen-2 plasmacytoid dendritic cells in atopic dermatitis. J. Allergy Clin. Immunol. 2004, 114, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Weng, D.X.; Li, T.A. Simultaneous determination of 5 active components in Fructus Cnidii by HPLC. Zhongguo Zhong Yao Za Zhi 2007, 32, 1883–1885. [Google Scholar] [PubMed]
- Jiang, Y.Q. Analysis of coumarins in Fructus Cnidii by HPLC-ESI-MS. Zhong Yao Cai 2006, 29, 1033–1035. [Google Scholar] [PubMed]
- Wu, M.H.; Zhao, L.H.; Song, Y.; Zhang, W.; Xiang, B.R.; Mei, L.H. Determination of five coumarins in Cnidii Fructus by micellar electrokinetic capillary chromatography. Planta Med. 2005, 71, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.J.; Sha, Z.F.; Gao, H. Determination of osthol and imperatorin in Cnidium monnieri (L.) cuss by fluorometry TLC scanning. Yao Xue Xue Bao 1990, 25, 530–533. [Google Scholar] [PubMed]
- Xu, J. A New Approach to Finding Natural Chemical Structure Classes. J. Med. Chem. 2002, 45, 5311–5320. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protocol. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Term | Count of Proteins | % | Proteins Associate with RSF | Proteins Associate with FC | p-Value |
|---|---|---|---|---|---|
| Intracellular signaling cascade | 69 | 73.40 | 57 | 56 | 1.05 × 10−50 |
| Second-messenger-mediated signaling | 41 | 43.61 | 31 | 33 | 1.09 × 10−46 |
| Cell surface receptor linked signal transduction | 69 | 73.40 | 55 | 56 | 1.97 × 10−39 |
| G-protein coupled receptor protein signaling pathway | 55 | 58.51 | 41 | 45 | 5.81 × 10−35 |
| Cyclic-nucleotide-mediated signaling | 27 | 28.72 | 18 | 24 | 8.46 × 10−32 |
| G-protein signaling, coupled to cyclic nucleotide second messenger | 26 | 27.65 | 17 | 23 | 1.48 × 10−31 |
| Positive regulation of catalytic activity | 36 | 38.29 | 34 | 28 | 3.49 × 10−26 |
| Positive regulation of molecular function | 37 | 39.36 | 34 | 29 | 1.32 × 10−25 |
| Protein kinase cascade | 31 | 32.97 | 28 | 26 | 1.21 × 10−24 |
| behavior | 32 | 34.04 | 28 | 28 | 8.15 × 10−23 |
| CAMP-mediated signaling | 18 | 19.14 | 13 | 17 | 4.94 × 10−20 |
| G-protein signaling, coupled to cAMP nucleotide second messenger | 17 | 18.08 | 12 | 16 | 2.82 × 10−19 |
| Cellular calcium ion homeostasis | 21 | 22.34 | 19 | 17 | 5.05 × 10−19 |
| Calcium ion homeostasis | 21 | 22.34 | 19 | 17 | 8.69 × 10−19 |
| Cellular metal ion homeostasis | 21 | 22.34 | 19 | 17 | 2.00 × 10−18 |
| KEGG Pathway | Count of Proteins | Proteins Associate with RSF | Proteins Associate with FC | p-Value | Class |
|---|---|---|---|---|---|
| Toll-like receptor signaling pathway | 12 | 12 | 10 | 8.77 × 10−7 | Organismal Systems; Immune system |
| Chemokine signaling pathway | 20 | 20 | 16 | 1.76 × 10−10 | Organismal Systems; Immune system |
| T cell receptor signaling pathway | 13 | 13 | 11 | 2.13 × 10−7 | Organismal Systems; Immune system |
| Fc epsilon RI signaling pathway | 11 | 11 | 9 | 6.19 × 10−7 | Organismal Systems; Immune system |
| Neurotrophin signaling pathway | 12 | 12 | 11 | 6.77 × 10−6 | Organismal Systems; Nervous system |
| Progesterone-mediated oocyte maturation | 11 | 11 | 11 | 1.56 × 10−6 | Organismal Systems; Endocrine system |
| Neuroactive ligand-receptor interaction | 44 | 31 | 37 | 8.44 × 10−34 | Environmental Information Processing; Signaling molecules and interaction |
| Calcium signaling pathway | 17 | 15 | 12 | 2.98 × 10−8 | Environmental Information Processing; Signal transduction |
| Jak-STAT signaling pathway | 14 | 13 | 10 | 1.73 × 10−6 | Environmental Information Processing; Signal transduction |
| VEGF signaling pathway | 10 | 10 | 10 | 4.13 × 10−6 | Environmental Information Processing; Signal transduction |
| Pancreatic cancer | 13 | 13 | 11 | 1.94 × 10−9 | Human Diseases; Cancers |
| Prostate cancer | 14 | 13 | 14 | 2.11 × 10−9 | Human Diseases; Cancers |
| Endometrial cancer | 9 | 9 | 9 | 2.16 × 10−6 | Human Diseases; Cancers |
| Renal carcinoma | 10 | 9 | 10 | 2.29 × 10−6 | Human Diseases; Cancers |
| Non-small cell lung cancer | 9 | 9 | 9 | 2.91 × 10−6 | Human Diseases; Cancers |
| Chronic myeloid leukemia | 10 | 10 | 10 | 4.13 × 10−6 | Human Diseases; Cancers |
| Acute myeloid leukemia | 9 | 9 | 9 | 5.06 × 10−6 | Human Diseases; Cancers |
| Pathways in cancer | 19 | 18 | 16 | 7.50 × 10−6 | Human Diseases; Cancers |
| Glioma | 9 | 9 | 9 | 9.54 × 10−6 | Human Diseases; Cancers |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, J.; Liu, Z.; Zhou, X.; Xu, J. Synergic Anti-Pruritus Mechanisms of Action for the Radix Sophorae Flavescentis and Fructus Cnidii Herbal Pair. Molecules 2017, 22, 1465. https://doi.org/10.3390/molecules22091465
Zhong J, Liu Z, Zhou X, Xu J. Synergic Anti-Pruritus Mechanisms of Action for the Radix Sophorae Flavescentis and Fructus Cnidii Herbal Pair. Molecules. 2017; 22(9):1465. https://doi.org/10.3390/molecules22091465
Chicago/Turabian StyleZhong, Jiali, Zhihong Liu, Xinxin Zhou, and Jun Xu. 2017. "Synergic Anti-Pruritus Mechanisms of Action for the Radix Sophorae Flavescentis and Fructus Cnidii Herbal Pair" Molecules 22, no. 9: 1465. https://doi.org/10.3390/molecules22091465





