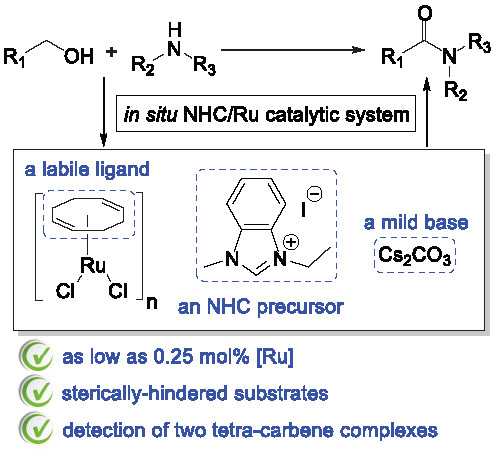
Ruthenium-Based Catalytic Systems Incorporating a Labile Cyclooctadiene Ligand with N-Heterocyclic Carbene Precursors for the Atom-Economic Alcohol Amidation Using Amines
Abstract
1. Introduction
2. Results and Discussion
3. Experimental
3.1. General Considerations
3.2. General Procedure for the Amide Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Humphrey, J.M.; Chamberlin, A.R. Chemical synthesis of natural product peptides: Coupling methods for the incorporation of noncoded amino acids into peptides. Chem. Rev. 1997, 97, 2243–2266. [Google Scholar] [CrossRef] [PubMed]
- Bode, J.W. Emerging methods in amide- and peptide-bond formation. Curr. Opin. Drug Discov. Dev. 2006, 9, 765–775. [Google Scholar] [CrossRef]
- Cupido, T.; Tulla-Puche, J.; Spengler, J.; Albericio, F. The synthesis of naturally occurring peptides and their analogs. Curr. Opin. Drug Discov. Dev. 2007, 10, 768–783. [Google Scholar]
- Valeur, E.; Bradley, M. Amide bond formation: Beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Punniyamurthy, T. Palladium-catalyzed one-pot conversion of aldehydes to amides. Adv. Synth. Catal. 2010, 352, 288–292. [Google Scholar] [CrossRef]
- Pattabiraman, V.R.; Bode, J.W. Rethinking amide bond synthesis. Nature 2011, 480, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Kim, Y.A. Recent development of peptide coupling reagents in organic synthesis. Tetrahedron 2004, 60, 2447–2467. [Google Scholar] [CrossRef]
- Kohn, M.; Breinbauer, R. The Staudinger ligation-A gift to chemical biology. Angew. Chem. Int. Ed. 2004, 43, 3106–3116. [Google Scholar] [CrossRef] [PubMed]
- Montalbetti, C.A.G.N.; Falque, V. Amide bond formation and peptide coupling. Tetrahedron 2005, 61, 10827–10852. [Google Scholar] [CrossRef]
- Kolakowski, R.V.; Shangguan, N.; Sauers, R.R.; Williams, L.J. Mechanism of thio acid/azide amidation. J. Am. Chem. Soc. 2006, 128, 5695–5702. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Murphy, J.A. Azide rearrangements in electron-deficient systems. Chem. Soc. Rev. 2006, 35, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, J.R.; Clark, T.P.; Watson, D.A.; Munday, R.H.; Buchwald, S.L. Palladium-catalyzed aminocarbonylation of aryl chlorides at atmospheric pressure: The dual role of sodium phenoxide. Angew. Chem. Int. Ed. 2007, 46, 8460–8463. [Google Scholar] [CrossRef] [PubMed]
- Owston, N.A.; Parker, A.J.; Williams, J.M.J. Iridium-catalyzed conversion of alcohols into amides via oximes. Org. Lett. 2007, 9, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.W.; Chan, P.W.H. Highly efficient ruthenium (II) porphyrin catalyzed amidation of aldehydes. Angew. Chem. Int. Ed. 2008, 47, 1138–1140. [Google Scholar] [CrossRef] [PubMed]
- Constable, D.J.C.; Dunn, P.J.; Hayler, J.D.; Humphrey, G.R.; Leazer, J.L., Jr.; Linderman, R.J.; Lorenz, K.; Manley, J.; Pearlman, B.A.; Wells, A.; et al. Key green chemistry research areas-a perspective from pharmaceutical manufacturers. Green Chem. 2007, 9, 411–420. [Google Scholar] [CrossRef]
- Allen, C.L.; Williams, J.M.J. Metal-catalysed approaches to amide bond formation. Chem. Soc. Rev. 2011, 40, 3405–3415. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hong, S.H. Oxidative amide synthesis directly from alcohols with amines. Org. Biomol. Chem. 2011, 9, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Gunanathan, C.; Milstein, D. Applications of acceptorless dehydrogenation and related transformations in chemical synthesis. Science 2013. [Google Scholar] [CrossRef] [PubMed]
