Quantitative Evaluation of Twelve Major Components of Sulfur-Fumigated Astragali Radix with Different Durations by UPLC-MS
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Sulfur-Fumigation of AR
2.3. Instruments
2.4. Standard Solutions Preparation
2.5. Sample Preparation
2.6. Method Validation
2.6.1. Calibration Curves, Limits of Detection and Quantification
2.6.2. Precision, Repeatability and Stability
2.6.3. Recovery Test
3. Results and Discussion
3.1. Optimization of Suitable LC-MS Conditions
3.2. Method Validation
3.2.1. Calibration Curves, Limits of Detection and Quantification
3.2.2. Precision, Repeatability and Stability
3.2.3. Accuracy
3.3. Quantification of the Major Components in AR with and without Sulfur-Fumigation
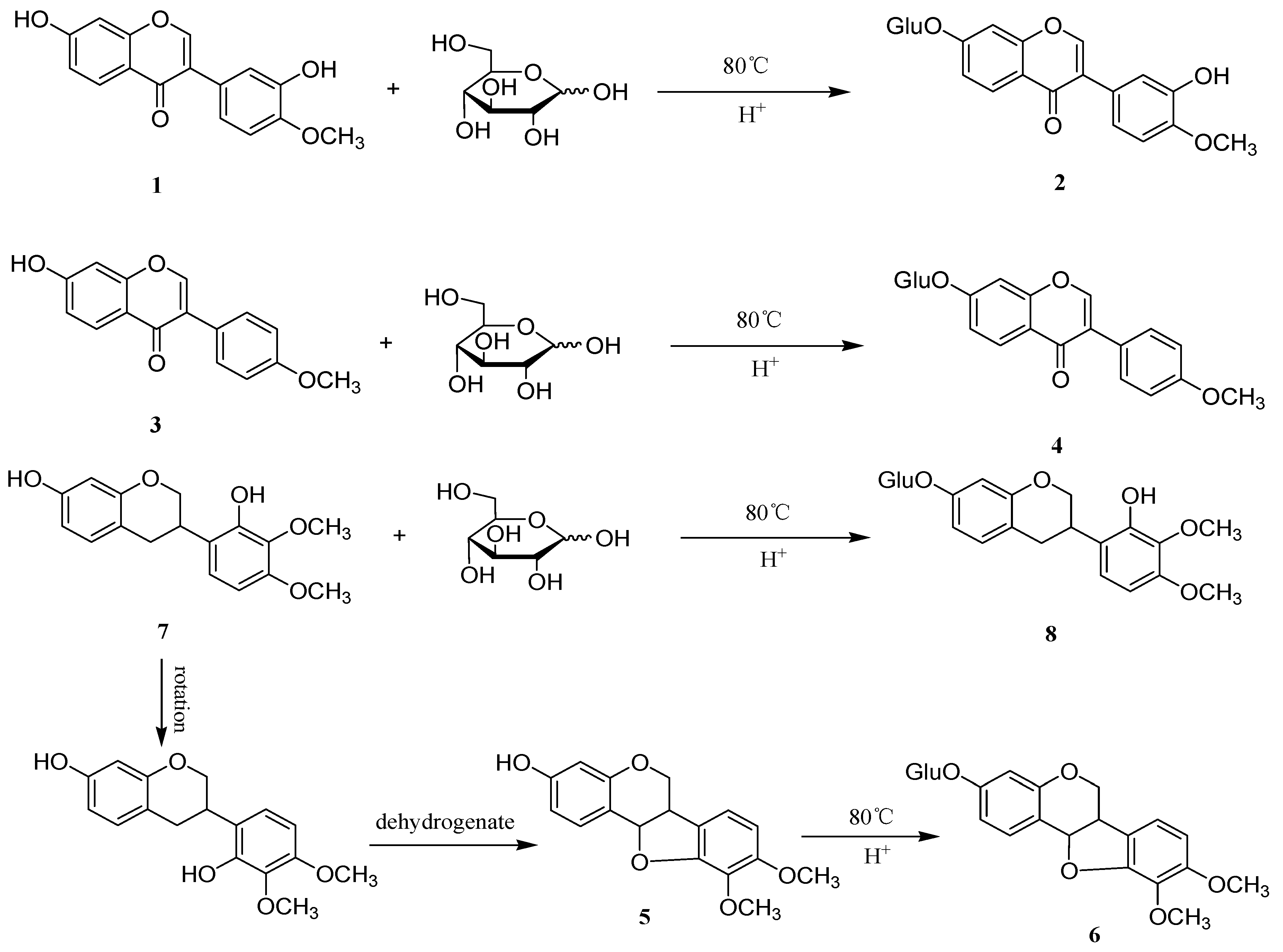
3.4. General Procedure for the Synthesis of Flavonoid Glycosides
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tao, X.; Gu, Z.N.; Sun, W. Effect of Astragalus membranaceus on reinforcing Kidney Qi. J. LiaoNing. Univ. TCM 2007, 9, 30–31. [Google Scholar]
- Sun, Z.H.; Shao, J.; Guo, M. A review on chemical components and pharmacological effects of Huangqi. Clin. J. Chin. Med. 2015, 7, 22–25. [Google Scholar]
- Huo, M.L.; Yuan, K.; Liang, X.R.; Li, X.; Li, G.M. Effect of Astragalus membranaceus (Fisch) Bunge extract on streptozocin-induced diabetic in rats. Trop. J. Pharm. Res. 2016, 15, 1465–1471. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, S.; Zhang, Y.M. Progresson pharmacological action and clinial application of radix astragali. Food Drug 2006, 9, 5–7. [Google Scholar]
- Cho, W.C.; Leung, M.N. In vitro and in vivo immunomodulating and immunorestorative effects of Astragalus membranaceus. J. Ethnopharmacol. 2007, 113, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.; Kim, E.H.; Chun, M.; Kang, S.; Shim, B.; Yu, Y.B.; Jeong, G.; Lee, J.S. Astragali radix elicits anti-inflammation via activation of MKP-1, concomitant with attenuation of p38 and Erk. J. Ethnopharmacol. 2008, 115, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C.; Leung, K.N. In vitro and in vivo anti-tumor effects of Astragalus membranaceus. Cancer Lett. 2007, 252, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Luan, Z.Q.; Zhao, P.L.; Cao, W.F. Research status of Astragali radix on nerve cells and nerve system diseases. Chin. Her. Med. 2012, 4, 271–275. [Google Scholar]
