Structural Determination of Ruthenium Complexes Containing Bi-Dentate Pyrrole-Ketone Ligands
Abstract
:1. Introduction
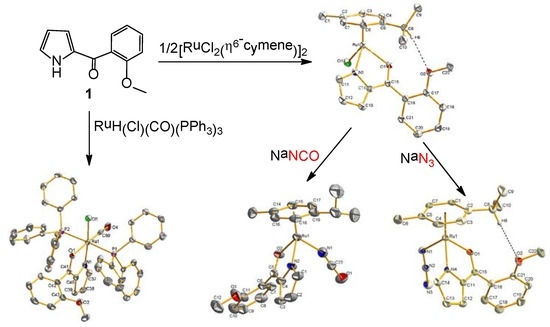
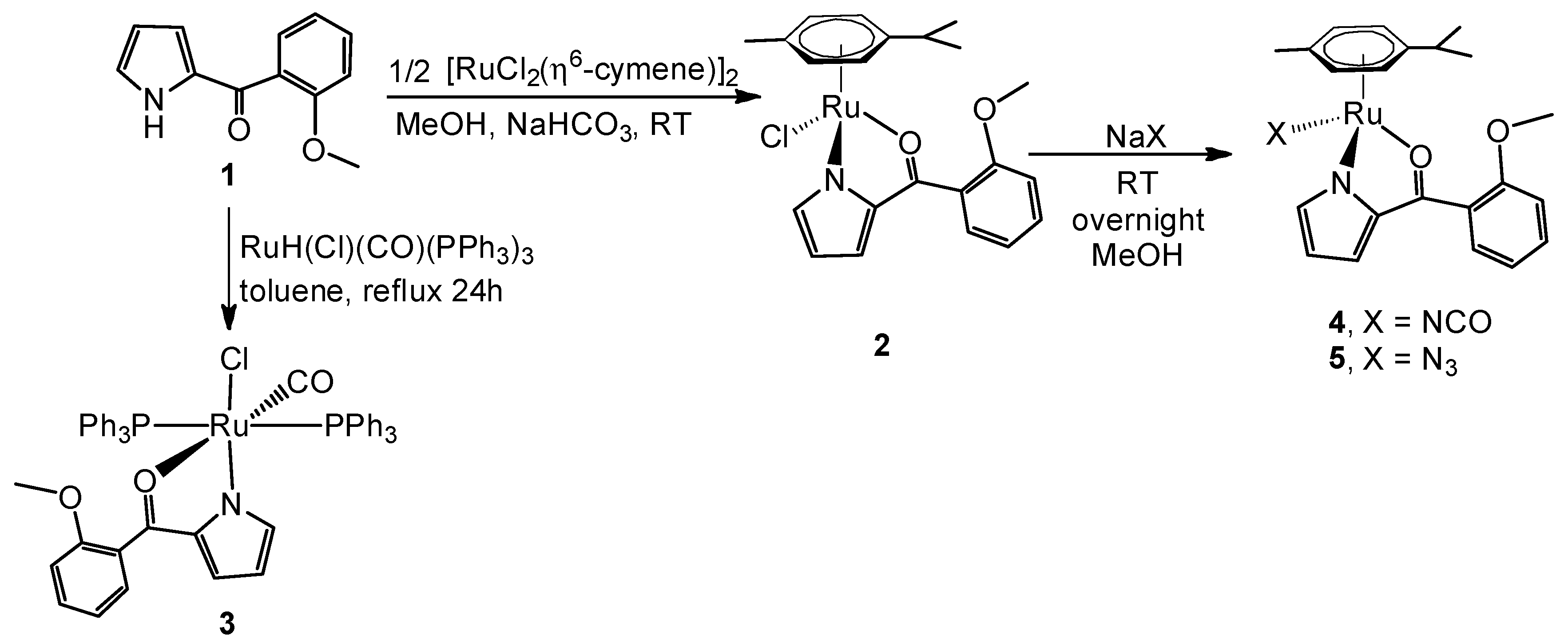
2. Results and Discussion
2.1. Synthesis and Characterization
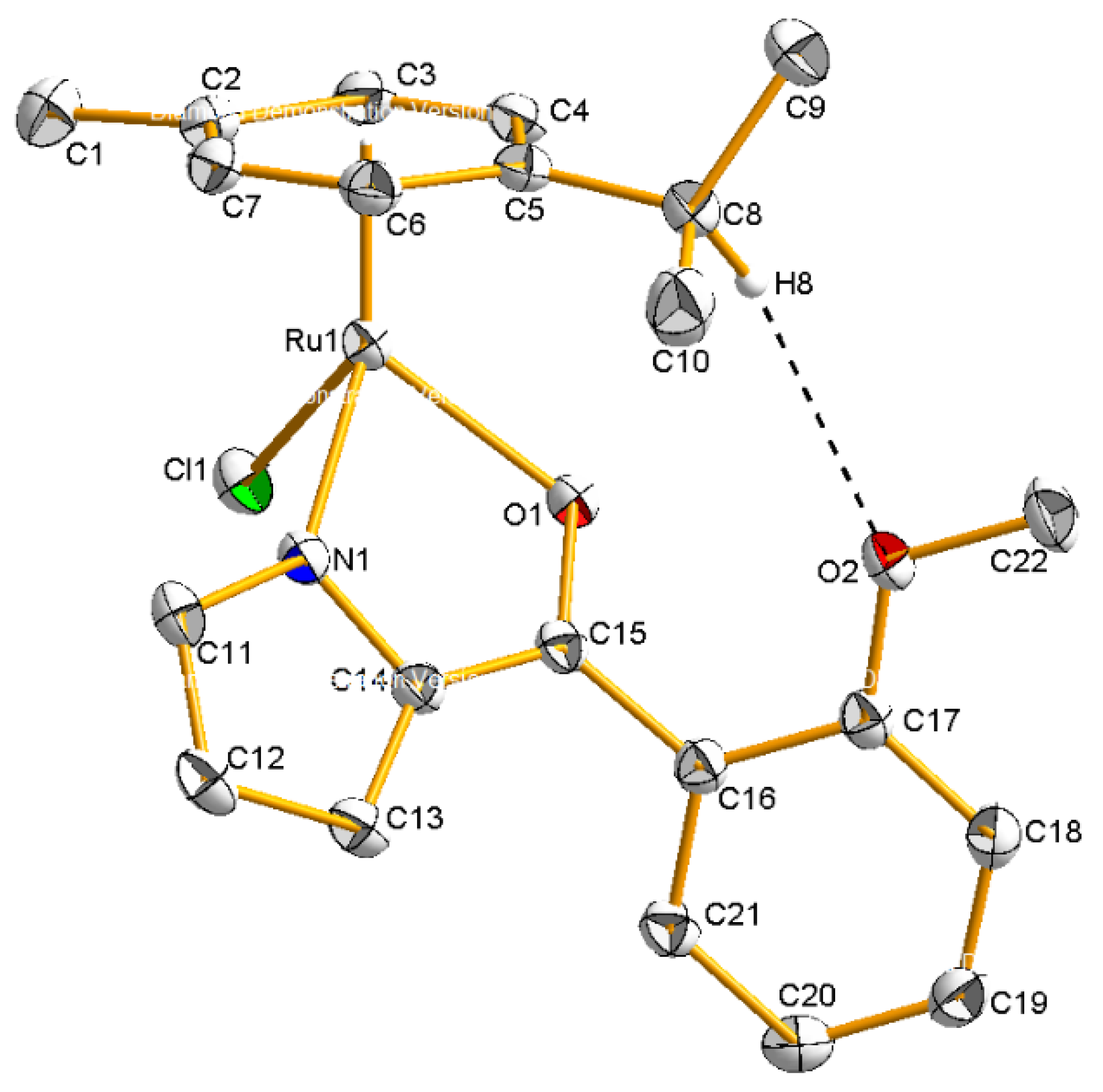
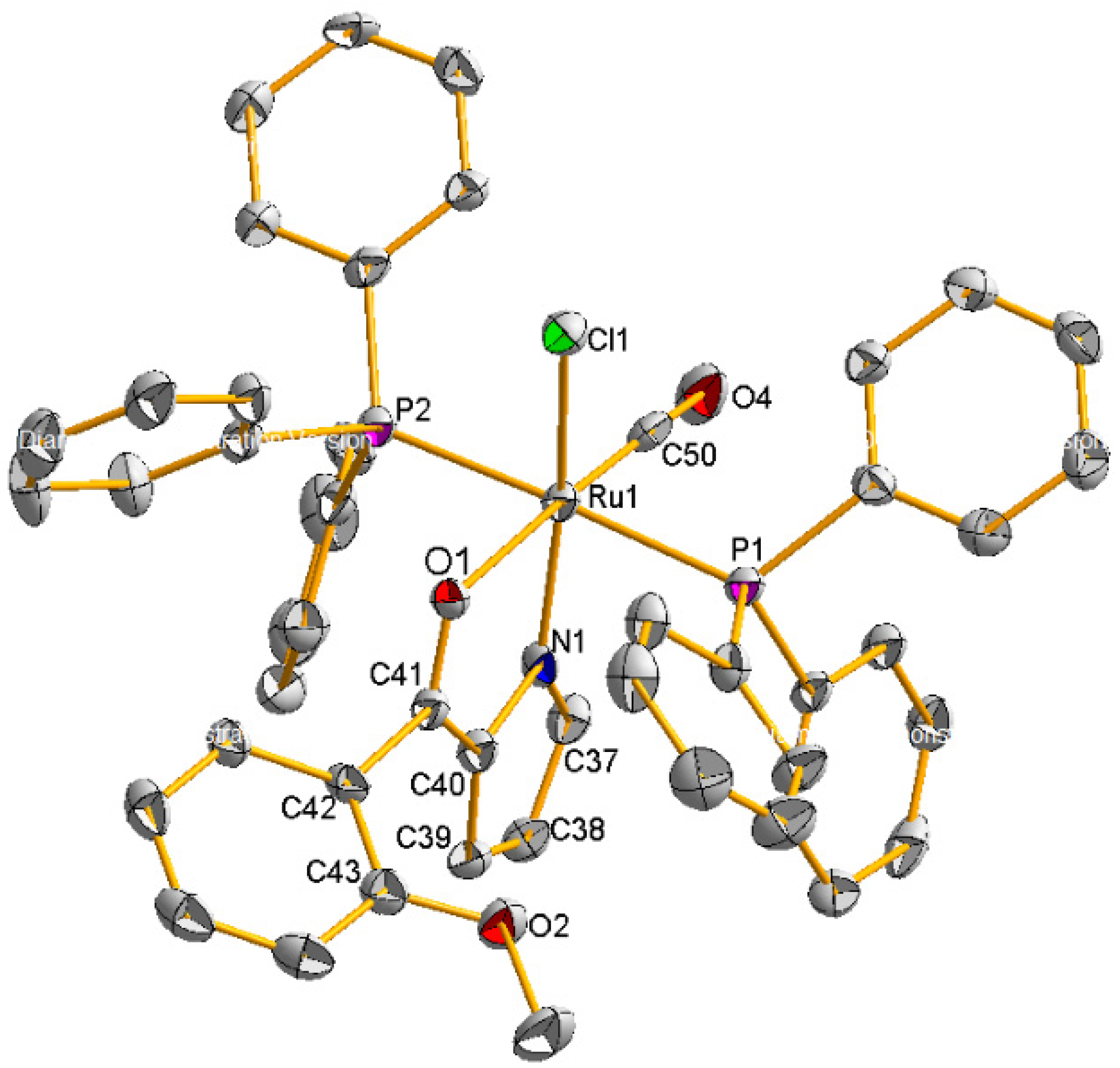
2.2. Molecular Geometries of Compounds 2–5
3. Experimental Section
3.1. General Consideration
3.1.1. Synthesis of the Ligand [C4H3NH-2-(CO-C6H4-2-OMe)] (1)
3.1.2. Synthesis of Compound {Ru(η6-cymene)[C4H3N-2-(CO-C6H4-2-OMe)]Cl} (2)
3.1.3. Synthesis of Compound {RuCl(CO)(PPh3)2[C4H3N-2-(COC6H4-2-OMe)]} (3)
3.1.4. Synthesis of Compound {Ru(η6-cymene)[C4H3N-2-(CO-C6H4-2-OMe)](OCN)} (4)
3.1.5. Synthesis of Compound {Ru(η6-cymene)[C4H3N-2-(CO-C6H4-2-OMe)](N3)} (5)
3.2. X-ray Crystallography
4. Conclusions
Supplementary Materials
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Dragutan, I.; Dragutan, V.; Demonceau, A. Editorial of special issue ruthenium complex: The expanding chemistry of the ruthenium complexes. Molecules 2015, 20, 17244–17274. [Google Scholar] [CrossRef] [PubMed]
- Storr, T. Ligand Design in Medicinal Inorganic Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2014. [Google Scholar]
- Holder, A.A.; Lilge, L.; Browne, W.R.; Lawrence, M.A.W.; Bullock, J.L., Jr. (Eds.) Ruthenium Complexes: Photochemical and Biomedical Applications; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Weiss, A.; Berndsen, R.H.; Dubois, M.; Muller, C.; Schibli, R.; Griffioen, A.W.; Dyson, P.J.; Nowak-Sliwinska, P. In vivo anti-tumor activity of the organometallic ruthenium(II)-arene complex [Ru(η6-p-cymene)Cl2(pta)] (RAPTA-C) in human ovarian and colorectal carcinomas. Chem. Sci. 2014, 5, 4742–4748. [Google Scholar] [CrossRef]
- Clavel, C.M.; Păunescu, E.; Nowak-Sliwinska, P.; Griffioen, A.W.; Scopelliti, R.; Dyson, P.J. Discovery of a highly tumor-selective rrganometallic ruthenium(II)-arene complex. J. Med. Chem. 2014, 57, 3546–3558. [Google Scholar] [CrossRef] [PubMed]