- Gunanathan, C.; Milstein, D. Bond activation and catalysis by ruthenium pincer complexes. Chem. Rev. 2014, 114, 12024–12087. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, R.M.; Suppo, J.S.; Campagne, J.M. Nonclassical routes for amide bond formation. Chem. Rev. 2016, 116, 12029–12122. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.Q.; Fan, G.M.; Zhu, R.J.; Shi, L.; Xiao, S.Y.; Bi, C. Highly efficient synthesis of amides. Prog. Chem. 2016, 28, 497–506. [Google Scholar]
- Gusey, D.G. Rethinking the dehydrogenative amide synthesis. ACS Catal. 2017, 7, 6656–6662. [Google Scholar]
- Chen, C.; Verpoort, F.; Wu, Q.Y. Atom-economic dehydrogenative amide synthesis via ruthenium catalysis. RSC Adv. 2016, 6, 55599–55607. [Google Scholar] [CrossRef]
- Naota, T.; Murahashi, S.I. Ruthenium-catalyzed transformations of amino-alcohols to lactams. Synlett 1991, 10, 693–694. [Google Scholar] [CrossRef]
- Gunanathan, C.; Ben-David, Y.; Milstein, D. Direct synthesis of amides from alcohols and amines with liberation of H-2. Science 2007, 317, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Gnanaprakasam, B.; Balaraman, E.; Ben-David, Y.; Milstein, D. Synthesis of peptides and pyrazines from β-Amino alcohols through extrusion of H2 catalyzed by ruthenium pincer complexes: Ligand-controlled selectivity. Angew. Chem. Int. Ed. 2011, 50, 12240–12244. [Google Scholar] [CrossRef] [PubMed]
- Gnanaprakasam, B.; Balaraman, E.; Gunanathan, C.; Milstein, D. Synthesis of polyamides from diols and diamines with liberation of H2. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1755–1765. [Google Scholar] [CrossRef]
- Srimani, D.; Balaraman, E.; Hu, P.; Ben-David, Y.; Milstein, D. Formation of tertiary amides and dihydrogen by dehydrogenative coupling of primary alcohols with secondary amines catalyzed by ruthenium bipyridine-based pincer complexes. Adv. Synth. Catal. 2013, 355, 2525–2530. [Google Scholar] [CrossRef]
- Nordstrøm, L.U.; Vogt, H.; Madsen, R. Amide synthesis from alcohols and amines by the extrusion of dihydrogen. J. Am. Chem. Soc. 2008, 130, 17672–17673. [Google Scholar] [CrossRef] [PubMed]
- Dam, J.H.; Osztrovszky, G.; Nordstrøm, L.U.; Madsen, R. Amide synthesis from alcohols and amines catalyzed by ruthenium N-heterocyclic carbene complexes. Chem. Eur. J. 2010, 16, 6820–6827. [Google Scholar] [CrossRef] [PubMed]
- Makarov, I.S.; Fristrup, P.; Madsen, R. Mechanistic investigation of the ruthenium-N-heterocyclic-carbene-catalyzed amidation of amines with alcohols. Chem. Eur. J. 2012, 18, 15683–15692. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.J.A.; Maxwell, A.C.; Williams, J.M.J. Ruthenium-catalyzed oxidation of alcohols into amides. Org. Lett. 2009, 11, 2667–2670. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.J.A.; Wakeham, R.J.; Maxwell, A.C.; Williams, J.M.J. Ruthenium-catalysed oxidation of alcohols to amides using a hydrogen acceptor. Tetrahedron 2014, 70, 3683–3690. [Google Scholar] [CrossRef]
