Steric Effect of Antioxidant Diels-Alder-Type Adducts: A Comparison of Sanggenon C with Sanggenon D
Abstract
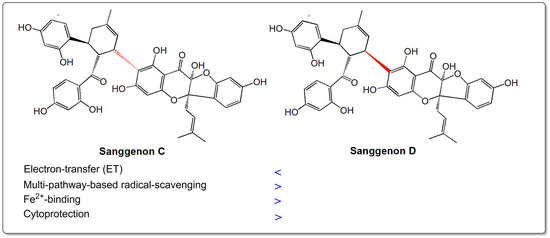
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Animals and Chemicals
3.2. FRAP Assay
3.3. Cu2+-Reducing Power Assay
3.4. ABTS•+-Scavenging Assay
3.5. DPPH•-Scavenging Assay
3.6. UV-Vis-Spectra Analysis of Fe2+-Binding With Sanggenons
3.7. Flow Cytometry Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Ethics Approval and Consent to Participate
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid diammonium) salt |
| bmMSCs | bone marrow-derived mesenchymal stem cells |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DPPH | Dulbecco’s modified Eagle’s medium |
| ET | electron transfer |
| FBS | fetal bovine serum |
| FRAP | ferric reducing antioxidant power |
| HAT | hydrogen atom transfer |
| PCET | proton-coupled electron transfer |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| SD | standard deviation |
| SEPT | sequential electron proton transfer |
| SPLET | sequential proton loss single electron transfer |
| TPTZ | 2,4,6-tris (2-pyridyl-s-triazine) |
| Trolox | (±)-6-hydroxyl-2,5,7,8-tetramethlychromane-2-carboxylic acid |
References
- Dai, S.J.; Lu, Z.M.; Chen, R.Y.; Yu, D.Q. Structure and spectral characteristics of Diels-Alder-type adducts from Morus. Yao Xue Xue Bao 2005, 40, 876–881. [Google Scholar] [PubMed]
- Nomura, T.; Fukai, T.; Hano, Y.; Uzawa, J. Structure of sanggenon D, a natural hypotensive Diels-Alder adduct from Chinese crude drug Sang-bai-pi. Heterocycles 1982, 17, 381–389. [Google Scholar] [CrossRef]
- Nomura, T.; Fukai, T.; Hano, Y.; Uzawa, J. Structure of sanggenon C, a natural hypotensive Diels-Alder adduct from Chinese crude drug Sang-bai-pi. Heterocycles 1981, 16, 2141–2148. [Google Scholar] [CrossRef]
- Chen, L.D.; Liu, Z.H.; Zhang, L.F.; Yao, J.N.; Wang, C.F. Sanggenon C induces apoptosis of colon cancer cells via inhibition of NO production, iNOS expression and ROS activation of the mitochondrial pathway. Oncol. Rep. 2017, 38, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, N.; Zhao, K.; Zhu, C.; Lu, X.; Li, S.; Lian, W.; Zhou, P.; Dong, X.; Zhao, C.; et al. Sanggenon C decreases tumor cell viability associated with proteasome inhibition. Front. Biosci. 2011, 3, 1315–1325. [Google Scholar] [CrossRef]
- Gu, Y.; Gao, L.; Chen, Y.; Xu, Z.; Yu, K.; Zhang, D.; Zhang, G.; Zhang, X. Sanggenon C protects against cardiomyocyte hypoxia injury by increasing autophagy. Mol. Med. Rep. 2017, 16, 8130–8136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narakornsak, S.; Aungsuchawan, S.; Pothacharoen, P.; Markmee, R.; Tancharoen, W.; Laowanitwattana, T.; Thaojamnong, C.; Peerapapong, L.; Boonma, N.; Tasuya, W.; et al. Encouraging effects on chondrogenic differentiation of human amniotic fluid-derived mesenchymal stem cells. Acta Histochem. 2017, 119, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Wei, G.; Wang, X.Z.; Liu, D.H.; Deng, R.D.; Li, H.; Zhou, J.H.; Li, Y.W.; Zeng, H.P.; Chen, D.F. Targeting of the sonic hedgehog pathway by atractylenolides promotes chondrogenic differentiation of mesenchymal stem cells. Biol. Pharm. Bull. 2012, 35, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Pati, S.; Lee, J.W. Concise Review: Mesenchymal Stem (Stromal) Cells: Biology and Preclinical Evidence for Therapeutic Potential for Organ Dysfunction Following Trauma or Sepsis. Stem Cells 2017, 35, 316–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.F.; Li, X.; Xu, Z.; Liu, X.; Du, S.H.; Li, H.; Zhou, J.H.; Zeng, H.P.; Hua, Z.C. Hexadecanoic Acid from Buzhong Yiqi Decoction Induces Proliferation of Bone Marrow Mesenchymal Stem Cells. J. Med. Food 2010, 13, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Zeng, H. Synthesis, Antioxidation Activity of (E)-9-p-Tolyl-3-[2-(8-hydroxy-quinol-2-yl)vinyl]-carbazole and (E)-9-(p-Anisyl)-3-[2-(8-hydroxy-quinol-2-yl)vinyl]-carbazole and Their Induction Proliferation of Mesenchymal Stem Cells. Acta Chim. Sin. 2009, 67, 974–982. [Google Scholar]