- Sáez, R.; Lorenzo, J.; Prieto, M.J.; Font-Bardia, M.; Calvet, T.; Omeñaca, N.; Vilaseca, M.; Moreno, V. Influence of PPh3 moiety in the anticancer activity of new organometallic ruthenium complexes. J. Inorg. Biochem. 2014, 136, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kilpin, K.J.; Crot, S.; Riedel, T.; Kitchen, J.A.; Dyson, P.J. Ruthenium(II) and osmium(II) 1,2,3-triazolylidene organometallics: A preliminary investigation into the biological activity of ‘click’ carbene complexes. Dalton Trans. 2014, 43, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Pettinari, R.; Pettinari, C.; Marchetti, F.; Clavel, C.M.; Scopelliti, R.; Dyson, P.J. Cytotoxicity of ruthenium–arene complexes containing β-ketoamine ligands. Organometallics 2013, 32, 309–316. [Google Scholar] [CrossRef]
- Pettinari, R.; Marchetti, F.; Pettinari, C.; Petrini, A.; Scopelliti, R.; Clavel, C.M.; Dyson, P.J. Synthesis, structure, and antiproliferative activity of ruthenium(II) arene complexes with N,O-chelating pyrazolone-based β-ketoamine ligands. Inorg. Chem. 2014, 53, 13105–13111. [Google Scholar] [CrossRef] [PubMed]
- Garza-Ortiz, A.; Maheswari, P.U.; Siegler, M.; Spek, A.I.; Reedijk, J. A new family of Ru(II) complexes with a tridentate pyridine Schiff-base ligand and bidentate co-ligands: Synthesis, characterization, structure and in vitro cytotoxicity studies. New J. Chem. 2013, 37, 3450–3460. [Google Scholar] [CrossRef]
- Chow, M.J.; Licona, C.; Wong, D.Y.Q.; Pastorin, G.; Gaiddon, C.; Ang, W.H. Discovery and investigation of anticancer ruthenium-arene Schiff-base complexes via water-promoted combinatorial three-component assembly. J. Med. Chem. 2014, 57, 6043–6059. [Google Scholar] [CrossRef] [PubMed]
- Allardyce, C.S.; Dyson, P.J. Ruthenium in medicine: Current clinical uses and future prospects. Platinum Metal. Rev. 2001, 45, 62–69. [Google Scholar]
- Zeng, L.; Gupta, P.; Chen, Y.; Wang, E.; Ji, L.; Chao, H.; Chen, Z.-S. The development of anticancer ruthenium(II) complexes: From single molecule compounds to nanomaterials. Chem. Soc. Rev. 2017, 46, 5771–5804. [Google Scholar] [CrossRef] [PubMed]
- Ronconi, L.; Sadler, P.J. Using coordination chemistry to design new medicines. Coord. Chem. Rev. 2007, 251, 1633–1648. [Google Scholar] [CrossRef]
- Dougan, S.J.; Sadler, P.J. The Design of Organometallic Ruthenium Arene Anticancer Agents. Chimia 2007, 61, 704–715. [Google Scholar] [CrossRef]