- Ghosh, S.C.; Muthaiah, S.; Zhang, Y.; Xu, X.Y.; Hong, S.H. Direct amide synthesis from alcohols and amines by phosphine-free ruthenium catalyst systems. Adv. Synth. Catal. 2009, 351, 2643–2649. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, C.; Ghosh, S.C.; Li, Y.X.; Hong, S.H. Well-defined N-heterocyclic carbene based ruthenium catalysts for direct amide synthesis from alcohols and amines. Organometallics 2010, 29, 1374–1378. [Google Scholar] [CrossRef]
- Muthaiah, S.; Ghosh, S.C.; Jee, J.E.; Chen, C.; Zhang, J.; Hong, S.H. Direct amide synthesis from either alcohols or aldehydes with amines: Activity of Ru (II) hydride and Ru (0) complexes. J. Org. Chem. 2010, 75, 3002–3006. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.C.; Hong, S.H. Simple RuCl3-catalyzed amide synthesis from alcohols and amines. Eur. J. Org. Chem. 2010, 4266–4270. [Google Scholar] [CrossRef]
- Zhang, J.; Senthilkumar, M.; Ghosh, S.C.; Hong, S.H. Synthesis of cyclic imides from simple diols. Angew. Chem. Int. Ed. 2010, 49, 6391–6395. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Y.; Hong, S.H. N-heterocyclic carbene based ruthenium-catalyzed direct amide synthesis from alcohols and secondary amines: Involvement of esters. J. Org. Chem. 2011, 76, 10005–10010. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hong, S.H. Selective catalytic sp3 C-O bond cleavage with C-N bond formation in 3-alkoxy-1-propanols. Org. Lett. 2012, 14, 2992–2995. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kang, B.; Hong, S.H. N-Heterocyclic carbene-based well-defined ruthenium hydride complexes for direct amide synthesis from alcohols and amines under base-free conditions. Tetrahedron 2015, 71, 4565–4569. [Google Scholar] [CrossRef]
- Kang, B.; Hong, S.H. Hydrogen acceptor- and base-free N-formylation of nitriles and amines using methanol as C-1 Source. Adv. Synth. Catal. 2015, 357, 834–840. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, S.H. Ruthenium-catalyzed urea synthesis using methanol as the C1 source. Org. Lett. 2016, 18, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Nova, A.; Balcells, D.; Schley, N.D.; Dobereiner, G.E.; Crabtree, R.H.; Eisenstein, O. An experimental-theoretical study of the factors that affect the switch between ruthenium-catalyzed dehydrogenative amide formation versus amine alkylation. Organometallics 2010, 29, 6548–6558. [Google Scholar] [CrossRef]
- Schley, N.D.; Dobereiner, G.E.; Crabtree, R.H. Oxidative synthesis of amides and pyrroles via dehydrogenative alcohol oxidation by ruthenium diphosphine diamine complexes. Organometallics 2011, 30, 4174–4179. [Google Scholar] [CrossRef]
- Prades, A.; Peris, E.; Albrecht, M. Oxidations and oxidative couplings catalyzed by triazolylidene ruthenium complexes. Organometallics 2011, 30, 1162–1167. [Google Scholar] [CrossRef]
- Zeng, H.; Guan, Z. Direct synthesis of polyamides via catalytic dehydrogenation of diols and diamines. J. Am. Chem. Soc. 2011, 133, 1159–1161. [Google Scholar] [CrossRef] [PubMed]
- Oldenhuis, N.J.; Dong, V.M.; Guan, Z. Catalytic acceptorless dehydrogenations: Ru-Macho catalyzed construction of amides and imines. Tetrahedron 2014, 70, 4213–4218. [Google Scholar] [CrossRef] [PubMed]
- Ortega, N.; Richter, C.; Glorius, F. N-formylation of amines by methanol activation. Org. Lett. 2013, 15, 1776–1779. [Google Scholar] [CrossRef] [PubMed]