- Li, X.C.; Hu, Q.P.; Jiang, S.X.; Li, F.; Lin, J.; Han, L.; Hong, Y.L.; Lu, W.B.; Gao, Y.X.; Chen, D.F. Flos chrysanthemi indici protects against hydroxyl-induced damages to DNA and MSCs via antioxidant mechanism. J. Saudi Chem. Soc. 2015, 19, 454–460. [Google Scholar] [CrossRef]
- Li, X.C.; Mai, W.Q.; Chen, D.F. Chemical study on protective effect against hydroxyl-induced DNA damage and antioxidant mechanism of myricitrin. J. Chin. Chem. Soc. 2014, 61, 383–391. [Google Scholar] [CrossRef]
- Kim, H.J.; Baburin, I.; Zaugg, J.; Ebrahimi, S.N.; Hering, S.; Hamburger, M. HPLC-based activity profiling-discovery of sanggenons as gabaa receptor modulators in the traditional Chinese drug sang bai pi (morus alba root bark). Planta Med. 2012, 78, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Q.; Fukai, T.; Sakagami, H.; Chang, W.J.; Yang, P.Q.; Wang, F.P.; Nomura, T. Cytotoxic Flavonoids with Isoprenoid Groups from Morus mongolica. J. Nat. Prod. 2001, 64, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.L.; Yu, D.O. 1H-NMR Spectroscopic Databook of Natural Products, 1st ed.; Chemical Industry Press: Beijing, China, 2011; pp. 1046–1051. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar]
- Li, X.C.; Han, W.; Mai, W.; Wang, L. Antioxidant Activity and mechanism of tetrahydroamentoflavone in vitro. Nat. Prod. Commun. 2013, 8, 787–789. [Google Scholar]
- Li, X.; Gao, Y.; Li, F.; Liang, A.; Xu, Z.; Bai, Y.; Mai, W.; Han, L.; Chen, D. Maclurin protects against hydroxyl radical-induced damages to mesenchymal stem cells: Antioxidant evaluation and mechanistic insight. Chem. Biol. Interact. 2014, 219, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, X.; Chen, L.; Lu, W.; Chen, X.; Han, L.; Chen, D. Protective effect against hydroxyl radical-induced DNA damage and antioxidant mechanism of [6]-gingerol: A Chemical Study. Bull. Korean Chem. Soc. 2014, 35, 1633–1638. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Lin, J.; Wang, T.; Huang, J.; Lin, Y.; Chen, D. Protective effects of dihydromyricetin against •OH-induced mesenchymal stem cells damage and mechanistic chemistry. Molecules 2016, 21, 604. [Google Scholar] [CrossRef] [PubMed]
- López-Munguía, A.; Hernández-Romero, Y.; Pedraza-Chaverri, J.; Miranda-Molina, A.; Regla, I.; Martínez, A.; Castillo, E. Phenylpropanoid glycoside analogues: Enzymatic synthesis, antioxidant activity and theoretical study of their free radical scavenger mechanism. PloS ONE 2011, 6, e20115. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.J.; Goeltz, J.C. Proton-Coupled Electron Transfer and Substituent Effects in Catechol-Based Deep Eutectic Solvents: Gross and Fine Tuning of Redox Activity. J. Phys. Chem. B 2017, 121, 10974–10978. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yoon, J. UV direct photolysis of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) in aqueous solution: Kinetics and mechanism. J. Photoch. Photobiol. A 2008, 197, 232–238. [Google Scholar] [CrossRef]
- Aliaga, C.; Lissi, E.A. Reactions of the radical cation derived from 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS·+) with amino acids. Kinetics and mechanism. Can. J. Chem. 2000, 78, 1052–1059. [Google Scholar] [CrossRef]
