Ab Initio Molecular Dynamics Simulation Study on the Stereo Reactions between Atomic Oxygen Anion and Methane
Abstract
:1. Introduction
2. Computational Method
3. Results and Discussion
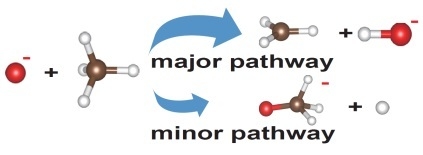
3.1. Intermolecular Interactions and Transition States
3.2. Stereodynamics of the Reactions
3.3. Reactions with Thermally Vibrating CH4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cui, X.; Li, H.; Wang, Y.; Hu, Y.; Hua, L.; Li, H.; Han, X.; Liu, Q.; Yang, F.; He, L.; et al. Room-temperature methane conversion by graphene-confined single iron atoms. Chemistry 2018, 4, 1902–1910. [Google Scholar] [CrossRef]
- Schwarz, H.; Shaik, S.; Li, J. Electronic effects on room-temperature, gas-phase C–H Bond activations by cluster oxides and metal carbides: The methane challenge. J. Am. Chem. Soc. 2017, 139, 17201–17212. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Kim, T.Y.; Kwon, G.; Yi, J.; Lee, H. Selective activation of methane on single-atom catalyst of rhodium dispersed on zirconia for direct conversion. J. Am. Chem. Soc. 2017, 139, 17694–17699. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, R.H.; Lei, A. Introduction: CH activation. Chem. Rev. 2017, 117, 8481–8482. [Google Scholar] [CrossRef] [PubMed]
- Schwach, P.; Pan, X.; Bao, X. Direct conversion of methane to value-added chemicals over heterogeneous catalysts: Challenges and prospects. Chem. Rev. 2017, 117, 8497–8520. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.N.; Ding, X.L.; Li, Z.Y.; Zhao, Y.X.; He, S.G. Hydrogen atom abstraction from CH4 by nanosized vanadium oxide cluster cations. J. Phys. Chem. 2014, 118, 24062–24071. [Google Scholar] [CrossRef]
- Ding, X.L.; Wu, X.N.; Zhao, Y.X.; He, S.G. C–H bond activation by oxygen-centered radicals over atomic clusters. Acc. Chem. Res. 2011, 45, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Dietl, N.; Schlangen, M.; Schwarz, H. Thermal hydrogen-atom transfer from methane: The role of radicals and spin states in oxo-cluster chemistry. Angew. Chem. Int. Ed. 2012, 51, 5544–5555. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Grabowski, J.J. Reactions of the atomic oxygen radical anion and the synthesis of organic reactive intermediates. Chem. Rev. 1992, 92, 1611–1647. [Google Scholar] [CrossRef]
- Fehsenfeld, F.; Schmeltekopf, A.; Schiff, H.; Ferguson, E. Laboratory measurements of negative ion reactions of atmospheric interest. Plan. Space Sci. 1967, 15, 373–379. [Google Scholar] [CrossRef]
- Fehsenfeld, F.; Ferguson, E.; Schmeltekopf, A. Thermal-energy associative-detachment reactions of negative ions. J. Chem. Phys. 1966, 45, 1844–1845. [Google Scholar] [CrossRef]
- Fehsenfeld, F.C.; Howard, C.J.; Schmeltekopf, A.L. Gas phase ion chemistry of HNO3. J. Chem. Phys. 1975, 63, 2835–2841. [Google Scholar] [CrossRef]
- Guo, Y.; Grabowski, J.J. Gas phase ion chemistry of the vinylidene radical anion and the acidity of the vinyl radical. Int. J. Mass Spectrom. Ion Process. 1990, 97, 253–264. [Google Scholar] [CrossRef]