- Jin, Y.; Chen, Q.; Li, X.; Fan, X.; Li, X. Astragali radix protects myocardium from ischemia injury by modulating energy metabolism. Int. J. Cardiol. 2014, 176, 1312–1315. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.L.; Bao, H.Y.; Liu, Y. Progress on chemical constituents and pharmacological effects of Astragali Radix in recent five year. Food Drug 2014, 16, 68–70. [Google Scholar]
- Duan, J.A.; Zhao, R.H.; Su, S.L.; Yan, H.; Guo, S. Basic understanding and suggestions on sulfur fumigation of medicinal materials. Mod. Chin. Med. 2011, 13, 3–5. [Google Scholar]
- Mao, C.Q.; Ji, L.; Lu, T.L.; Dan, X.; Li, L.; Liu, H.N.; Ning, Z.W.; Song, Y.; Zhu, J.J. Research development of harmful substances and its harm of traditional Chinese medicine after sulfur fumigation. Chin. J. Chin. Mater. Med. 2014, 39, 2801–2806. [Google Scholar]
- Zhai, Y.Y.; Hu, M.X.; Chen, A.J.; Guo, B.L.; Huang, W.H.; Cao, X.J.; Hou, M.L. Effects of sulphur fumigation on flavonoids and saponins in Astragali radix. Xian Dai Yao Wu Yu Lin Chuang 2014, 29, 489–493. [Google Scholar]
- Jin, X.; Zhu, L.Y.; Shen, H.; Xu, J.; Li, S.L.; Jia, X.B.; Cai, H.; Cai, B.C.; Yan, R. Influence of sulphur-fumigation on the quality of white ginseng: A quantitative evaluation of major ginsenosides by high performance liquid chromatography. Food Chem. 2012, 135, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Kan, W.L.; Zhu, H.; Li, S.L.; Lin, G. Sulfur fumigation reducing systemic exposure of ginsenosides and weakening immunomodulatory activity of ginseng. J. Ethnopharmacol. 2016, 195, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Liu, X.; Li, S.L.; Cai, B.C.; Cai, H. Current situation in studies on traditional Chinese medicinal materials and Yinpian by sulfur-fumigated process. Chin. Tradit. Herbal. Drugs 2010, 41, 1403–1406. [Google Scholar]
Sample Availability: Samples of the compounds 1–12 are available from the authors. |


| No. | Analyte | Precursor Ion (m/z) | Product Ion (m/z) | DP a (V) | CE b (V) |
|---|---|---|---|---|---|
| 1 | Calycosin | 285.2 | 213.1 | 70 | 45 |
| 2 | Calycosin-7-glucoside | 447.3 | 285.4 | 60 | 20 |
| 3 | Formononetin | 269.0 | 167.2 | 70 | 47 |
| 4 | Ononin | 431.1 | 269.1 | 65 | 20 |
| 5 | Methylnissolin | 301.2 | 167.2 | 54 | 20 |
| 6 | Astraisoflavan-7-O-β-d-glucoside | 463.3 | 167.4 | 60 | 40 |
| 7 | 7,2′-dihydroxy-3′,4′-dimethoxyisoflavane | 303.0 | 167.2 | 55 | 19 |