- Malineni, J.; Merkens, C.; Keul, H.; Möller, M. An efficient N-heterocyclic carbene based ruthenium-catalyst: Application towards the synthesis of esters and amides. Catal. Commun. 2013, 40, 80–83. [Google Scholar] [CrossRef]
- Malineni, J.; Keul, H.; Möller, M. An efficient N-heterocyclic carbene-ruthenium complex: Application towards the synthesis of polyesters and polyamides. Macromol. Rapid Commun. 2015, 36, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Sengupta, G.; Sarbajna, A.; Dutta, I.; Bera, J.K. Amide synthesis from alcohols and amines catalyzed by a Ru-II-N-heterocyclic carbene (NHC)-carbonyl complex. J. Organomet. Chem. 2014, 771, 124–130. [Google Scholar] [CrossRef]
- Xie, X.K.; Huynh, H.V. Tunable dehydrogenative amidation versus amination using a single ruthenium-NHC catalyst. ACS Catal. 2015, 5, 4143–4151. [Google Scholar] [CrossRef]
- Nirmala, M.; Viswanathamurthi, P. Design and synthesis of ruthenium (II) OCO pincer type NHC complexes and their catalytic role towards the synthesis of amides. J. Chem. Sci. 2016, 128, 9–21. [Google Scholar] [CrossRef]
- Selvamurugan, S.; Ramachandran, R.; Prakash, G.; Viswanathamurthi, P.; Malecki, J.G.; Endo, A. Ruthenium (II) carbonyl complexes containing bidentate 2-oxo-1,2-dihydroquinoline-3-carbaldehyde hydrazone ligands as efficient catalysts for catalytic amidation reaction. J. Organomet. Chem. 2016, 803, 119–127. [Google Scholar] [CrossRef]
- Selvamurugan, S.; Ramachandran, R.; Prakash, G.; Nirmala, M.; Viswanathamurthi, P.; Fujiwara, S.; Endo, A. Ruthenium (II) complexes encompassing 2-oxo-1,2-dihydroquinoline-3-carbaldehyde thiosemicarbazone hybrid ligand: A new versatile potential catalyst for dehydrogenative amide synthesis. Inorg. Chim. Acta 2017, 454, 46–53. [Google Scholar] [CrossRef]
- Higuchi, T.; Tagawa, R.; Iimuro, A.; Akiyama, S.; Nagae, H.; Mashima, K. Tunable ligand effects on ruthenium catalyst activity for selectively preparing imines or amides by dehydrogenative coupling reactions of alcohols and amines. Chem. Eur. J. 2017, 23, 12795–12804. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xiong, M.Q.; Cheng, C.X.; Wang, H.J.; Lu, Q.; Liu, H.F.; Yao, F.B.; Chen, C.; Verpoort, F. In situ generated ruthenium catalyst systems bearing diverse N-heterocyclic carbene precursors for atom-economic amide synthesis from alcohols and amines. Chem. Asian J. 2018, 13, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xiong, M.Q.; Zhang, N.; Wang, H.J.; Miao, Y.; Su, W.; Yuan, Y.; Chen, C.; Verpoort, F. Efficient N-heterocyclic carbene/ruthenium catalytic systems for the alcohol amidation with amines: Involvement of poly-carbene complexes? ChemCatChem 2018. [Google Scholar] [CrossRef]
- Maji, M.; Chakrabarti, K.; Paul, B.; Roy, B.C.; Kundu, S. Ruthenium(II)-NNN-pincer-complex-catalyzed reactions between various alcohols and amines for sustainable C-N and C-C bond formation. Adv. Synth. Catal. 2018, 360, 722–729. [Google Scholar] [CrossRef]
- Huynh, H.V.; Han, Y.; Jothibasu, R.; Yang, J.A. 13C-NMR spectroscopic determination of ligand donor strengths using N-heterocyclic carbene complexes of palladium (II). Organometallics 2009, 28, 5395–5404. [Google Scholar] [CrossRef]
- Chen, C.; Kim, M.H.; Hong, S.H. N-heterocyclic carbene-based ruthenium-catalyzed direct amidation of aldehydes with amines. Org. Chem. Front. 2015, 2, 241–247. [Google Scholar] [CrossRef]