- Osman, A.M.; Wong, K.; Fernyhough, A. ABTS radical-driven oxidation of polyphenols: Isolation and structural elucidation of covalent adducts. Biochem. Biophys. Res. Commun. 2006, 34, 6321–6329. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.M. Multiple pathways of the reaction of 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) with (+)-catechin: Evidence for the formation of a covalent adduct between DPPH and the oxidized form of the polyphenol. Biochem. Biophys. Res. Commun. 2011, 412, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C. 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO•) radical-scavenging: A new and simple antioxidant assay in vitro. J. Agric. Food Chem. 2017, 65, 6288–6297. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron chelating. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Jiang, Q.; Wang, T.T.; Liu, J.J.; Chen, D.F. Comparison of the Antioxidant Effects of Quercitrin and Isoquercitrin: Understanding the Role of the 6″-OH Group. Molecules 2016, 21, 1246. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Li, X.; Chen, D.; Mai, Y.; Wen, B.; Wang, X. Concordance between antioxidant activities in vitro and chemical components of Radix Astragali (Huangqi). Nat. Prod. Res. 2012, 26, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Zeng, G.C.; Li, X.C.; Zeng, H.P. In vitro studies on the antioxidant and protective effect of 2-substituted -8-hydroxyquinoline derivatives against H2O2-induced oxidative stress in BMSCs. Chem. Biol. Drug Des. 2010, 75, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Li, X.C.; Lin, J.; Li, Y.R.; Wang, T.T.; Jiang, Q.; Chen, D.F. Sarcandra glabra (Caoshanhu) protects mesenchymal stem cells from oxidative stress: A bioevaluation and mechanistic chemistry. BMC Complement. Altern. Med. 2016, 16, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, X.C.; Tian, Y.G.; Lin, Q.; Xie, H.; Lu, W.B.; Chi, Y.G.; Chen, D.F. Lyophilized aqueous extracts of Mori Fructus and Mori Ramulus protect Mesenchymal stem cells from •OH–treated damage: Bioassay and antioxidant mechanism. BMC Complement. Altern. Med. 2017, 17, 242. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Han, L.; Li, Y.R.; Zhang, J.; Chen, J.M.; Lu, W.B.; Zhao, X.J.; Lai, Y.T.; Chen, D.F.; Wei, G. Protective Effect of Sinapine against Hydroxyl Radical-Induced Damage to Mesenchymal Stem Cells and Possible Mechanisms. Chem. Pharm. Bull. 2016, 64, 319–325. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of sanggenon D is available from the authors. |



| Assays | Sanggenon C μg/mL (μM) | Sanggenon D μg/mL (μM) | Trolox µg/mL (μM) |
|---|---|---|---|
| FRAP | 285.2 ± 18.3 (402.5 ± 25.8 c) | 151.4 ± 21.2 (213.7 ± 30.0 b) | 20.8 ± 1.4 (83.1 ± 5.5 a) |
| Cu2+-reducing | 7.6 ± 0.2 (10.8 ± 0.3 b) | 5.8 ± 0.0 (8.2 ± 0.0 a) | 5.7 ± 0.0 (22.8 ± 0.0 c) |
| ABTS•+-scavenging | 3.8 ± 0.3 (5.4 ± 0.4 a) | 7.2 ± 0.5 (10.2 ± 0.7 b) | 5.9 ± 0.1 (23.7 ± 0.2 c) |
| DPPH•-scavenging | 81.4 ± 1.9 (114.9 ± 2.7 b) | 104.0 ± 1.2 (146.8 ± 1.7 c) | 4.8 ± 0.1 (19.4 ± 0.6 a) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Ren, Z.; Wu, Z.; Fu, Z.; Xie, H.; Deng, L.; Jiang, X.; Chen, D. Steric Effect of Antioxidant Diels-Alder-Type Adducts: A Comparison of Sanggenon C with Sanggenon D. Molecules 2018, 23, 2610. https://doi.org/10.3390/molecules23102610
Li X, Ren Z, Wu Z, Fu Z, Xie H, Deng L, Jiang X, Chen D. Steric Effect of Antioxidant Diels-Alder-Type Adducts: A Comparison of Sanggenon C with Sanggenon D. Molecules. 2018; 23(10):2610. https://doi.org/10.3390/molecules23102610
Chicago/Turabian StyleLi, Xican, Zhenxing Ren, Zimei Wu, Zhen Fu, Hong Xie, Langyu Deng, Xiaohua Jiang, and Dongfeng Chen. 2018. "Steric Effect of Antioxidant Diels-Alder-Type Adducts: A Comparison of Sanggenon C with Sanggenon D" Molecules 23, no. 10: 2610. https://doi.org/10.3390/molecules23102610