Ginger Phytochemicals Inhibit Cell Growth and Modulate Drug Resistance Factors in Docetaxel Resistant Prostate Cancer Cell
Abstract
:1. Introduction
2. Results
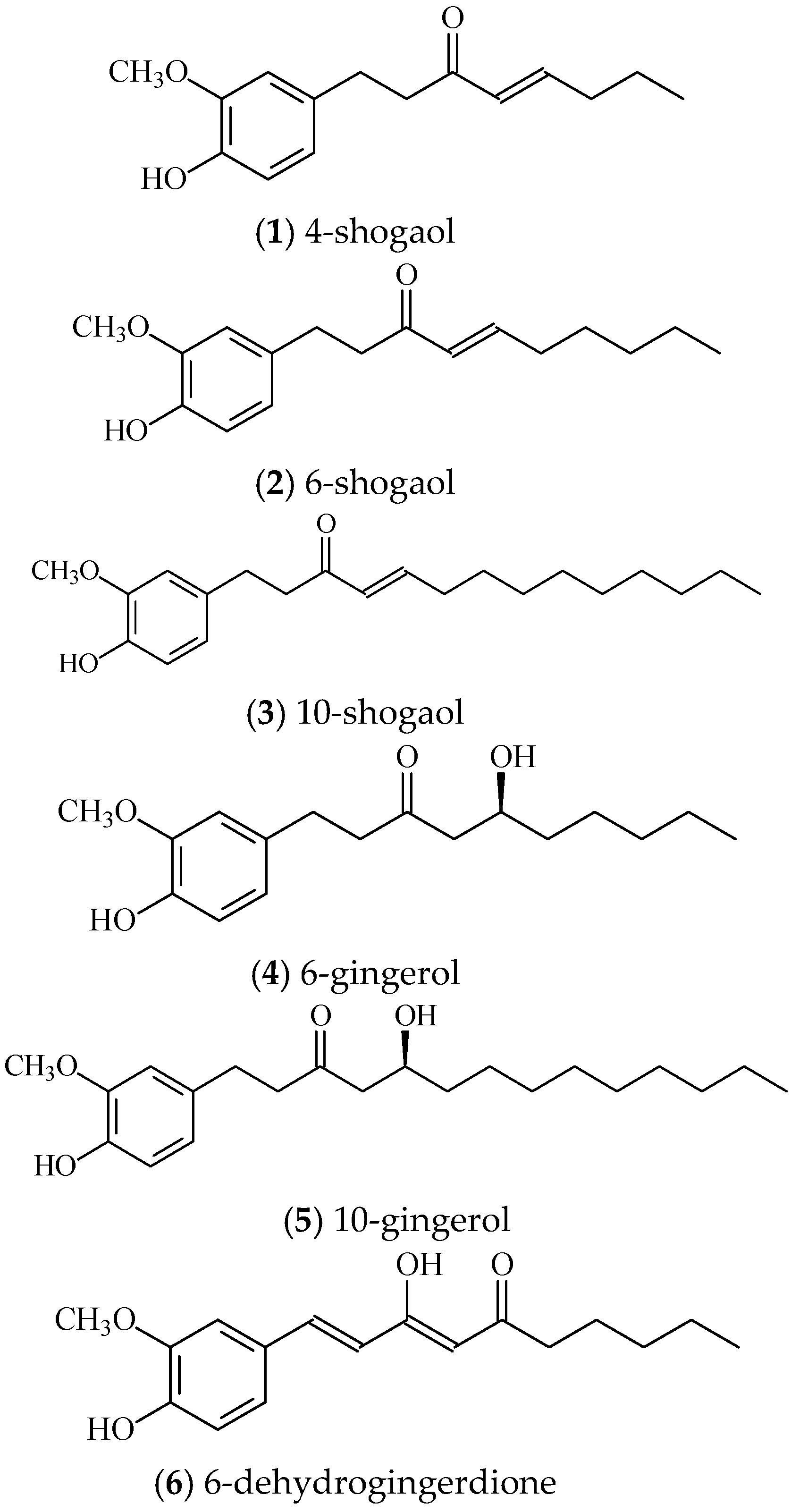
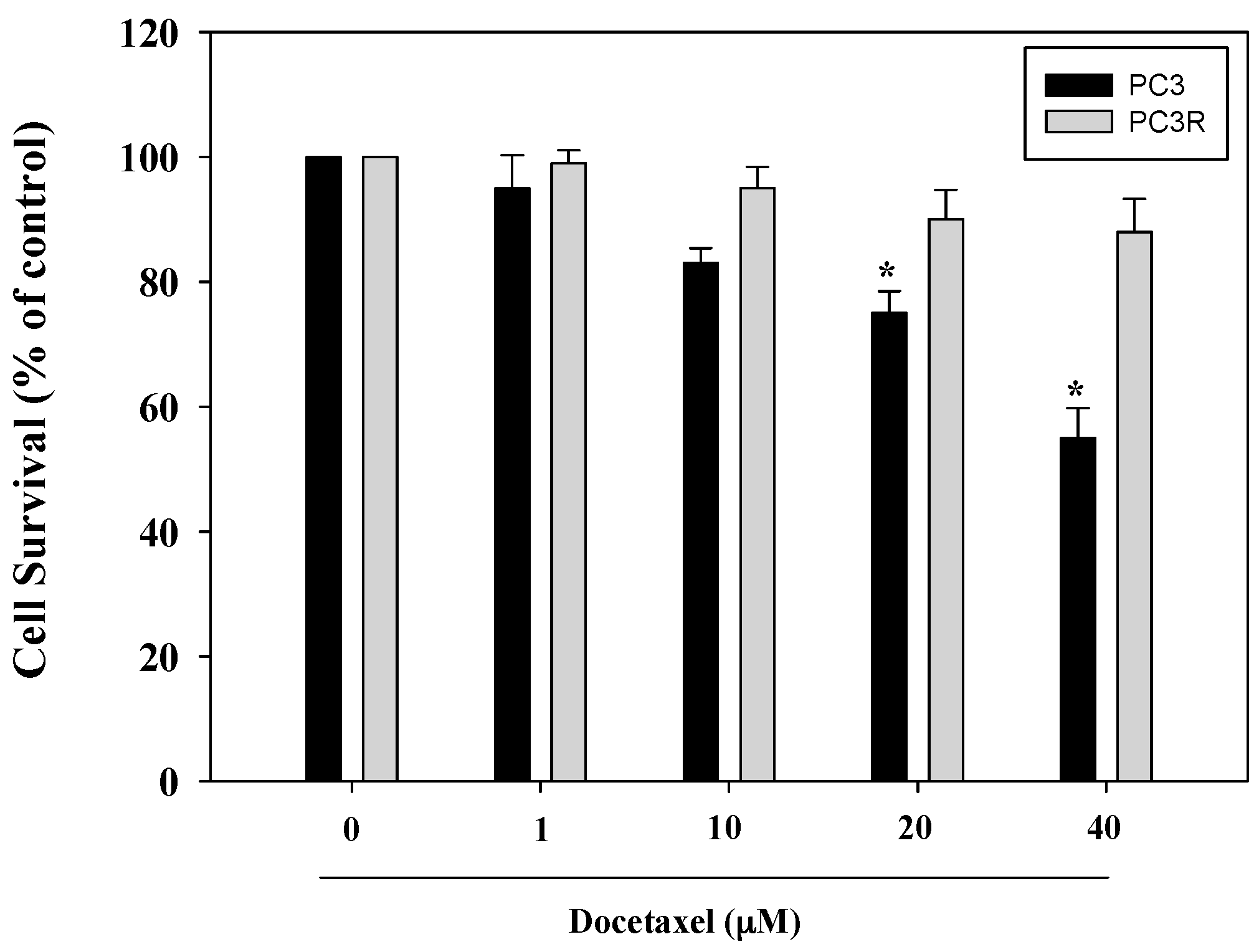
2.1. Docetaxel Resistant PC3 Cell
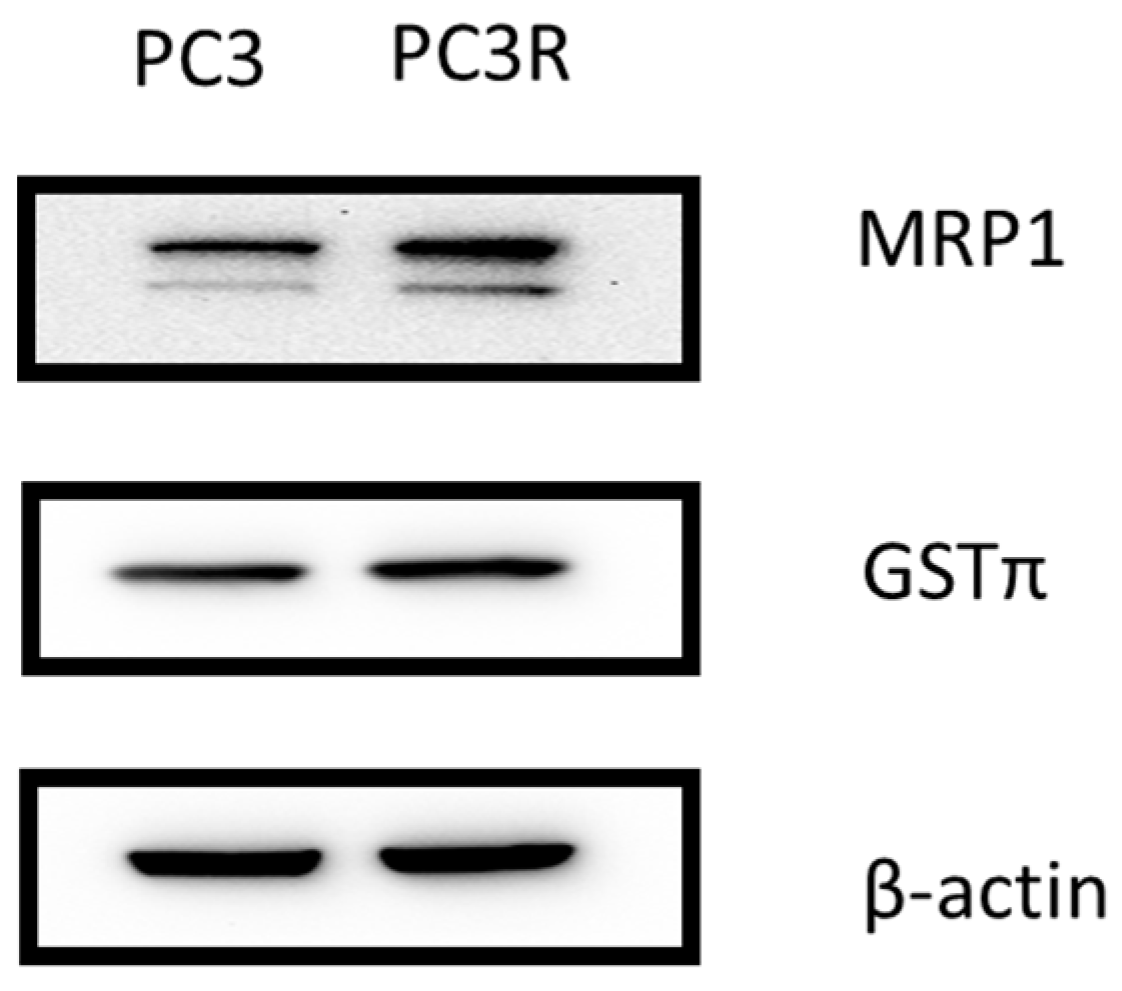
Docetaxel Increases GSTπ and MRP1 Protein Expression in Prostate Cancer Cell Line
2.2. The Pharmacological Activities of Ginger Phytochemicals
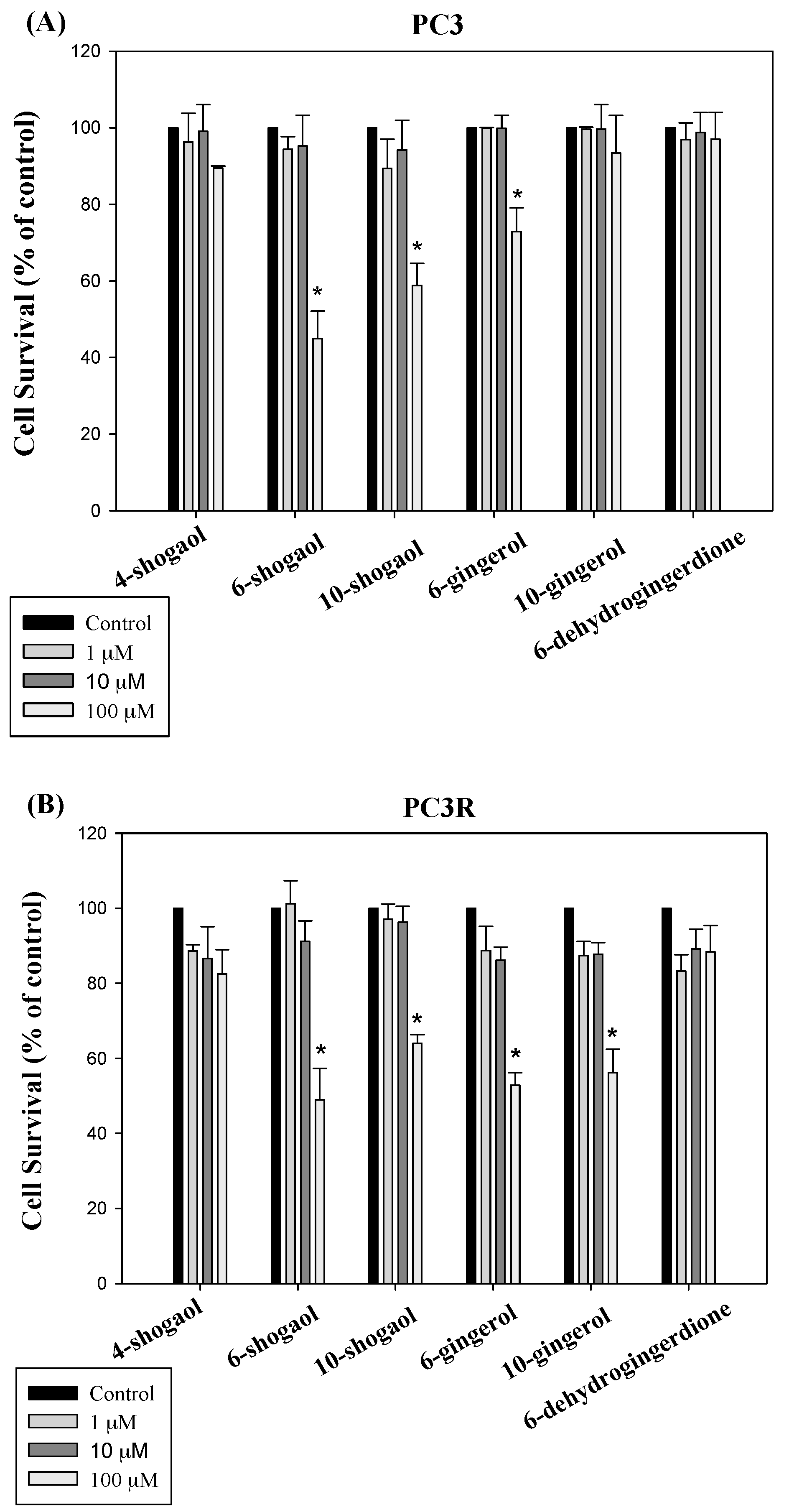
2.2.1. Ginger Phytochemicals Inhibit Prostate Cancer Cell Proliferation
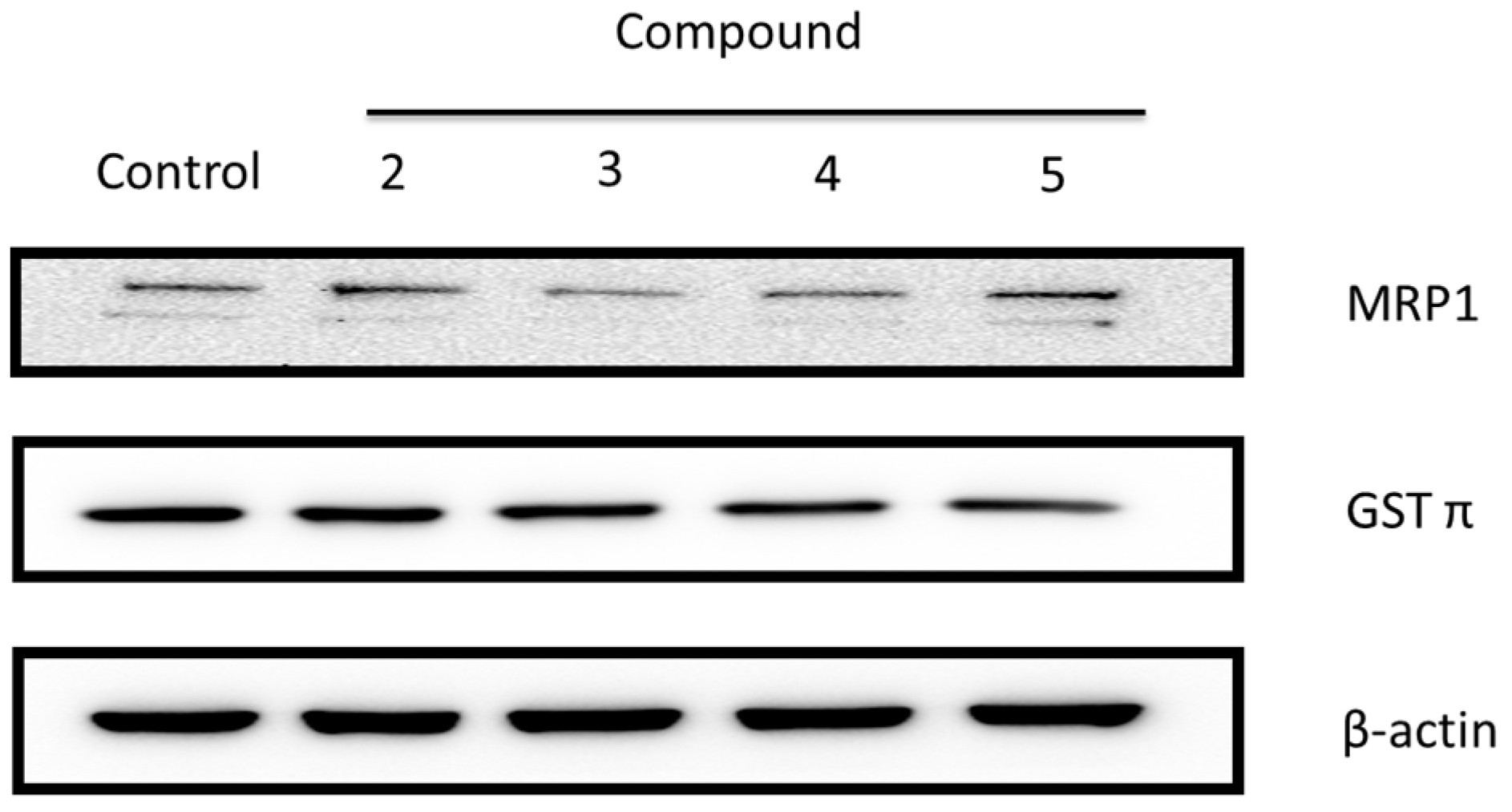
2.2.2. Ginger Phytochemicals Disturbed GST and MRP-1 Protein Expression in PC3R Cells
3. Discussion
4. Materials and Methods
4.1. Extraction and Isolation
Plant Material
4.2. Pharmacological Assay
4.2.1. Cell Culture
4.2.2. Establishment of Drug Resistance Prostate Cell Line
4.2.3. Cell Proliferation Assay
4.2.4. Western Blot
4.3. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lewis, R.; Hornberger, B. Beyond the PSA test: How to better stratify a patient’s risk of prostate cancer. JAAPA Off. J. Am. Acad. Phys. Assist. 2017, 30, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Eastham, J. Prostate cancer screening. Investig. Clin. Urol. 2017, 58, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Stefanovski, D.; Tang, G.; Wawrowsky, K.; Boston, R.C.; Lambrecht, N.; Tajbakhsh, J. Prostate cancer diagnosis using epigenetic biomarkers, 3D high-content imaging and probabilistic cell-by-cell classifiers. Oncotarget 2017, 8, 57278–57301. [Google Scholar] [CrossRef]