- Sheeba, M.M.; Tamizh, M.M.; Farrugia, L.J.; Endo, A.; Karvembu, R. Chiral (η6-p-Cymene)ruthenium(II) complexes containing monodentate acylthiourea ligands for efficient asymmetric transfer hydrogenation of ketones. Organometallics 2014, 33, 540–550. [Google Scholar] [CrossRef]
- Guo, R.; Lough, A.J.; Morris, R.H.; Song, D. Asymmetric hydrogenation of ketones catalyzed by ruthenium hydride complexes of a beta-aminophosphine ligand derived from norephedrine. Organometallics 2004, 23, 5524–5529. [Google Scholar] [CrossRef]
- Kalutharage, N.; Yi, C.S. Scope and mechanistic analysis for chemoselective hydrogenolysis of carbonyl compounds catalyzed by a cationic ruthenium hydride complex with a tunable phenol ligand. J. Am. Chem. Soc. 2015, 137, 11105–11114. [Google Scholar] [CrossRef] [PubMed]
- Benneth, M.A.; Smith, A.K. Arene ruthenium(II) complexes formed by dehydrogenation of cyclohexadienes with ruthenium(III) trichloride. J. Chem. Soc. Dalton Trans. 1974, 233–241. [Google Scholar] [CrossRef]
- Chatt, J.; Leigh, G.J.; Mingos, D.M.P.; Paske, R.J. Complexes of osmium, ruthenium, rhenium, and iridium halides with some tertiary monophosphines and monoarsines. J. Chem. Soc. A 1968, 2636–2641. [Google Scholar] [CrossRef]
- Allardyce, C.S.; Dyson, P.J.; Ellis, D.J.; Heath, S.L. [Ru(η6-p-cymene)Cl2(pta)] (pta = 1,3,5-triaza-7-phosphatricyclo-[3.3.1.1]decane): A water soluble compound that exhibits pH dependent DNA binding providing selectivity for diseased cells. Chem. Commun 2001, 1396–1397. [Google Scholar] [CrossRef]
- Adeniyi, A.A.; Ajibade, P.A. Development of ruthenium-based complexes as anticancer agents: Toward a rational design of alternative receptor targets. Rev. Inorg. Chem. 2016, 36, 53–75. [Google Scholar] [CrossRef]
- Clarke, M.J. Ruthenium metallopharmaceuticals. Coord. Chem. Rev. 2003, 236, 209–233. [Google Scholar] [CrossRef]
- Dyson, P.J. Systematic Design of a Targeted Organometallic Antitumour Drug in Pre-clinical Development. Chimia 2007, 61, 698–703. [Google Scholar] [CrossRef]
- Cox, M.; Lehninger, A.L.; Nelson, D.R. Principles of Biochemistry; Worth Publishers: New York, NY, USA, 2000. [Google Scholar]
