Synthesis, Characterization, and Antifungal Activity of Pyridine-Based Triple Quaternized Chitosan Derivatives
Abstract
:1. Introduction
2. Results
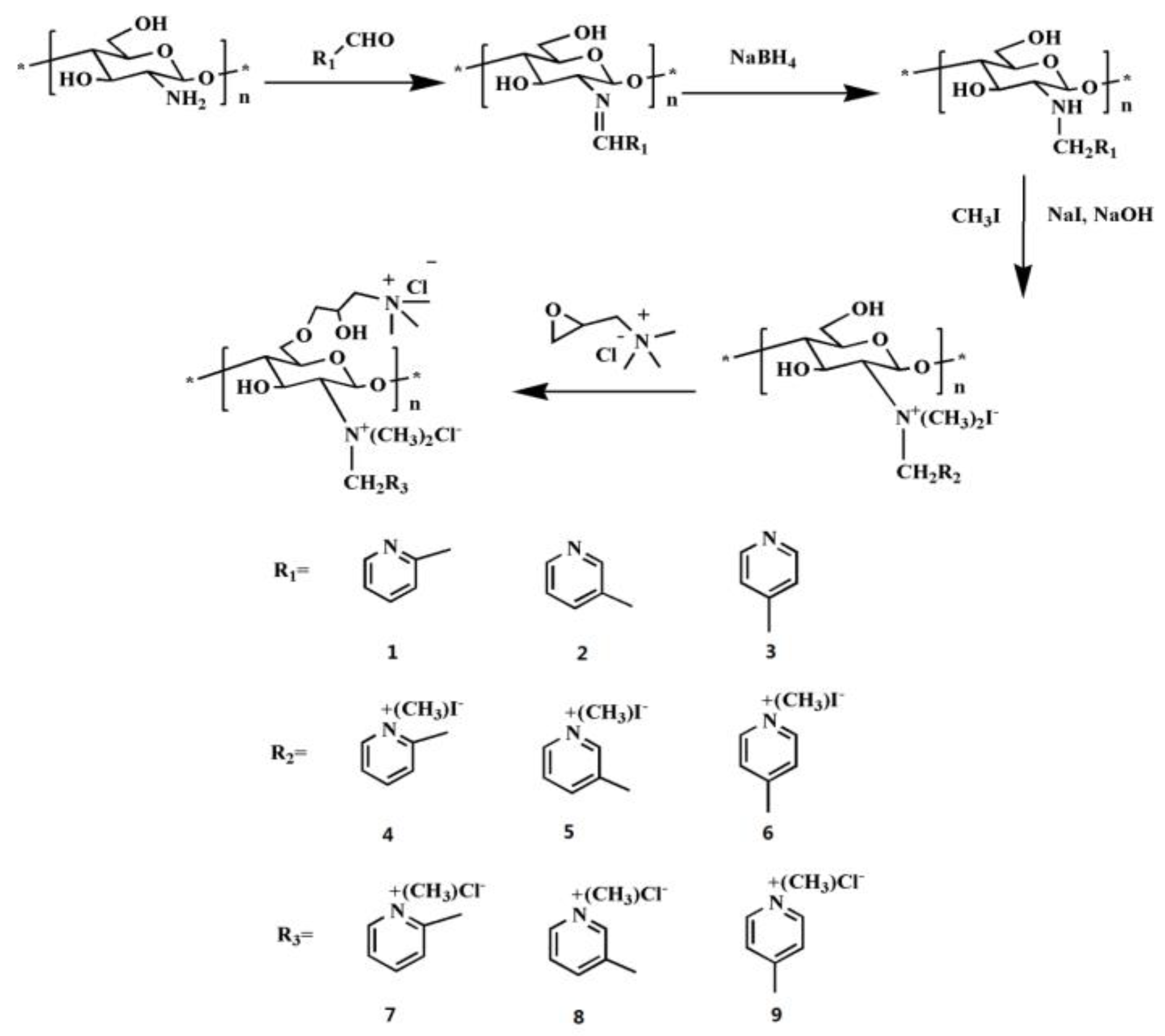
2.1. Structure of the Chitosan Derivative
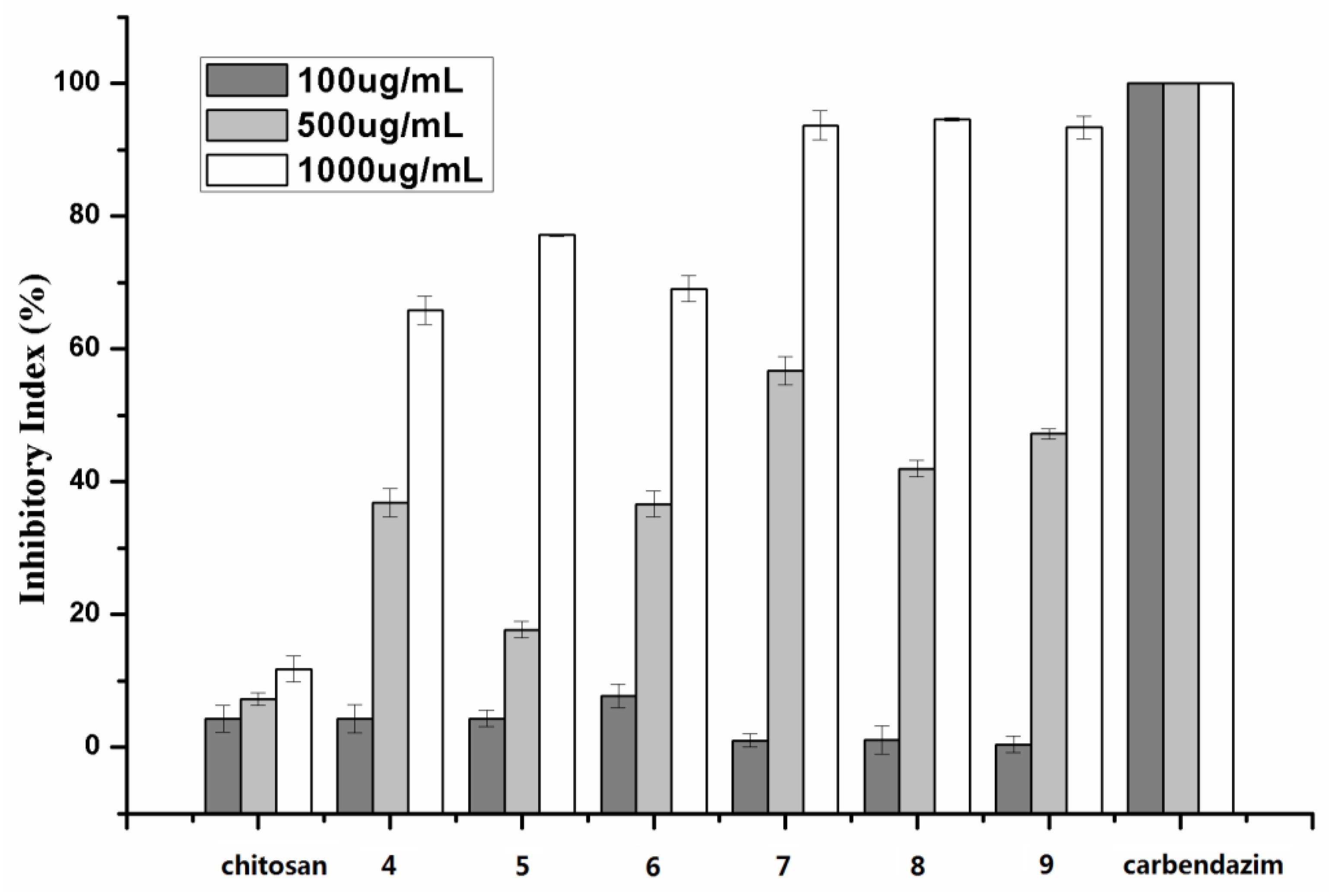
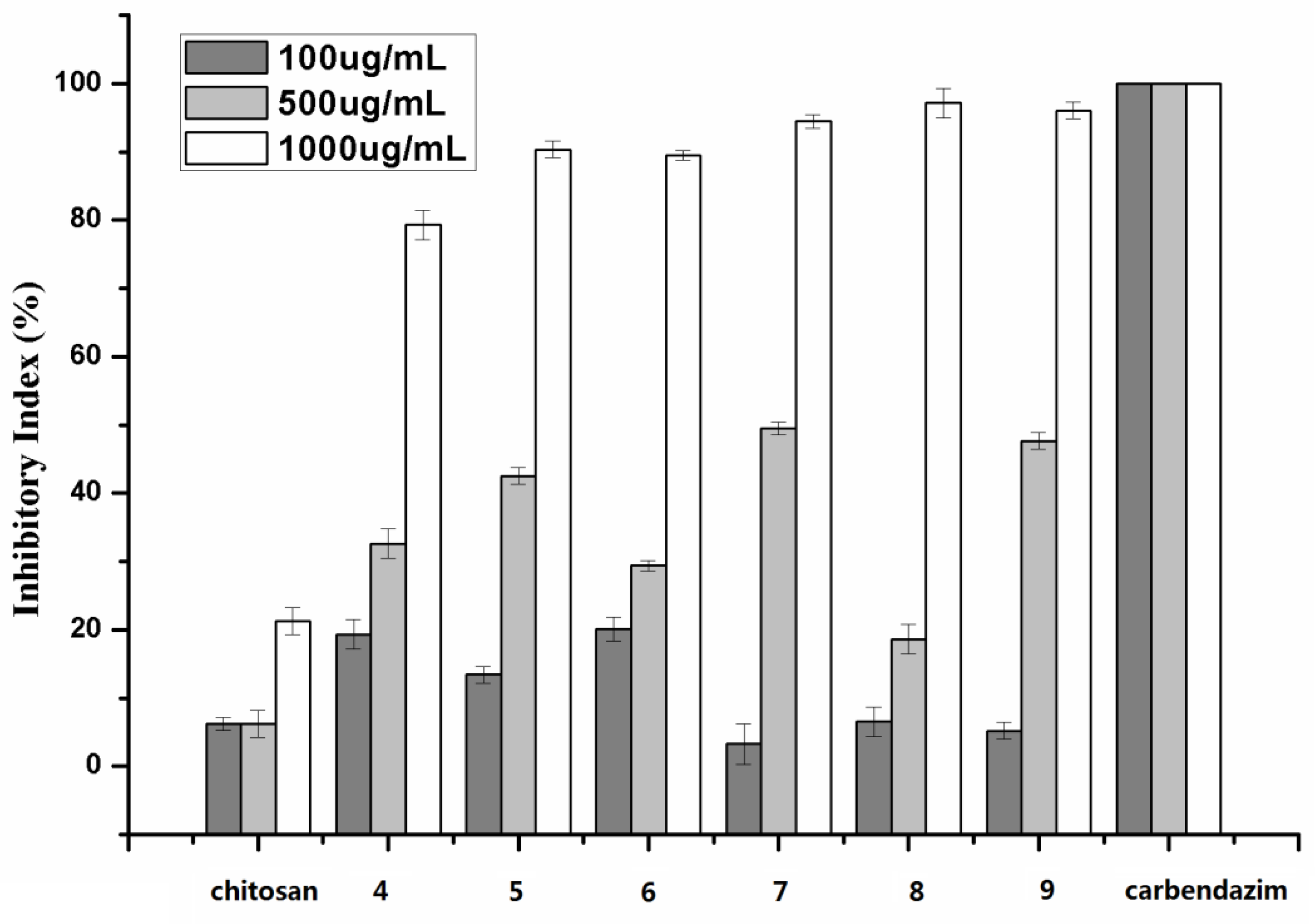
2.2. Antifungal Activity
2.3. Cytotoxicity Analysis
3. Materials and Methods
3.1. Materials
3.2. Analytical Methods
3.3. Synthesis of Pyridine-Based Double Quaternized Chitosan Derivatives 4, 5 and 6
3.4. Synthesis of Triple Quaternized Chitosan Derivatives 7, 8 and 9
3.5. Antifungal Assay
3.6. Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Q.; Qiu, L.; Tan, W.; Gu, G.; Guo, Z. Novel 1,2,3-triazolium-functionalized inulin derivatives: Synthesis, free radical-scavenging activity, and antifungal activity. RSC Adv. 2017, 7, 42225–42232. [Google Scholar] [CrossRef]
- Li, Q.; Ren, J.; Dong, F.; Feng, Y.; Gu, G.; Guo, Z. Synthesis and antifungal activity of thiadiazole-functionalized chitosan derivatives. Carbohydr. Res. 2013, 373, 103–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everts, K.L.; Egel, D.S.; Langston, D.; Zhou, X.-G. Chemical management of Fusarium wilt of watermelon. Crop Prot. 2014, 66, 114–119. [Google Scholar] [CrossRef]
- Lievens, B.; Claes, L.; Vakalounakis, D.J.; Vanachter, A.C.R.C.; Thomma, B.P.H.J. A robust identification and detection assay to discriminate the cucumber pathogens Fusarium oxysporum f. sp. cucumerinum and f. sp. radicis-cucumerinum. Environ. Microbiol. 2007, 9, 2145–2161. [Google Scholar]
- Lan, C.; Ruan, H.; Yang, X.; Yao, J.; Jiang, J. Development of a loop-mediated isothermal amplification assay for sensitive and specific detection of Fusarium oxysporum f. sp. cucumerinum Owen. Phytoparasitica 2018, 46, 283–293. [Google Scholar] [CrossRef]