| 8 | 7,2′-dihydroxy-3′,4′-dimethoxy isoflavan-7-O-β-d-glucopyranoside | 463.3 | 301.0 | −74 | −24 |
| 9 | Astragaloside I | 867.9 | 59.1 | −250 | −83 |
| 10 | Astragaloside II | 825.7 | 59.1 | −110 | −70 |
| 11 | Astragaloside III | 783.8 | 160.9 | −150 | −47 |
| 12 | Astragaloside IV | 783.8 | 101.0 | −115 | −57 |
| Analytes | Regression Equation (μg/mL) | Linear Range (μg/mL) | Correlation Coefficient (R2) | LOQ (ng/mL) | LOD (ng/mL) | Precision RSD a (%) | Repeatability RSD (%) | Stability RSD (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | ||||||||
| Calycosin | y = 446765x + 322603 | 0.4752~11.88 | 0.9989 | 0.59 | 0.06 | 2.06% | 3.16% | 4.47% | 3.93% |
| Calycosin-7-glucoside | y = 202558x + 365088 | 0.864~21.6 | 0.9992 | 1.08 | 0.11 | 1.31% | 2.09% | 3.43% | 2.95% |
| Formononetin | y = 469388x + 141426 | 0.3482~17.41 | 0.9986 | 0.87 | 0.35 | 1.65% | 1.69% | 4.09% | 3.37% |
| Ononin | y = 51545x + 4316.5 | 0.31~15.5 | 0.9994 | 3.10 | 1.55 | 1.42% | 3.86% | 4.63% | 4.05% |
| Methylnissolin | y = 329224x + 84783 | 0.6976~17.44 | 0.9993 | 0.09 | 0.02 | 1.46% | 2.53% | 4.31% | 2.99% |
| Astraisoflavan-7-O-β-d-glucoside | y = 130675x + 208291 | 1.5226~38.064 | 0.9991 | 0.08 | 0.04 | 2.03% | 2.78% | 4.49% | 3.73% |
| 7,2′-dihydroxy-3′,4′-dimethoxyisoflavane | y = 57437x + 11250 | 0.2571~6.427 | 0.9990 | 0.32 | 0.35 | 1.96% | 3.53% | 4.92% | 3.77% |
| 7,2′-dihydroxy-3′,4′-dimethoxy isoflavan-7-O-β-d-glucopyranoside | y = 127060x + 236161 | 2.496~62.4 | 0.9988 | 0.01 | 0.001 | 1.25% | 2.37% | 3.68% | 1.50% |
| Astragaloside I | y = 1009.3x − 10607 | 8.56~214 | 0.9991 | 0.09 | 0.04 | 2.36% | 3.18% | 4.84% | 2.52% |
| Astragaloside II | y = 10750x + 25435 | 1.028~25.2 | 0.9993 | 0.03 | 0.002 | 1.65% | 3.78% | 2.66% | 2.03% |
| Astragaloside III | y = 12640x + 30515 | 1.128~28.2 | 0.9993 | 0.03 | 0.01 | 1.41% | 2.19% | 2.65% | 1.87% |
| Astragaloside IV | y = 6748.6x + 164720 | 4.584~114.6 | 0.9989 | 0.06 | 0.01 | 2.18% | 1.98% | 4.67% | 2.06% |
| Analyte | Initial Amount (μg) | Added Amount (μg) | Detected Amount (μg) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| 0.61 | 1.90 | 103.50% | 3.2 | ||
| Calycosin | 1.22 | 1.22 | 2.48 | 101.50% | 1.5 |
| 1.83 | 3.01 | 98.50% | 2.3 | ||
| 3.735 | 4.79 | 96.70% | 2.7 | ||
| Calycosin-7-glucoside | 7.47 | 7.47 | 14.51 | 97.10% | 0.9 |
| 11.205 | 19.28 | 103.20% | 1.7 | ||
| 1.21 | 3.47 | 95.40% | 1.6 | ||