- Karamouzis, M.V.; Papavassiliou, K.A.; Adamopoulos, C.; Papavassiliou, A.G. Targeting Androgen/Estrogen Receptors Crosstalk in Cancer. Trends Cancer 2016, 2, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Crona, D.J.; Whang, Y.E. Androgen Receptor-Dependent and -Independent Mechanisms Involved in Prostate Cancer Therapy Resistance. Cancers 2017, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, T.; Yang, J.C.; Gao, A.C.; Evans, C.P. Targeting molecular resistance in castration-resistant prostate cancer. BMC Med. 2015, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Galletti, G.; Leach, B.I.; Lam, L.; Tagawa, S.T. Mechanisms of resistance to systemic therapy in metastatic castration-resistant prostate cancer. Cancer Treat. Rev. 2017, 57, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N. New agents and strategies for the hormonal treatment of castration-resistant prostate cancer. Expert Opin. Investig. Drugs 2010, 19, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Teply, B.A.; Hauke, R.J. Chemotherapy options in castration-resistant prostate cancer. Indian J. Urol. IJU J. Urol. Soc. India 2016, 32, 262–270. [Google Scholar]
- Gameiro, M.; Silva, R.; Rocha-Pereira, C.; Carmo, H.; Carvalho, F.; Bastos, M.L.; Remiao, F. Cellular Models and In Vitro Assays for the Screening of modulators of P-gp, MRP1 and BCRP. Molecules 2017, 22, 600. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, S.; Chen, Z.S.; Kitazono, M.; Sumizawa, T.; Furukawa, T.; Aikou, T. Mechanisms for resistance to anticancer agents and the reversal of the resistance. Hum. Cell 1999, 12, 95–102. [Google Scholar] [PubMed]
- Joshi, P.; Vishwakarma, R.A.; Bharate, S.B. Natural alkaloids as P-gp inhibitors for multidrug resistance reversal in cancer. Eur. J. Med. Chem. 2017, 138, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Yu, A.M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Di, Y.M.; Zhou, Z.W.; Mo, S.L.; Zhou, S.F. Multidrug resistance-associated proteins and implications in drug development. Clin. Exp. Pharm. Phys. 2010, 37, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.F.; Pokharel, D.; Bebawy, M. MRP1 and its role in anticancer drug resistance. Drug Metab. Rev. 2015, 47, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Sodani, K.; Patel, A.; Kathawala, R.J.; Chen, Z.S. Multidrug resistance associated proteins in multidrug resistance. Chin. J. Cancer 2012, 31, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ke, W.; Bao, R.; Hu, X.; Chen, F. Beneficial effects of ginger Zingiber officinale Roscoe on obesity and metabolic syndrome: A review. Ann. N. Y. Acad. Sci. 2017, 