- Elena, K. First report of Phomopsis asparagi causing stem blight of asparagus in Greece. Plant Pathol. 2006, 55, 300. [Google Scholar] [CrossRef]
- Nguyen, D.-M.-C.; Seo, D.-J.; Lee, H.-B.; Kim, I.-S.; Kim, K.-Y.; Park, R.-D.; Jung, W.-J. Antifungal activity of gallic acid purified from Terminalia nigrovenulosa bark against Fusarium solani. Microb. Pathog. 2013, 56, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Xu, P.-Y.; Zhang, Y.-J.; Wang, P.-P.; Yu, H.; Jiang, J.-H. Synthesis of biocontrol macromolecules by derivative of chitosan with surfactin and antifungal evaluation. Int. J. Biol. Macromol. 2014, 66, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, K.; Li, P. Synthesis, characterization, and antifungal evaluation of diethoxyphosphoryl polyaminoethyl chitosan derivatives. Carbohydr. Polym. 2018, 190, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xing, R.; Liu, S.; Zhong, Z.; Ji, X.; Wang, L.; Li, P. The influence of molecular weight of quaternized chitosan on antifungal activity. Carbohydr. Polym. 2008, 71, 694–697. [Google Scholar] [CrossRef]
- Yang, J.; Xie, Q.; Zhu, J.; Zou, C.; Chen, L.; Du, Y.; Li, D. Preparation and in vitro antioxidant activities of 6-amino-6-deoxychitosan and its sulfonated derivatives. Biopolymers 2015, 103, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Natio, P.-K.; Ogawa, Y.; Sawada, D.; Nishiyama, Y.; Iwata, T.; Wada, M. X-ray crystal structure of anhydrous chitosan at atomic resolution. Biopolymers 2016, 105, 361–368. [Google Scholar]
- Ilium, L. Chitosan and Its Use as a Pharmaceutical Excipient. Pharm. Res. 1998, 15, 1326–1331. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- El-Sawy, N.M.; Abd El-Rehim, H.A.; Elbarbary, A.M.; Hegazy, E.-S.A. Radiation-induced degradation of chitosan for possible use as a growth promoter in agricultural purposes. Carbohydr. Polym. 2010, 79, 555–562. [Google Scholar] [CrossRef]