| Formononetin | 2.42 | 2.42 | 5.10 | 105.20% | 3.1 |
| 3.63 | 6.01 | 99.20% | 2.7 | ||
| 0.87 | 2.61 | 99.60% | 0.4 | ||
| Ononin | 1.74 | 1.74 | 3.53 | 101.40% | 0.9 |
| 2.61 | 4.56 | 104.70% | 1.5 | ||
| 2.29 | 7.12 | 103.90% | 3.6 | ||
| Methylnissolin | 4.57 | 4.57 | 9.36 | 102.30% | 3.7 |
| 6.86 | 12.06 | 105.50% | 3.0 | ||
| 8.54 | 26.67 | 104.10% | 2.6 | ||
| Astraisoflavan-7-O-β-d-glucoside | 17.08 | 17.08 | 34.02 | 99.60% | 3.4 |
| 25.62 | 42.23 | 98.90% | 2.6 | ||
| 1.99 | 6.24 | 104.50% | 1.5 | ||
| 7,2′-dihydroxy-3′,4′-dimethoxyisoflavane | 3.98 | 3.98 | 8.15 | 102.30% | 1.6 |
| 5.97 | 10.11 | 101.60% | 3.2 | ||
| 1.26 | 3.87 | 102.70% | 3.1 | ||
| 7,2′-dihydroxy-3′,4′-dimethoxy | 2.51 | 2.51 | 4.96 | 98.80% | 2.3 |
| 3.77 | 6.39 | 101.80% | 5.7 | ||
| isoflavan-7-O-β-d-glucopyranoside | 2.01 | 6.12 | 101.70% | 0.6 | |
| Isoflavan-7-O-β-d-glucopyranoside | 4.01 | 4.01 | 7.90 | 98.40% | 2.3 |
| 6.02 | 9.58 | 95.50% | 2.6 | ||
| 1.23 | 3.79 | 103.10% | 3.1 | ||
| Astragaloside I | 2.45 | 2.45 | 4.88 | 99.60% | 4.1 |
| 3.68 | 6.06 | 98.90% | 2.8 | ||
| 1.07 | 3.16 | 98.60% | 1.5 | ||
| Astragaloside III | 2.14 | 2.14 | 4.40 | 102.90% | 2.7 |
| 3.21 | 5.30 | 99.10% | 3.1 | ||
| 4.33 | 13.50 | 104.10% | 1.8 | ||
| Astragaloside IV | 8.65 | 8.65 | 16.83 | 97.30% | 3.1 |
| 12.98 | 22.44 | 103.80% | 2.2 |
| Compounds | AR with and without Sulfur-Fumigation. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 4 h | 6 h | 8 h | 12 h | 16 h | 24 h | 36 h | 48 h | 60 h | 72 h | Non-Fumigated | |
| 1 | 7.49 ± 0.03 | 5.04 ± 0.04 | 6.40 ± 0.02 | 7.36 ± 0.07 | 4.91 ± 0.00 | 5.97 ± 0.01 | 7.44 ± 0.10 | 5.37 ± 0.02 | 5.42 ± 0.05 | 4.86 ± 0.01 | 5.28 ± 0.06 | 6.03 ± 0.03 | 13.55 ± 0.68 |
| 2 | 4.24 ± 0.05 | 5.45 ± 0.02 | 5.10 ± 0.00 | 4.83 ± 0.01 | 4.61 ± 0.04 | 4.27 ± 0.02 | 4.07 ± 0.05 | 4.39 ± 0.03 | 4.33 ± 0.04 | 4.48 ± 0.01 | 3.92 ± 0.02 | 3.87 ± 0.02 | 3.21 ± 0.01 |
| 3 | 0.59 ± 0.01 | 0.59 ± 0.03 | 0.75 ± 0.02 | 0.78 ± 0.00 | 0.50 ± 0.02 | 0.64 ± 0.01 | 0.50 ± 0.03 | 0.62 ± 0.00 | 0.60 ± 0.02 | 0.56 ± 0.01 | 0.61 ± 0.02 | 0.63 ± 0.01 | 1.37 ± 0.04 |
| 4 | 0.81 ± 0.02 | 1.19 ± 0.09 | 1.04 ± 0.01 | 1.05 ± 0.04 | 0.88 ± 0.05 | 0.80 ± 0.07 | 1.06 ± 0.02 | 0.89 ± 0.00 | 0.91 ± 0.02 | 0.95 ± 0.01 | 0.76 ± 0.02 | 0.75 ± 0.01 | 0.40 ± 0.02 |
| 5 | 0.30 ± 0.01 | 0.25 ± 0.00 | 0.27 ± 0.02 | 0.27 ± 0.00 | 0.21 ± 0.00 | 0.22 ± 0.01 | 0.35 ± 0.02 | 0.22 ± 0.05 | 0.23 ± 0.01 | 0.22 ± 0.02 | 0.23 ± 0.00 | 0.22 ± 0.01 | 0.24 ± 0.01 |
