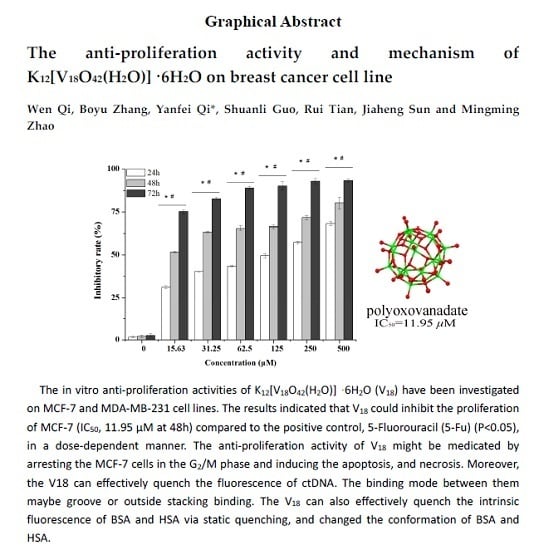
The Anti-Proliferation Activity and Mechanism of Action of K12[V18O42(H2O)]∙6H2O on Breast Cancer Cell Lines
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Growth Inhibition Effect in Vitro Assays
2.2. Morphological Analysis
2.3. Flow Cytometric Analysis for Cell Cycle Distribution and Apoptosis
2.4. The Expression of Apoptosis-Related Molecules
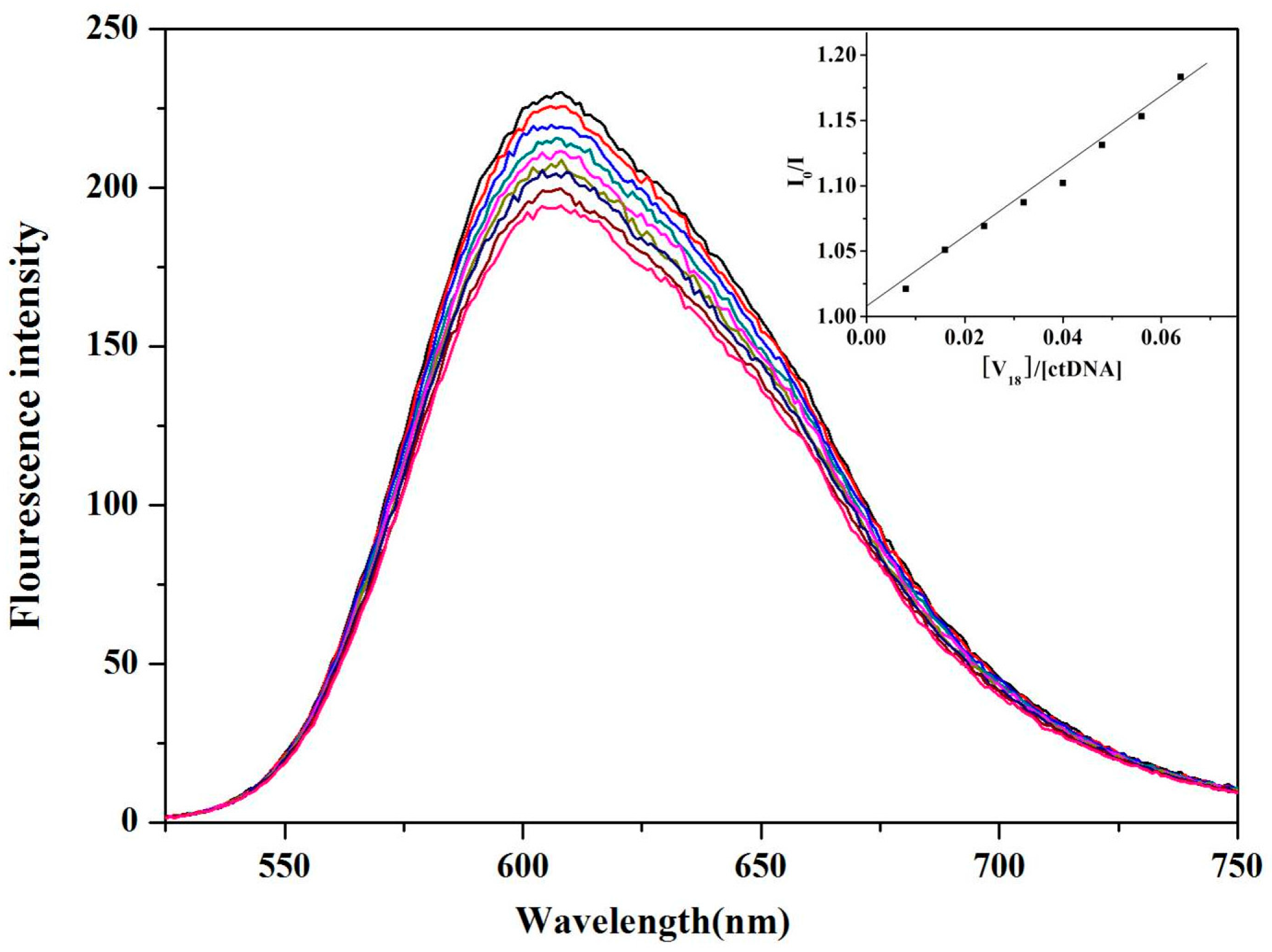
2.5. ctDNA Binding of V18
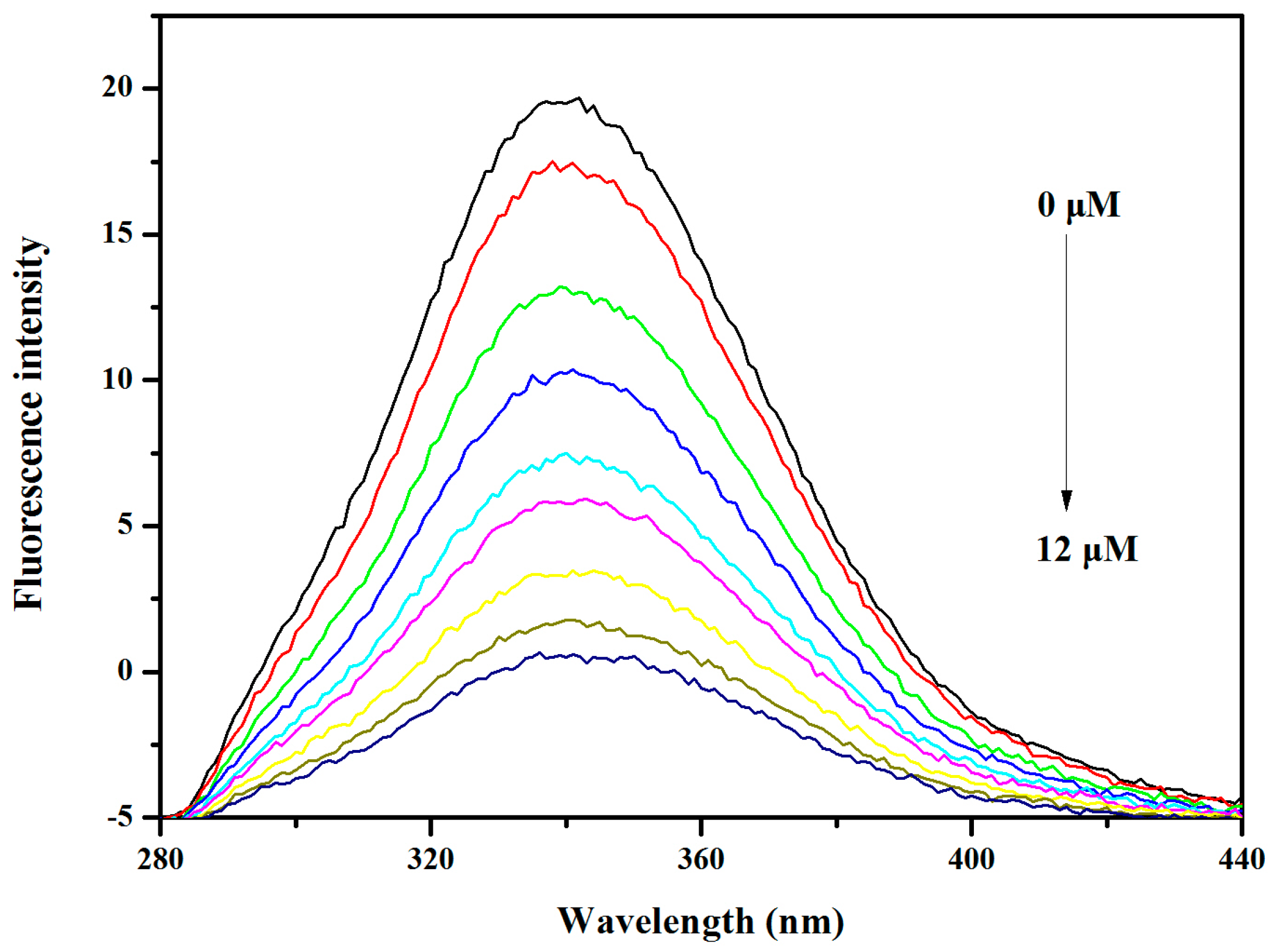
2.6. BSA and HSA Binding of V18
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Synthesis of K12[V18O42(H2O)]∙6H2O
3.3. Cell Culture and the Antitumor Activity in Vitro Assay
3.4. Morphological Observation
3.5. Flow Cytometric Analysis of Cell Cycle Distribution and Apoptosis
3.6. Western Blot Analysis
3.7. ctDNA Binding Experiments
3.8. BSA and HSA Binding Experiments
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pope, M.T. Polyoxometalates. In Encyclopedia of Inorganic & Bioinorganic Chemistry, 1st ed.; Scott, R.A., Ed.; John Wiley and Sons, Inc.: Somerset, NJ, USA, 2011. [Google Scholar]
- Rhule, J.T.; Hill, C.L.; Judd, D.A.; Schinazi, R.F. Polyoxometalates in Medicine. Chem. Rev. 1998, 98, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Wang, X.L.; Qin, C.; Zhang, Z.M.; Li, Y.G.; Chen, W.L.; Wang, E.B. Polyoxometalate-assisted synthesis of transition-metal cubane clusters as artificial mimics of the oxygen evolving center of photosystem. Chem. Soc. Rev. 2016, 313, 94–110. [Google Scholar] [CrossRef]
- Li, M.; Xu, C.; Wu, L.; Ren, J.S.; Wang, E.B.; Qu, X.G. Self-assembled peptide polyoxometalate hybrid nanospheres: Two in one enhances targeted inhibition of amyloid β-peptide aggregation associated with Alzheimer’s disease. Small 2013, 9, 3455–3461. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.F.; Zhang, H.; Wang, J.; Jiang, Y.F.; Li, J.H.; Yuan, Y.; Zhang, S.Y.; Xu, K.; Li, Y.G.; Li, J. In vitro anti-hepatitis B and SARS virus activities of a titanium-substituted-heteropolytungstate. Antivir. Res. 2012, 93, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Z.; Zhou, M.C.; Jing, J.B.; Li, W.; Wang, Y.; Wu, L.X.; Wang, L.Y.; Wang, Y.Q.; Lee, M. Polyoxometalate driven self-assembly of short peptides into multivalent nanofibers with enhanced antibacterial activity. Angew. Chem. Int. Ed. 2016, 55, 2592–2595. [Google Scholar] [CrossRef] [PubMed]
- Thomadaki, H.; Karaliota, A.; Litos, C.; Scorilas, A. Enhanced antileukemic activity of the novel complex 2,5-dihydroxybenzoate molybdenum(VI) against 2,5-dihydroxybenzoate, polyoxometalate of Mo(VI), and tetraphenylphosphonium in the human HL-60 and K562 leukemic cell lines. J. Med. Chem. 2007, 50, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Gao, H.; Yan, M.; Li, X.Y.; Dai, Z.F.; Liu, S.Q. Polyoxometalate-based organic-inorganic hybrids as Anti-tumor drugs. Small 2015, 11, 2938–2945. [Google Scholar] [CrossRef] [PubMed]
- Yamase, T. Photo- and electrochromism of polyoxometalates and related materials. Chem. Rev. 1998, 98, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Yamase, T. Anti-tumor, -viral, and -bacterial activities of polyoxometalates for realizing an inorganic drug. J. Mater. Chem. 2005, 15, 4773–4782. [Google Scholar] [CrossRef]
- Judd, D.A.; Nettles, J.H.; Nevins, N.; Snyder, J.P.; Liotta, D.C.; Tang, J.; Ermolieff, J.; Schinazi, R.F.; Hill, C.L. Polyoxometalate HIV-1 protease inhibitors. A new mode of protease inhibition. J. Am. Chem. Soc. 2001, 123, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Stephan, H.; Kubeil, M.; Emmerling, F.; Müller, C.E. Polyoxometalates as versatile enzyme Inhibitors. Eur. J. Inorg. Chem. 2013, 2013, 1585–1594. [Google Scholar] [CrossRef]