- Shimizu, S. Recent advances in subporphyrins and triphyrin analogues: Contracted porphyrins comprising three pyrrole rings. Chem. Rev. 2017, 117, 2730–2784. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Gross, D.E.; Cho, D.-G.; Lynch, V.M.; Sessler, J.L. N-Tosylpyrrolidine calix[4]pyrrole: Synthesis and ion binding studies. J. Org. Chem. 2011, 76, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Bando, Y. Recent progress in research on anion-responsive pyrrole-based π-conjugated acyclic molecules. Chem. Commun. 2013, 49, 4100–4113. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, R.; Dham, S.; Kapoor, K.K. Active methylenes in the synthesis of a pyrrole motif: An imperative structural unit of pharmaceuticals, natural products and optoelectronic materials. RSC Adv. 2016, 6, 37039–37066. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Gumber, D.; Abbot, V.; Dhiman, S.; Sharma, P. Pyrrole: A resourceful small molecule in key medicinal hetero-aromatics. RSC Adv. 2015, 5, 15233–15266. [Google Scholar] [CrossRef]
- Al-Mourabit, A.; Zancanella, M.A.; Tilvi, S.; Romo, D. Biosynthesis, asymmetric synthesis, and pharmacology, including cellular targets, of the pyrrole-2-aminoimidazole marine alkaloids. Nat. Prod. Rep. 2011, 28, 1229–1260. [Google Scholar] [CrossRef] [PubMed]
- Trimm, H.H.; Hunter, W., Jr. Dyes and Drugs-New Uses and Implications; Apple Academic Press: Toronto, ON, Canada, 2011. [Google Scholar]
- Kanakis, A.A.; Sarli, V. Total synthesis of (±)-marinopyrrole A via copper-mediated N-arylation. Org. Lett. 2010, 12, 4872–4875. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.C.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Structures, reactivities, and antibiotic properties of the marinopyrroles A−F. J. Org. Chem. 2010, 75, 3240–3250. [Google Scholar] [CrossRef] [PubMed]
- Petruso, S.; Bonanno, S.; Caronna, S.; Ciofalo, M.; Maggio, B.; Schillaci, D. Oxidative halogenation of substituted pyrroles with Cu(II). Part IV. Bromination of 2-(2′-hydroxybenzoyl)pyrrole. A new synthesis of bioactive analogs of monodeoxypyoluteorin. J. Heterocycl. Chem. 1994, 31, 941. [Google Scholar] [CrossRef]
- Ghigo, G.; Ciofalo, M.; Gagliardi, L.; La Manna, G.; Cramer, C. The electronic spectra of 2-(2′-hydroxybenzoyl)pyrrole and 2-(2’-methoxybenzoyl)pyrrole: A theoretical study. J. Phys. Org. Chem. 2005, 18, 1099–1106. [Google Scholar] [CrossRef]