1398, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.P.; Deol, P.K.; Kondepudi, K.K.; Bishnoi, M. Anticancer Potential of Ginger: Mechanistic and Pharmaceutical Aspects. Curr. Pharm. Des. 2016, 22, 4160–4172. [Google Scholar] [CrossRef] [PubMed]
- Hashem, R.M.; Rashed, L.A.; Hassanin, K.M.A.; Hetta, M.H.; Ahmed, A.O. Effect of 6-gingerol on AMPK-NF-kappaB axis in high fat diet fed rats. Biomed. Pharm. 2017, 88, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Mohd Yusof, Y.A. Gingerol and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 177–207. [Google Scholar] [PubMed]
- Chen, C.Y.; Chiu, C.C.; Wu, C.P.; Chou, Y.T.; Wang, H.M. Enhancements of skin cell proliferations and migrations via 6-dehydrogingerdione. J. Agric. Food Chem. 2013, 61, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, C.H.; Kung, C.H.; Kuo, S.H.; Kuo, S.Y. [6]-gingerol induces Ca2+ mobilization in Madin-Darby canine kidney cells. J. Nat. Prod. 2008, 71, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Blando, J.; Silver, E.; Beltran, L.; Sessler, J.; DiGiovanni, J. 6-Shogaol from dried ginger inhibits growth of prostate cancer cells both in vitro and in vivo through inhibition of STAT3 and NF-kappaB signaling. Cancer Prev. Res. 2014, 7, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Brahmbhatt, M.; Gundala, S.R.; Asif, G.; Shamsi, S.A.; Aneja, R. Ginger phytochemicals exhibit synergy to inhibit prostate cancer cell