- Lee, S.Y.; Fiene, A.; Li, W.J.; Hanck, T.; Brylev, KA.; Fedorov, VE.; Lecka, J.; Haider, A.; Pietzsch, H.J.; Zimmermann, H. Polyoxometalates potent and selective ecto nucleotidase inhibitors. Biochem. Pharmacol. 2015, 93, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.F.; Wu, Y.; Gao, W.; Eniyoji, K.; Csizmadia, E.; Muller, C.E.; Murakami, T.; Robson, S.C. CD39/ENTPD1 expression by CD4+ Foxp3+, regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology 2010, 139, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Ahmedin, J.D.; Yu, X.Q. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Dianat, S.; Bordbar, A.K.; Tangestaninejad, S.; Yadollahi, B.; Zarkesh-Esfahani, S.H.; Habibi, P. ctDNA binding affinity and in vitro antitumor activity of three Keggin type polyoxotungestates. J. Photochem. Photobiol. B 2013, 124, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Qin, P.; Liu, R.; Wang, J. Characterizing the Interaction between tartrazine and two serum albumins by a hybrid spectroscopic approach. J. Agric. Food Chem. 2011, 59, 6650–6656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, L.; Xu, Z.; Liang, Z.; Su, J.; Huang, J.; Li, B. Investigation of the interaction of naringin palmitate with bovine serum albumin: Spectroscopic analysis and molecular docking. PLoS ONE 2013, 8, e59106. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Qing, Y.; Qi, Y.F.; Guo, L.; Li, J. In vitro antitumor activity of a keggin vanadium-substituted polyoxomolybdate and its ctDNA binding properties. J. Chem. 2015, 2015, 1–6. [Google Scholar]