- Wang, H.-G.; Amin, S. Mcl-1 Modulating Compositions. Patent WO2013/112878 A1, 1 August 2013. [Google Scholar]
- Trisuwan, K.; Rukachaisirikul, V.; Sukpondma, Y.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. Furo[3,2-h]isochroman, furo[3,2-h]-isoquinoline, isochroman, phenol, pyranone, and pyrone derivatives from the sea fan-derived fungus Penicillium sp. PSU-F40. Tetrahedron 2010, 66, 4484–4489. [Google Scholar] [CrossRef]
- Chen, T.; Li, H.; Qu, S.; Zheng, B.; He, L.; Lai, Z.; Wang, Z.-X.; Huang, K.-W. Hydrogenation of esters catalyzed by ruthenium PN3-pincer complexes containing an aminophosphine arm. Organometallics 2014, 33, 4152–4155. [Google Scholar] [CrossRef]
- Anaby, A.; Butschke, B.; Ben-David, Y.; Shimon, L.J.W.; Leitus, G.; Feller, M.; Milstein, D. B–H bond cleavage via metal–ligand cooperation by dearomatized ruthenium pincer complexes. Organometallics 2014, 33, 3716–3726. [Google Scholar] [CrossRef]
- Raja, M.U.; Gowri, N.; Ramesh, R. Synthesis, crystal structure and catalytic activity of ruthenium(II) carbonyl complexes containing ONO and ONS donor ligands. Polyhedron 2010, 29, 1175–1181. [Google Scholar] [CrossRef]
- Gusev, D.G.; Dolgushin, F.M.; Antipin, M.Y. Hydride, borohydride, and dinitrogen pincer complexes of ruthenium. Organometallics 2000, 19, 3429–3434. [Google Scholar] [CrossRef]
- Veljković, D.Z.; Janjića, G.V.; Zarić, S.D. Are C–H⋯O interactions linear? The case of aromatic CH donors. CrystEngComm 2011, 13, 5005–5010. [Google Scholar] [CrossRef]
- Manikandan, T.S.; Saranya, S.; Ramesh, R. Synthesis and catalytic evaluation of ruthenium(II) benzhydrazone complex in transfer hydrogenation of ketones. Tetrahedron Lett. 2016, 57, 3764–3769. [Google Scholar] [CrossRef]
- McGuiggan, M.F.; Pignolet, L.H. Synthesis and X-ray structural characterization of an ortho-metalated ruthenium complex of acetophenone. Inorg. Chem. 1982, 21, 2523–2526. [Google Scholar] [CrossRef]
- Flower, K.R.; Garrould, M.W.; Leal, L.G.; Mangold, C.; O’Malley, P.J.; Pritchard, R.G. The synthesis, reactivity and modelling studies of [RuCl(CO)(η2-C,O-C6H4-2-CHO)(PPh3)2]: Crystal and molecular structures of [RuCl(CO)(η2-C,O-C6H4-2-CHO)(PPh3)2]·3.5CHCl3 and [Ru(NCtBu)(CO)(η2-C,O-C6H4-2-CHO)(PPh3)2][BF4]·2CHCl3. J. Organomet. Chem. 2008, 693, 408–416. [Google Scholar] [CrossRef]