- Şenel, S.; McClure, S.J. Potential applications of chitosan in veterinary medicine. Adv. Drug Deliv. Rev. 2004, 56, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan Chemistry and Pharmaceutical Perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-H.; Hudson, S.M. Application of a fiber-reactive chitosan derivative to cotton fabric as an antimicrobial textile finish. Carbohydr. Res. 2004, 56, 227–234. [Google Scholar] [CrossRef]
- Li, Q.; Tan, W.; Zhang, C.; Gu, G.; Guo, Z. Novel triazolyl-functionalized chitosan derivatives with different chain lengths of aliphatic alcohol substituent: Design, synthesis, and antifungal activity. Carbohydr. Res. 2015, 418, 44–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, K.; Li, P. Synthesis, characterization and antifungal efficacy of chitosan derivatives with triple quaternary ammonium groups. Int. J. Biol. Macromol. 2018, 114, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, H.; Chen, X.; Ji, X.; Li, P. Hydroxyl radicals scavenging activity of N-substituted chitosan and quaternized chitosan. Bioorg. Med. Chem. Lett. 2006, 16, 6348–6350. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Shen, D.; Xu, W. Synthesis and antibacterial activities of quaternary ammonium salt of chitosan. Carbohydr. Res. 2001, 333, 1–6. [Google Scholar] [CrossRef]
- Amaral, I.F.; Granja, P.L.; Barbosa, M.A. Chemical modification of chitosan by phosphorylation: An XPS, FT-IR and SEM study. J. Biomat. Sci. Polym. 2005, 16, 1575–1593. [Google Scholar] [CrossRef]
- Oyervides-Muñoz, E.; Pollet, E.; Ulrich, G.; De Jesús Sosa-Santillán, G.; Avérous, L. Original method for synthesis of chitosan-based antimicrobial agent by quaternary ammonium grafting. Carbohydr. Polym. 2017, 157, 1922–1932. [Google Scholar] [CrossRef] [PubMed]