- Cheng, Z. Studies on the interaction between scopoletin and two serum albumins by spectroscopic methods. J. Lumin. 2012, 132, 2719–2729. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, R.R.; Wang, H.C.; Pu, H.L. Studies on the binding of vinpocetine to human serum albumin by molecular spectroscopy and modeling. Chin. Chem. Lett. 2012, 23, 599–602. [Google Scholar] [CrossRef]
- Gao, P.F.; Zhang, S.; Li, H.W.; Zhang, T.; Wu, W.; Wu, L. A Two-Step Binding Process of Eu-Containing Polyoxometalates to Bovine Serum Albumin. Langmuir 2015, 31, 10888–10896. [Google Scholar] [CrossRef] [PubMed]
- Geisberger, G.; Gyenge, E.B.; Hinger, D.; Bösiger, P.; Maake, C.; Patzke, G.R. Synthesis, characterization and bioimaging of fluorescent labeled polyoxometalates. J. Dalton Trans. 2013, 42, 9914–9920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, A.; Sessoli, R.; Krickemeyer, E.; Bögge, H.; Meyer, J.; Gatteschi, D.; Pardi, L.; Westphal, J.; Hovemeier, K.; Rohlfing, R.; et al. Polyoxovanadates: High-Nuclearity Spin Clusters with Interesting Host−Guest Systems and Different Electron Populations. Synthesis, Spin Organization, Magnetochemistry, and Spectroscopic Studies. Inorg. Chem. 1997, 36, 5239–5250. [Google Scholar] [CrossRef]
- Li, C.Y.; Cao, H.Q.; Sun, J.H.; Tian, R.; Li, D.B.; Qi, Y.F.; Yang, W.; Li, J. Antileukemic activity of an arsenomolybdate in the human HL-60 and U937 leukemia cells. J. Inorg. Biochem. 2017, 168, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Cindri, M.K.; Kraljević, T.; Kralj, M.; Kamenar, B. Structural and antitumor activity study of γ-octamolybdates containing aminoacids and peptides. Inorg. Chim. Acta 2006, 359, 1673–1680. [Google Scholar] [CrossRef]
- Dablemont, C.; Proust, A.; Thouvenot, R.; Afonso, C.; Fournier, F.; Tabet, J.C. Functionalization of polyoxometalates: From Lindqvist to Keggin derivatives. 1. Synthesis, solution studies, and spectroscopic and ESI mass spectrometry characterization of the rhenium phenylimido tungstophosphate [PW11O39(ReNC6H5)]4−. Inorg. Chem. Commun. 2004, 43, 3514–3520. [Google Scholar] [CrossRef] [PubMed]