- Flower, K.R.; Leal, L.G.; Pritchard, R.G. Definitive proof of a Ru-Cl⋯ClCHCl interaction in the solid state: Crystal and molecular structure of [RuCl(CO)(η2-C,N-C6H4CH=NC6H4-4-Me)(PPh3)2]·CHCl3. Inorg. Chem. Commun. 2004, 7, 729–730. [Google Scholar] [CrossRef]
- Shivakumar, M.; Pramanik, K.; Ghosh, P.; Chakravorty, A. Isolation and structure of the first azo anion radical complexes of ruthenium. Inorg. Chem. 1998, 37, 5968–5969. [Google Scholar] [CrossRef] [PubMed]
- Cadierno, V.; Diez, J.; Garcia-Garrido, S.E.; Garcia-Granda, S.; Gimeno, J. Ruthenium(II) and ruthenium(IV) complexes containing hemilabile heterodifunctional iminophosphorane-phosphine ligands Ph2PCH2P(=NR)Ph2. J. Chem. Soc. Dalton Trans. 2002, 1465–1472. [Google Scholar] [CrossRef]
- Cadierno, V.; Crochet, P.; Diez, J.; Garcia-Alvarez, J.; Garcia-Garrido, S.E.; Gimeno, J.; Garcia-Granda, S.; Rodriguez, M.A. Ruthenium(II) and ruthenium(IV) complexes containing κ1-P-, κ2-P,O-, and κ3-P,N,O-iminophosphorane-phosphine ligands Ph2PCH2P{=NP(=O)(OR)2}Ph2 (R = Et, Ph): synthesis, reactivity, theoretical studies, and catalytic activity in transfer hydrogenation of cyclohexanone. Inorg. Chem. 2003, 42, 3293–3307. [Google Scholar] [PubMed]
- Singh, T.; Kishan, R.; Nethaji, M.; Thirupathi, N. Synthesis, reactivity Studies, structural aspects, and solution behavior of half sandwich ruthenium(II) N,N′,N′′-triarylguanidinate complexes. Inorg. Chem. 2012, 51, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Nongbri, S.L.; Das, B.; Rao, K.M. Arene ruthenium β-diketonato triazolato derivatives: Synthesis and spectral studies (β-diketones: 1-phenyl-3-methyl-4-benzoyl pyrazol-5-one, acetylacetone derivatives). J. Organomet. Chem. 2009, 694, 3881–3891. [Google Scholar] [CrossRef]
- Yadav, M.; Singh, A.K.; Maiti, B.; Pandey, D.S. Heteroleptic arene ruthenium complexes based on meso-substituted dipyrrins: Synthesis, structure, reactivity, and electrochemical studies. Inorg. Chem. 2009, 48, 7593–7603. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.S.; Kreisel, K.A.; Yap, G.P.A.; Kollipara, M.R. Synthesis of arene ruthenium triazolato complexes by cycloaddition of the corresponding arene ruthenium azido complexes with activated alkynes or with fumaronitrile. J. Organomet. Chem. 2006, 691, 3509–3518. [Google Scholar] [CrossRef]