| 6 | 0.55 ± 0.00 | 0.90 ± 0.01 | 0.69 ± 0.01 | 0.64 ± 0.02 | 0.59 ± 0.00 | 0.58 ± 0.05 | 0.53 ± 0.07 | 0.58 ± 0.00 | 0.52 ± 0.02 | 0.67 ± 0.00 | 0.47 ± 0.00 | 0.44 ± 0.01 | 0.32 ± 0.02 |
| 7 | 4.18 ± 0.06 | 2.61 ± 0.01 | 2.99 ± 0.05 | 3.44 ± 0.00 | 2.93 ± 0.02 | 3.29 ± 0.04 | 3.06 ± 0.01 | 2.51 ± 0.00 | 3.17 ± 0.02 | 2.60 ± 0.00 | 2.54 ± 0.00 | 3.24 ± 0.01 | 4.47 ± 0.10 |
| 8 | 0.86 ± 0.00 | 1.59 ± 0.00 | 1.38 ± 0.05 | 1.20 ± 0.07 | 1.13 ± 0.06 | 1.16 ± 0.02 | 0.95 ± 0.01 | 1.27 ± 0.00 | 1.08 ± 0.04 | 1.42 ± 0.06 | 0.97 ± 0.00 | 0.75 ± 0.00 | 0.68 ± 0.00 |
| 9 | 0.71 ± 0.01 | 0.74 ± 0.02 | 0.70 ± 0.00 | 0.71 ± 0.05 | 0.70 ± 0.00 | 0.70 ± 0.02 | 0.74 ± 0.04 | 0.69 ± 0.07 | 0.70 ± 0.00 | 0.68 ± 0.01 | 0.67 ± 0.02 | 0.70 ± 0.04 | 0.73 ± 0.04 |
| 10 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 11 | 0.78 ± 0.06 | 0.84 ± 0.02 | 0.79 ± 0.01 | 0.70 ± 0.04 | 0.71 ± 0.00 | 0.73 ± 0.01 | 0.75 ± 0.00 | 0.59 ± 0.02 | 0.60 ± 0.00 | 0.64 ± 0.01 | 0.57 ± 0.02 | 0.58 ± 0.01 | 0.95 ± 0.03 |
| 12 | 3.97 ± 0.00 | 4.17 ± 0.02 | 3.73 ± 0.01 | 3.38 ± 0.01 | 3.59 ± 0.00 | 3.53 ± 0.02 | 3.61 ± 0.01 | 2.68 ± 0.05 | 2.81 ± 0.02 | 3.03 ± 0.04 | 2.62 ± 0.00 | 2.58 ± 0.01 | 4.94 ± 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, X.; Sun, Z.; Yang, M.; Zhu, N.; Yang, J.; Ma, G.; Xu, X. Quantitative Evaluation of Twelve Major Components of Sulfur-Fumigated Astragali Radix with Different Durations by UPLC-MS. Molecules 2018, 23, 2609. https://doi.org/10.3390/molecules23102609
Xing X, Sun Z, Yang M, Zhu N, Yang J, Ma G, Xu X. Quantitative Evaluation of Twelve Major Components of Sulfur-Fumigated Astragali Radix with Different Durations by UPLC-MS. Molecules. 2018; 23(10):2609. https://doi.org/10.3390/molecules23102609
Chicago/Turabian StyleXing, Xiaoyan, Zhonghao Sun, Meihua Yang, Nailiang Zhu, Junshan Yang, Guoxu Ma, and Xudong Xu. 2018. "Quantitative Evaluation of Twelve Major Components of Sulfur-Fumigated Astragali Radix with Different Durations by UPLC-MS" Molecules 23, no. 10: 2609. https://doi.org/10.3390/molecules23102609
APA StyleXing, X., Sun, Z., Yang, M., Zhu, N., Yang, J., Ma, G., & Xu, X. (2018). Quantitative Evaluation of Twelve Major Components of Sulfur-Fumigated Astragali Radix with Different Durations by UPLC-MS. Molecules, 23(10), 2609. https://doi.org/10.3390/molecules23102609