- Gabas, I.M.; Stepien, G.; Moros, M.; Mitchell, S.G.; de la Fuente, J.M. In vitro cell cytotoxicity profile and morphological response to polyoxometalate-stabilised gold nanoparticles. New J. Chem. 2016, 40, 1039–1047. [Google Scholar] [CrossRef]
- Dianat, S.; Bordbar, A.K.; Tangestaninejad, S.; Yadeollahi, B.; Zarkesh-Esfahani, S.H.; Habibi, P. In vitro, antitumor activity of parent and nano-encapsulated mono cobalt-substituted Keggin polyoxotungstate and its ctDNA binding properties. Chem. Biol. Interact. 2014, 215, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Hemmateenejad, B.; Shamsipur, M.; Samari, F.; Khayamian, T.; Ebrahimi, M.; Rezaei, Z. Combined fluorescence spectroscopy and molecular modeling studies on the interaction between harmalol and human serum albumin. Pharmaceut. Biomed. 2012, 67, 201–208. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |











© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, W.; Zhang, B.; Qi, Y.; Guo, S.; Tian, R.; Sun, J.; Zhao, M. The Anti-Proliferation Activity and Mechanism of Action of K12[V18O42(H2O)]∙6H2O on Breast Cancer Cell Lines. Molecules 2017, 22, 1535. https://doi.org/10.3390/molecules22091535
Qi W, Zhang B, Qi Y, Guo S, Tian R, Sun J, Zhao M. The Anti-Proliferation Activity and Mechanism of Action of K12[V18O42(H2O)]∙6H2O on Breast Cancer Cell Lines. Molecules. 2017; 22(9):1535. https://doi.org/10.3390/molecules22091535
Chicago/Turabian StyleQi, Wen, Boyu Zhang, Yanfei Qi, Shuanli Guo, Rui Tian, Jiaheng Sun, and Mingming Zhao. 2017. "The Anti-Proliferation Activity and Mechanism of Action of K12[V18O42(H2O)]∙6H2O on Breast Cancer Cell Lines" Molecules 22, no. 9: 1535. https://doi.org/10.3390/molecules22091535