proliferation. Nutr. Cancer 2013, 65, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Kubatka, P.; Kello, M.; Kajo, K.; Kruzliak, P.; Vybohova, D.; Mojzis, J.; Adamkov, M.; Fialova, S.; Veizerova, L.; Zulli, A.; et al. Oregano demonstrates distinct tumour-suppressive effects in the breast carcinoma model. Eur. J. Nutr. 2017, 56, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lee, B.Y.; Brown, D.A.; Molloy, M.P.; Marx, G.M.; Pavlakis, N.; Boyer, M.J.; Stockler, M.R.; Kaplan, W.; Breit, S.N.; et al. Identification of candidate biomarkers of therapeutic response to docetaxel by proteomic profiling. Cancer Res. 2009, 69, 7696–7703. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.E.; Dorff, T.B.; Quinn, D.I.; Diaz, P.M.; Castellanos, O.O.; Agus, D.B. Safety and Efficacy of Docetaxel, Bevacizumab, and Everolimus for Castration-resistant Prostate Cancer (CRPC). Clin. Genitourin. Cancer 2017. [Google Scholar] [CrossRef] [PubMed]
- Amawi, H.; Ashby, C.R., Jr.; Tiwari, A.K. Cancer chemoprevention through dietary flavonoids: What’s limiting? Chin. J. Cancer 2017, 36, 50. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, H. Cancer Preventive Activities of Tea Catechins. Molecules 2016, 21, 1679. [Google Scholar] [CrossRef] [PubMed]
- Rowles, J.L., III; Ranard, K.M.; Smith, J.W.; An, R.; Erdman, J.W., Jr. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017. [Google Scholar] [CrossRef]