- Doncel-Pérez, E.; Aranaz, I.; Bastida, A.; Revuelta, J.; Camacho, C.; Acosta, N.; Garrido, L.; Civera, C.; García-Junceda, E.; Heras, A.; et al. Synthesis, physicochemical characterization and biological evaluation of chitosan sulfate as heparan sulfate mimics. Carbohydr. Polym. 2018, 191, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Malinak, D.; Dolezal, R.; Marek, J.; Salajkova, S.; Soukup, O.; Vejsova, M.; Korabecny, J.; Honegr, J.; Penhaker, M.; Musilek, K.; et al. 6-Hydroxyquinolinium salts differing in the length of alkyl side-chain: Synthesis and antimicrobial activity. Bioorg. Med. Chem. Lett. 2014, 24, 5238–5524. [Google Scholar] [CrossRef] [PubMed]
- Kotzé, A.F.; Lueßen, H.L.; de Boer, A.G.; Verhoef, J.C.; Junginger, H.E. Chitosan for enhanced intestinal permeability: Prospects for derivatives soluble in neutral and basic environments. Eur. J. Pharm. Sci. 1999, 7, 145–151. [Google Scholar] [CrossRef]
- Thanou, M.; Verhoef, J.C.; Junginger, H.E. Chitosan and its derivatives as intestinal absorption enhancers. Adv. Drug Deliv. Rev. 2001, 50, S91–S101. [Google Scholar] [CrossRef]
- Guo, Z.; Xing, R.; Liu, S.; Zhong, Z.; Ji, X.; Wang, L.; Li, P. Antifungal properties of Schiff bases of chitosan, N-substituted chitosan and quaternized chitosan. Carbohydr. Res. 2007, 342, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, Q.; Tan, W.; Dong, F.; Luan, F.; Guo, Z. Synthesis, Characterization, and the Antioxidant Activity of Double Quaternized Chitosan Derivatives. Molecules 2017, 22, 501. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Z.; Bao, X.; Xu, G.; Yao, P. Fatty acid and quaternary ammonium modified chitosan nanoparticles for insulin delivery. Colloids Surf. B 2018, 170, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Xin, M.; Li, M.; Huang, H.; Zhou, S.; Liu, J. Synthesis, characterization, and antibacterial activity of N,O-quaternary ammonium chitosan. Carbohydr. Res. 2011, 346, 2445–2450. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Guo, Z.; Jiang, P. Synthesis, characterization, and antifungal activity of novel quaternary chitosan derivatives. Carbohydr. Res. 2010, 345, 1896–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, K.; Li, P. C-coordinated O-carboxymethyl chitosan metal complexes: Synthesis, characterization and antifungal efficacy. Int. J. Biol. Macromol. 2018, 106, 68–77. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds double quaternized chitosan derivatives (4, 5 and 6) and triple quaternized chitosan derivatives (7, 8 and 9) are available from the authors. |




| Compounds | Yields (%) | Elemental Analyses (%) | Degrees of Substitution (%) | Deacetylation (%) | ||
|---|---|---|---|---|---|---|
| C | N | C/N | ||||
| Chitosan | 43.42 | 7.98 | 5.44 | 82.6 | ||
| 4 | 86.3 | 34.17 | 5.60 | 6.10 | 50.4 | |
| 5 | 84.6 | 30.83 | 5.03 | 6.13 | 53.2 | |
| 6 | 86.2 | 33.01 | 5.42 | 6.09 | 49.0 | |
| 7 | 78.3 | 34.78 | 5.89 | 5.91 | 90.3 | |
| 8 | 80.6 | 39.22 | 6.61 | 5.93 | 92.6 | |
| 9 | 76.2 | 40.63 | 6.87 | 5.91 | 91.4 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, L.; Chen, Y.; Tan, W.; Li, Q.; Gu, G.; Dong, F.; Guo, Z. Synthesis, Characterization, and Antifungal Activity of Pyridine-Based Triple Quaternized Chitosan Derivatives. Molecules 2018, 23, 2604. https://doi.org/10.3390/molecules23102604
Wei L, Chen Y, Tan W, Li Q, Gu G, Dong F, Guo Z. Synthesis, Characterization, and Antifungal Activity of Pyridine-Based Triple Quaternized Chitosan Derivatives. Molecules. 2018; 23(10):2604. https://doi.org/10.3390/molecules23102604
Chicago/Turabian StyleWei, Lijie, Yuan Chen, Wenqiang Tan, Qing Li, Guodong Gu, Fang Dong, and Zhanyong Guo. 2018. "Synthesis, Characterization, and Antifungal Activity of Pyridine-Based Triple Quaternized Chitosan Derivatives" Molecules 23, no. 10: 2604. https://doi.org/10.3390/molecules23102604