- Chou, P.K.; Kass, S.R. C4H4 negative ions: Formation of the bicyclo[1.1.0]but-1(3)-ene radical anion and an experimental determination of the heat of formation of bicyclo[1.1.0]but-1(3)-ene. J. Am. Chem. Soc. 1991, 113, 697–698. [Google Scholar] [CrossRef]
- Guo, Y.; Grabowski, J.J. Reactions of the benzyne radical anion in the gas phase, the acidity of the phenyl radical, and the heat of formation of o-benzyne. J. Am. Chem. Soc. 1991, 113, 5923–5931. [Google Scholar] [CrossRef]
- Carrascosa, E.; Meyer, J.; Wester, R. Imaging the dynamics of ion–molecule reactions. Chem. Soc. Rev. 2017, 46, 7498–7516. [Google Scholar] [CrossRef] [Green Version]
- Meyer, J.; Wester, R. Ion–Molecule Reaction Dynamics. Annu. Rev. Phys. Chem. 2017, 68, 333–353. [Google Scholar] [CrossRef] [PubMed]
- Comer, J.; Schulz, G.J. Measurements of electron-detachment cross sections from O− and S−. Phys. Rev. A 1974, 10, 2100–2106. [Google Scholar] [CrossRef]
- Lindinger, W.; Albritton, D.L.; Fehsenfeld, F.C.; Ferguson, E.E. Reactions of O− with N2, N2O, SO2, NH3, CH4, and C2H4 and C2H2− with O2 from 300 K to relative kinetic energies of ~2 eV. J. Chem. Phys. 1975, 63, 3238–3242. [Google Scholar] [CrossRef]
- Bohme, D.K.; Fehsenfeld, F.C. Thermal reactions of O− ions with saturated hydrocarbon molecules. Can. J. Chem. 1969, 47, 2717–2719. [Google Scholar] [CrossRef]
- Viggiano, A.A.; Morris, R.A.; Miller, T.M.; Friedman, J.F.; Menedez-Barreto, M.; Paulson, J.F.; Michels, H.H.; Hobbs, R.H. Reaction on the O− + CH4 potential energy surface: Dependence on translational and internal energy and on isotopic composition, 93–1313 K. J. Chem. Phys. 1997, 106, 8455–8463. [Google Scholar] [CrossRef]
- Ferguson, E.E.; Fehsenfeld, F.C.; Albritton, D.L. Ion chemistry of the earth’s atmosphere. In Gas Phase Ion Chemistry; Bowers, M.T., Ed.; Academic Press: New York, NY, USA, 1979; Chapter 2; pp. 45–82. [Google Scholar]
- Carpenter, M.A.; Farrar, J.M. Dynamics of hydrogen atom abstraction in the O− + CH4 reaction: Product energy disposal and angular distributions. J. Chem. Phys. 1997, 106, 5951–5960. [Google Scholar] [CrossRef]
- Diken, E.G.; Weddle, G.H.; Headrick, J.M.; Weber, J.M.; Johnson, M.A. Argon cluster-mediated trapping and vibrational spectroscopic characterization of an OH−·HCH2• intermediate in the O•− + CH4 reaction. J. Phys. Chem. A 2004, 108, 10116–10121. [Google Scholar] [CrossRef]
- Arai, H.; Kato, S.; Koda, S. Ab initio potential surfaces for the atomic oxygen(1D) + methane reaction. J. Phys. Chem. 1994, 98, 12–16. [Google Scholar] [CrossRef]
- Head-Gordon, M.; Pople, J.A.; Frisch, M.J. MP2 energy evaluation by direct methods. Chem. Phys. Lett. 1988, 153, 503–506. [Google Scholar] [CrossRef]
- Head-Gordon, M.; Head-Gordon, T. Analytic MP2 frequencies without fifth-order storage. Theory and application to bifurcated hydrogen bonds in the water hexamer. Chem. Phys. Lett. 1994, 220, 122–128. [Google Scholar] [CrossRef]
- Sæbø, S.; Almlöf, J. Avoiding the integral storage bottleneck in LCAO calculations of electron correlation. Chem. Phys. Lett. 1989, 154, 83–89. [Google Scholar] [CrossRef]
- Frisch, M.J.; Head-Gordon, M.; Pople, J.A. A direct MP2 gradient method. Chem. Phys. Lett. 1990, 166, 275–280. [Google Scholar] [CrossRef]