- Rivera, M.; Ramos, Y.; Rodriguez-Valentin, M.; Lopez-Acevedo, S.; Cubano, L.A.; Zou, J.; Zhang, Q.; Wang, G.; Boukli, N.M. Targeting multiple pro-apoptotic signaling pathways with curcumin in prostate cancer cells. PLoS ONE 2017, 12, e0179587. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, E.E.; Dilda, P.J. Glutathione S-conjugates as prodrugs to target drug-resistant tumors. Front. Pharm. 2014, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Van Brussel, J.P.; van Steenbrugge, G.J.; Romijn, J.C.; Schroder, F.H.; Mickisch, G.H. Chemosensitivity of prostate cancer cell lines and expression of multidrug resistance-related proteins. Eur. J. Cancer 1999, 35, 664–671. [Google Scholar] [CrossRef]
- Theyer, G.; Schirmbock, M.; Thalhammer, T.; Sherwood, E.R.; Baumgartner, G.; Hamilton, G. Role of the MDR-1-encoded multiple drug resistance phenotype in prostate cancer cell lines. J. Urol. 1993, 150, 1544–1547. [Google Scholar] [CrossRef]
- Liu, D.; Wu, M.; Lu, Y.; Xian, T.; Wang, Y.; Huang, B.; Zeng, G.; Huang, Q. Protective effects of 6-Gingerol on vascular endothelial cell injury induced by high glucose via activation of PI3K-AKT-eNOS pathway in human umbilical vein endothelial cells. Biomed. Pharm. 2017, 93, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.B.; Lin, C.C.; Tsay, G.J. 6-Gingerol Inhibits Growth of Colon Cancer Cell LoVo via Induction of G2/M Arrest. Evid. Based Complement. Altern. Med. eCAM 2012, 2012, 326096. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tyagi, A.K. Ginger and its constituents: Role in prevention and treatment of gastrointestinal cancer. Gastroenterol. Res. Pract. 2015, 2015, 142979. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of compounds 1–6 are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-M.; Kao, C.-L.; Tseng, Y.-T.; Lo, Y.-C.; Chen, C.-Y. Ginger Phytochemicals Inhibit Cell Growth and Modulate Drug Resistance Factors in Docetaxel Resistant Prostate Cancer Cell. Molecules 2017, 22, 1477. https://doi.org/10.3390/molecules22091477
Liu C-M, Kao C-L, Tseng Y-T, Lo Y-C, Chen C-Y. Ginger Phytochemicals Inhibit Cell Growth and Modulate Drug Resistance Factors in Docetaxel Resistant Prostate Cancer Cell. Molecules. 2017; 22(9):1477. https://doi.org/10.3390/molecules22091477
Chicago/Turabian StyleLiu, Chi-Ming, Chiu-Li Kao, Yu-Ting Tseng, Yi-Ching Lo, and Chung-Yi Chen. 2017. "Ginger Phytochemicals Inhibit Cell Growth and Modulate Drug Resistance Factors in Docetaxel Resistant Prostate Cancer Cell" Molecules 22, no. 9: 1477. https://doi.org/10.3390/molecules22091477
APA StyleLiu, C.-M., Kao, C.-L., Tseng, Y.-T., Lo, Y.-C., & Chen, C.-Y. (2017). Ginger Phytochemicals Inhibit Cell Growth and Modulate Drug Resistance Factors in Docetaxel Resistant Prostate Cancer Cell. Molecules, 22(9), 1477. https://doi.org/10.3390/molecules22091477