- Sheldirck, G.M. Area-Detector Absorption Correction (SADABS); Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Sheldrick, G.M. SHELX97-Programs for Crystal Structure Analysis: Structure Determination (SHELXS); University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELX97-Programs for Crystal Structure Analysis: Refinement (SHELXL); University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
Sample Availability: Samples of the Compounds 2–5 are available from the authors. |
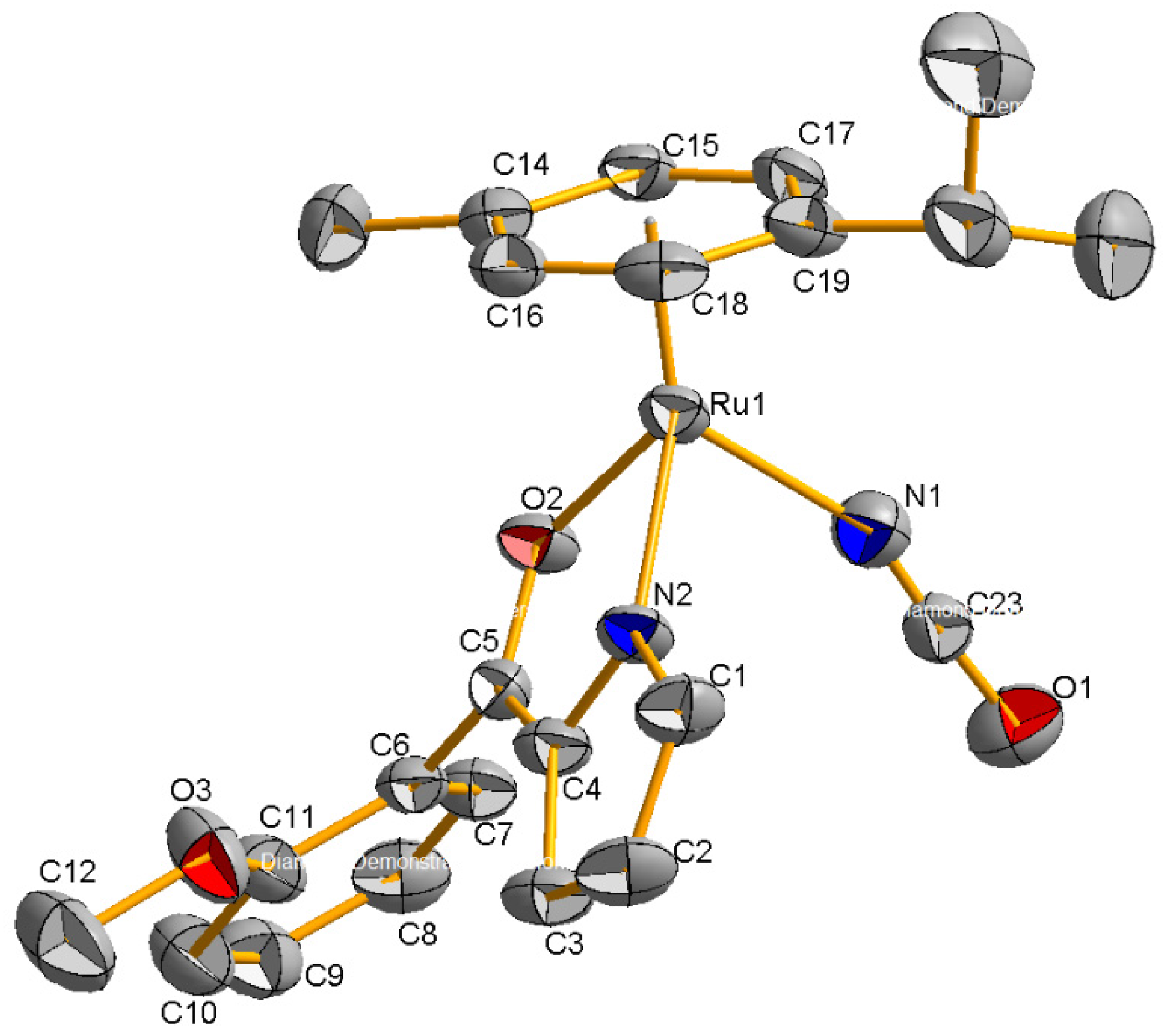
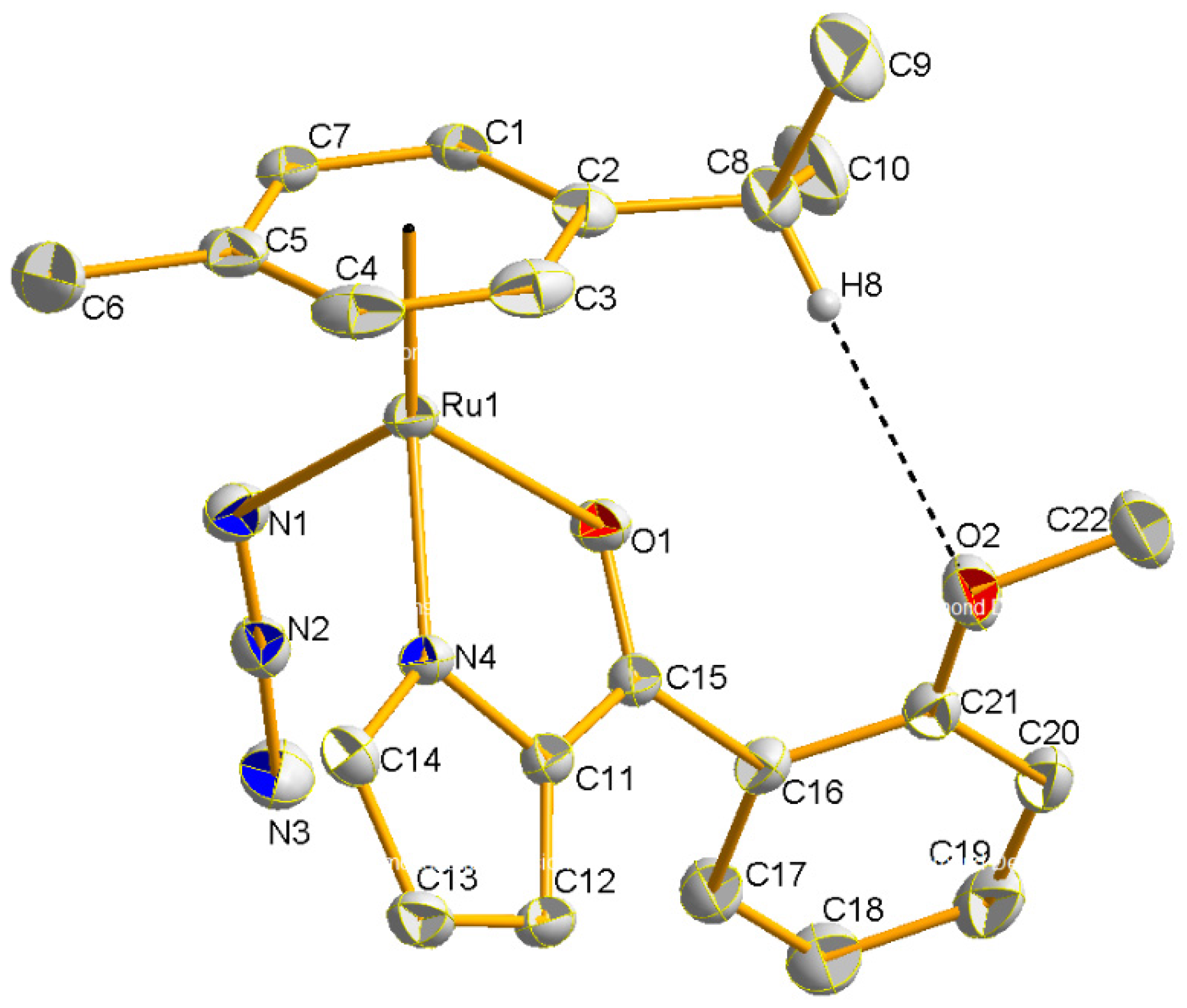
| 2 | 3 THF | 4 | 5 | |
|---|---|---|---|---|
| formula | C22H24ClNO2Ru | C53H48ClNO4P2Ru | C23H24N2O3Ru | C22H24N4O2Ru |
| FW | 470.94 | 961.38 | 477.51 | 477.52 |
| T [K] | 150(2) | 150(2) | 150(2) | 150(2) |
| crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| space group | P21/n | P21/n | P21/c | P21/c |
| a [Å] | 10.4657(4) | 10.2279(5) | 10.157(5) | 17.3849(14) |
| b [Å] | 9.1505(3) | 17.5822(9) | 15.836(7) | 9.2423(7) |
| c [Å] | 21.1478(8) | 25.9234(13) | 14.019(8) | 13.8157(11) |
| α [°] | 90 | 90 | 90 | 90 |
| β [°] | 99.097(2) | 97.693(3) | 105.44(3) | 112.379(4) |
| γ [°] | 90 | 90 | 90 | 90 |
| V [Å3] | 1999.78(13) | 4619.8(4) | 2173.5(19) | 2052.7(3) |
| Z | 4 | 4 | 4 | 4 |
| ρc [Mg m−3] | 1.564 | 1.382 | 1.465 | 1.545 |
| μ [mm−1] | 0.934 | 0.513 | 0.748 | 0.787 |
| F(000) | 960 | 1824 | 980 | 976.0 |
| rflns collected | 28,629 | 49,633 | 26,439 | 18,124 |
| independent rflns | 5176 [Rint = 0.0263] | 8132 [Rint = 0.0979] | 5380 [Rint = 0.0893] | 5243 [Rint = 0.0298] |
| data/restraints/parameters | 5176/0/248 | 8132/0/560 | 5380/0/266 | 5243/0/266 |
| goodness-of-fit on F2 | 1.027 | 0.880 | 0.968 | 1.067 |
| R1, wR2 (I > 2σ(I)) | R1 = 0.0208 | R1 = 0.0591 | R1 = 0.0568 | R1 = 0.0306 |
| wR2 = 0.0494 | wR2 = 0.1682 | wR2 = 0.1449 | wR2 = 0.0783 | |
| R1, wR2 (all data) | R1 = 0.0260 | R1 = 0.0879 | R1 = 0.0890 | R1 = 0.0356 |
| wR2 = 0.0520 | wR2 = 0.1925 | wR2 = 0.1724 | wR2 = 0.0810 | |