- Frisch, M.J.; Head-Gordon, M.; Pople, J.A. Semi-direct algorithms for the MP2 energy and gradient. Chem. Phys. Lett. 1990, 166, 281–289. [Google Scholar] [CrossRef]
- Hratchian, H.P.; Schlegel, H.B. Accurate reaction paths using a Hessian based predictor–corrector integrator. J. Chem. Phys. 2004, 120, 9918–9924. [Google Scholar] [CrossRef] [PubMed]
- Hratchian, H.P.; Schlegel, H.B. Using hessian updating to increase the efficiency of a hessian based predictor-corrector reaction path following method. J. Chem. Theory Comput. 2005, 1, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Millam, J.M.; Bakken, V.R.; Chen, W.; Hase, W.L.; Schlegel, H.B. Ab initio classical trajectories on the Born–Oppenheimer surface: Hessian-based integrators using fifth-order polynomial and rational function fits. J. Chem. Phys. 1999, 111, 3800–3805. [Google Scholar] [CrossRef]
- Bakken, V.; Millam, J.M.; Bernhard Schlegel, H. Ab initio classical trajectories on the Born–Oppenheimer surface: Updating methods for Hessian-based integrators. J. Chem. Phys. 1999, 111, 8773–8777. [Google Scholar] [CrossRef]
- Gaussian 09, Revision D.01; Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. (Eds.) Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Boys, S.F.; Bernardi, F.D. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Simon, S.; Duran, M.; Dannenberg, J.J. How does basis set superposition error change the potential surfaces for hydrogen-bonded dimers? J. Chem. Phys. 1996, 105, 11024–11031. [Google Scholar] [CrossRef]
- Xie, J.; Otto, R.; Mikosch, J.; Zhang, J.; Wester, R.; Hase, W.L. Identification of atomic-level mechanisms for gas-phase X– + CH3Y SN2 reactions by combined experiments and simulations. Acc. Chem. Res. 2014, 47, 2960–2969. [Google Scholar] [CrossRef] [PubMed]
- Mikosch, J.; Trippel, S.; Eichhorn, C.; Otto, R.; Lourderaj, U.; Zhang, J.; Hase, W.; Weidemüller, M.; Wester, R. Imaging nucleophilic substitution dynamics. Science 2008, 319, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Hua-Gen, Y. An ab initio molecular dynamics study of the roaming mechanism of the H2 + HOC+ reaction. Phys. Scr. 2011, 84, 028104. [Google Scholar]
- Li, A.; Li, J.; Guo, H. Quantum manifestation of roaming in H + MgH → Mg + H2: The birth of roaming resonances. J. Phys. Chem. A 2013, 117, 5052–5060. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |










© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Feng, W.; Wang, W.; Li, P. Ab Initio Molecular Dynamics Simulation Study on the Stereo Reactions between Atomic Oxygen Anion and Methane. Molecules 2018, 23, 2495. https://doi.org/10.3390/molecules23102495
Wang W, Feng W, Wang W, Li P. Ab Initio Molecular Dynamics Simulation Study on the Stereo Reactions between Atomic Oxygen Anion and Methane. Molecules. 2018; 23(10):2495. https://doi.org/10.3390/molecules23102495
Chicago/Turabian StyleWang, Weihua, Wenling Feng, Wenliang Wang, and Ping Li. 2018. "Ab Initio Molecular Dynamics Simulation Study on the Stereo Reactions between Atomic Oxygen Anion and Methane" Molecules 23, no. 10: 2495. https://doi.org/10.3390/molecules23102495