- Kaufhold, S.; Petermann, L.; Staehle, R.; Rau, S. Transition metal complexes with N-heterocyclic carbene ligands: From organometallic hydrogenation reactions toward water splitting. Coord. Chem. Rev. 2015, 304, 73–87. [Google Scholar] [CrossRef]
Sample Availability: Samples of compounds 3a–3t are available from the authors. |



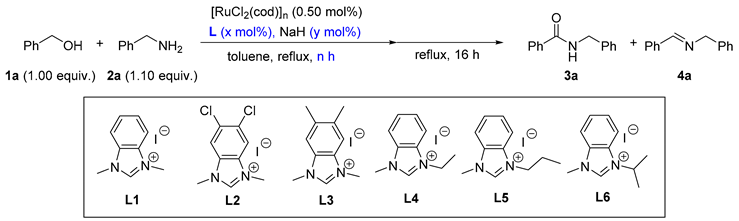
| Entry | L | x | y | n | Yields (%) b | ||
|---|---|---|---|---|---|---|---|
| 3a | 4a | Unreacted 1a | |||||
| 1 | L1 | 2.00 | 3.50 | 0.5 | 62 | 15 | 18 |
| 2 | L2 | 2.00 | 3.50 | 0.5 | 28 | 30 | 39 |
| 3 | L3 | 2.00 | 3.50 | 0.5 | 63 | 15 | 16 |
| 4 | L4 | 2.00 | 3.50 | 0.5 | 78 | 10 | 8 |
| 5 | L5 | 2.00 | 3.50 | 0.5 | 72 | 12 | 8 |
| 6 | L6 | 2.00 | 3.50 | 0.5 | 28 | 30 | 39 |
| 7 | L4 | 2.00 | 3.50 | 0.0 | 57 | 10 | 31 |
| 8 | L4 | 2.00 | 3.50 | 1.0 | 79 | 6 | 8 |
| 9 | L4 | 2.00 | 3.50 | 1.5 | 81 | 5 | 7 |
| 10 | L4 | 2.00 | 3.50 | 2.0 | 83 | 4 | 5 |
| 11 | L4 | 2.00 | 3.50 | 2.5 | 82 | 4 | 6 |
| 12 | L4 | 0.00 | 1.50 | 2.0 | 0 | 19 | 76 |
| 13 | L4 | 0.50 | 2.00 | 2.0 | 37 | 10 | 51 |
| 14 | L4 | 1.00 | 2.50 | 2.0 | 60 | 11 | 28 |
| 15 | L4 | 1.50 | 3.00 | 2.0 | 75 | 7 | 16 |
| 16 | L4 | 2.50 | 4.00 | 2.0 | 86 | 4 | 9 |
| 17 | L4 | 3.00 | 4.50 | 2.0 | 81 | 6 | 3 |
| 18 c | L4 | 2.50 | 4.00 | 2.0 | 93 | 5 | 0 |

| Entry | Base | x | y | Yields (%) b | ||
|---|---|---|---|---|---|---|
| 3a | 4a | Unreacted 1a | ||||
| 1 | NaH | 2.00 | 1.50 | 65 | 7 | 24 |
| 2 | KHMDS | 2.00 | 1.50 | 27 | 11 | 57 |
| 3 | KOtBu | 2.00 | 1.50 | 45 | 15 | 32 |
| 4 | Cs2CO3 | 2.00 | 1.50 | 86 | 7 | 5 |
| 5 | Cs2CO3 | 2.00 | 0.50 | 57 | 18 | 22 |
| 6 | Cs2CO3 | 2.00 | 1.00 | 71 | 13 | 12 |
| 7 | Cs2CO3 | 2.00 | 2.00 | 69 | 16 | 13 |
| 8 | Cs2CO3 | 2.00 | 2.50 | 45 | 38 | 15 |
| 9 | Cs2CO3 | 1.50 | 1.50 | 66 | 15 | 12 |
| 10 | Cs2CO3 | 1.75 | 1.50 | 90 | 7 | 2 |
| 11 | Cs2CO3 | 2.25 | 1.50 | 81 | 10 | 8 |
| 12 | Cs2CO3 | 2.50 | 1.50 | 72 | 12 | 15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Miao, Y.; De Winter, K.; Wang, H.-J.; Demeyere, P.; Yuan, Y.; Verpoort, F. Ruthenium-Based Catalytic Systems Incorporating a Labile Cyclooctadiene Ligand with N-Heterocyclic Carbene Precursors for the Atom-Economic Alcohol Amidation Using Amines. Molecules 2018, 23, 2413. https://doi.org/10.3390/molecules23102413
Chen C, Miao Y, De Winter K, Wang H-J, Demeyere P, Yuan Y, Verpoort F. Ruthenium-Based Catalytic Systems Incorporating a Labile Cyclooctadiene Ligand with N-Heterocyclic Carbene Precursors for the Atom-Economic Alcohol Amidation Using Amines. Molecules. 2018; 23(10):2413. https://doi.org/10.3390/molecules23102413
Chicago/Turabian StyleChen, Cheng, Yang Miao, Kimmy De Winter, Hua-Jing Wang, Patrick Demeyere, Ye Yuan, and Francis Verpoort. 2018. "Ruthenium-Based Catalytic Systems Incorporating a Labile Cyclooctadiene Ligand with N-Heterocyclic Carbene Precursors for the Atom-Economic Alcohol Amidation Using Amines" Molecules 23, no. 10: 2413. https://doi.org/10.3390/molecules23102413
APA StyleChen, C., Miao, Y., De Winter, K., Wang, H.-J., Demeyere, P., Yuan, Y., & Verpoort, F. (2018). Ruthenium-Based Catalytic Systems Incorporating a Labile Cyclooctadiene Ligand with N-Heterocyclic Carbene Precursors for the Atom-Economic Alcohol Amidation Using Amines. Molecules, 23(10), 2413. https://doi.org/10.3390/molecules23102413