| largest diff. peak, hole [eÅ−3] | 0.406 and −0.367 | 0.811 and −0.925 | 1.071 and −2.014 | 0.870 and −0.699 |
| 2 | |||
| Ru(1)-Cl(1) | 2.4092(5) | Ru(1)-N(1) | 2.060(1) |
| Ru(1)-O(1) | 2.103(1) | Ru(1)-Cymene | 1.6598(1) |
| O(1)-C(15) | 1.273(2) | N(1)-Ru(1)-O(1) | 77.09(5) |
| Cl(1)-Ru(1)-N(1) | 85.41(4) | Cl(1)-Ru(1)-O(1) | 85.53(3) |
| 3 | |||
| Ru(1)-C(50) | 1.845(7) | Ru(1)-N(1) | 2.038(5) |
| Ru(1)-O(1) | 2.139(4) | Ru(1)-P(1) | 2.4058(15) |
| O(1)-C(15) | 1.273(2) | Ru(1)-P(2) | 2.3908(15) |
| Ru(1)-Cymene | 1.6598(1) | Ru(1)-Cl(1) | 2.4185(15) |
| O(1)-C(41) | 1.280(7) | C(50)-O(4) | 1.128(8) |
| C(50)-Ru(1)-O(1) | 172.9(2) | P(2)-Ru(1)-P(1) | 177.38(5) |
| N(1)-Ru(1)-Cl(1) | 166.70(14) | N(1)-Ru(1)-O(1) | 78.32(16) |
| 4 | |||
| Ru(1)-N(1) | 2.070(5) | Ru(1)-N(2) | 2.057(3) |
| Ru(1)-O(2) | 2.124(3) | Ru(1)-Cymene | 1.6674(7) |
| O(2)-C(5) | 1.278(5) | N(1)-C(23) | 1.155(6) |
| O(1)-C(23) | 1.218(6) | Ru(1)-N(1)-C(23) | 156.9(4) |
| N(1)-Ru(1)-N(2) | 84.53(16) | N(2)-Ru(1)-O(2) | 77.03(13) |
| N(1)-Ru(1)-O(2) | 83.61(16) | N(1)-C(23)-O(1) | 176.3(6) |
| 5 | |||
| Ru(1)-N(1) | 2.109(2) | Ru(1)-N(2) | 2.0640(19) |
| Ru(1)-O(1) | 2.1114(15) | Ru(1)-Cymene | 1.6610(2) |
| O(1)-C(15) | 1.276(3) | N(1)-N(2) | 1.204(3) |
| N(2)-N(3) | 1.157(3) | N(4)-Ru(1)-O(1) | 77.08(7) |
| N(1)-Ru(1)-N(4) | 86.02(8) | N(1)-Ru(1)-O(1) | 86.62(7) |
| Ru(1)-N(1)-N(2) | 120.43(17) | N(1)-N(2)-N(3) | 176.7(3) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-W.; Chen, Y.-F.; Li, Y.-J.; Chen, K.-H.; Lin, C.-H.; Huang, J.-H. Structural Determination of Ruthenium Complexes Containing Bi-Dentate Pyrrole-Ketone Ligands. Molecules 2018, 23, 159. https://doi.org/10.3390/molecules23010159
Tsai Y-W, Chen Y-F, Li Y-J, Chen K-H, Lin C-H, Huang J-H. Structural Determination of Ruthenium Complexes Containing Bi-Dentate Pyrrole-Ketone Ligands. Molecules. 2018; 23(1):159. https://doi.org/10.3390/molecules23010159
Chicago/Turabian StyleTsai, Ya-Wen, Yun-Fan Chen, Yong-Jie Li, Kuan-Hung Chen, Chia-Her Lin, and Jui-Hsien Huang. 2018. "Structural Determination of Ruthenium Complexes Containing Bi-Dentate Pyrrole-Ketone Ligands" Molecules 23, no. 1: 159. https://doi.org/10.3390/molecules23010159