A New Environmentally-Friendly Colorimetric Probe for Formaldehyde Gas Detection under Real Conditions
Abstract
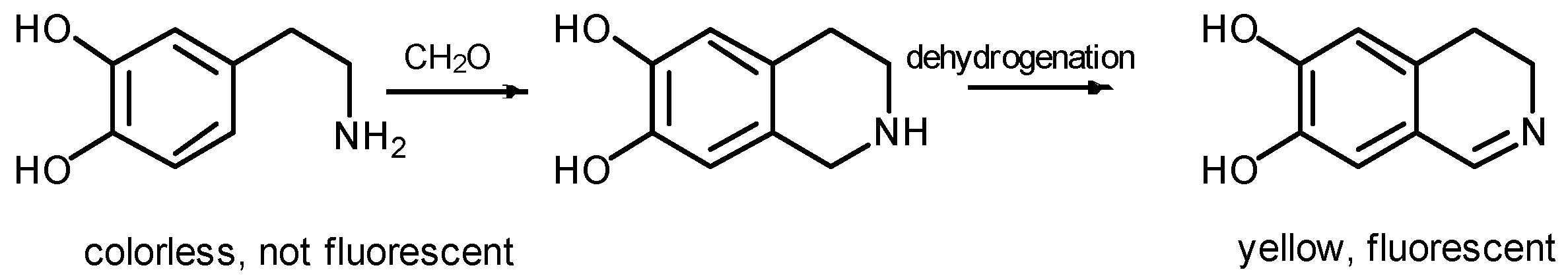
:1. Introduction
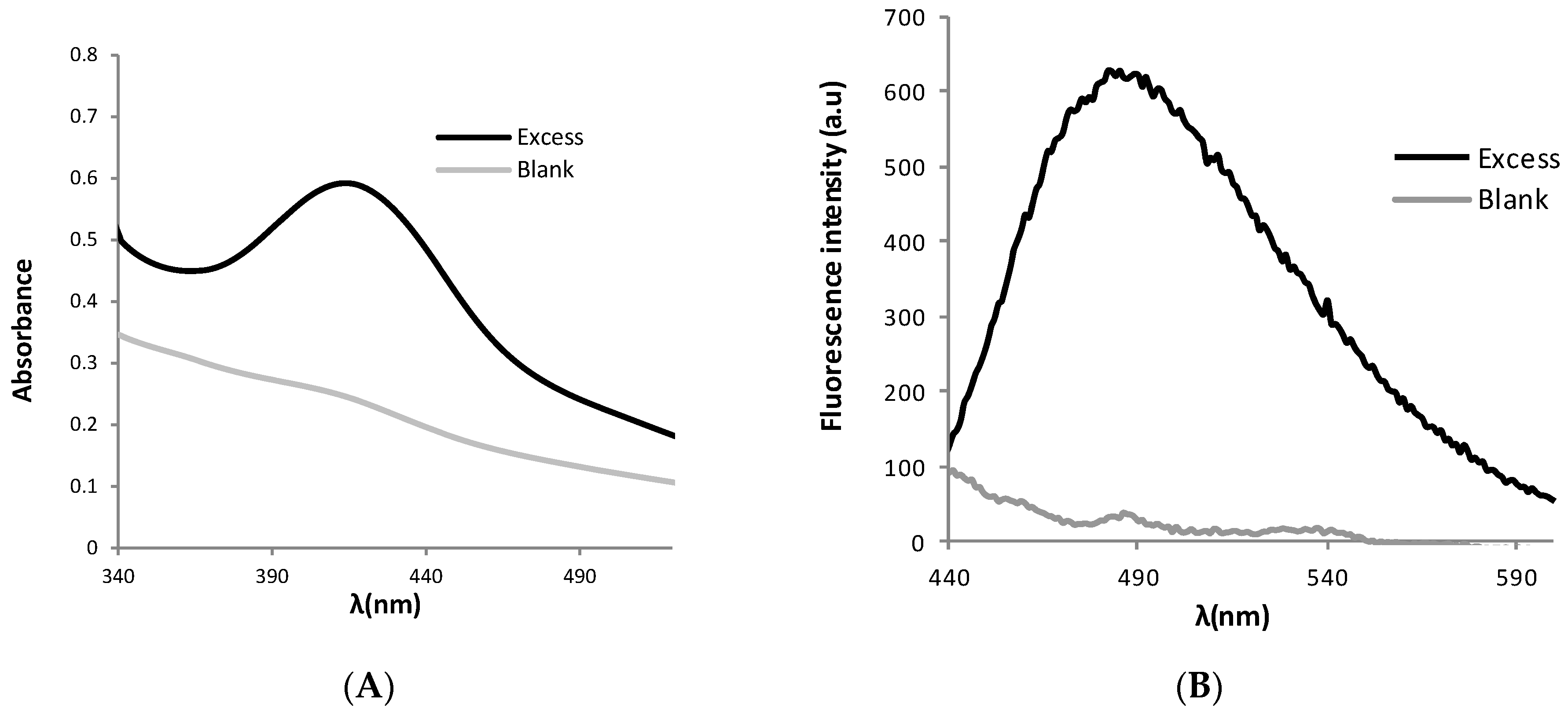
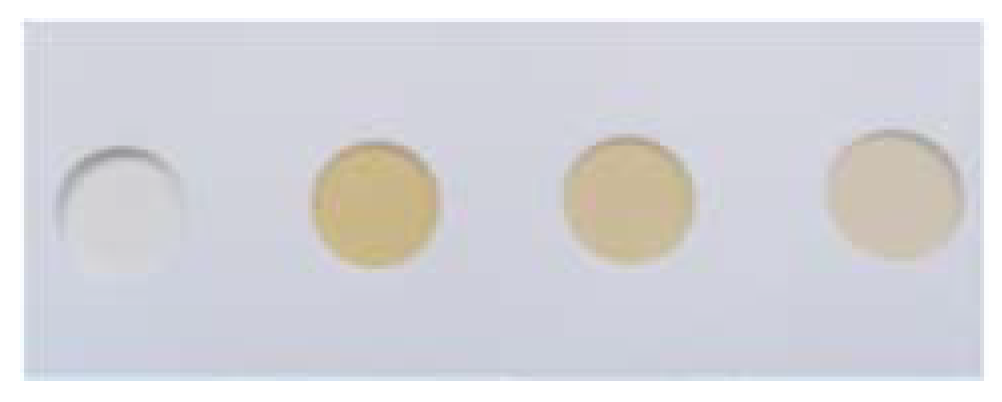
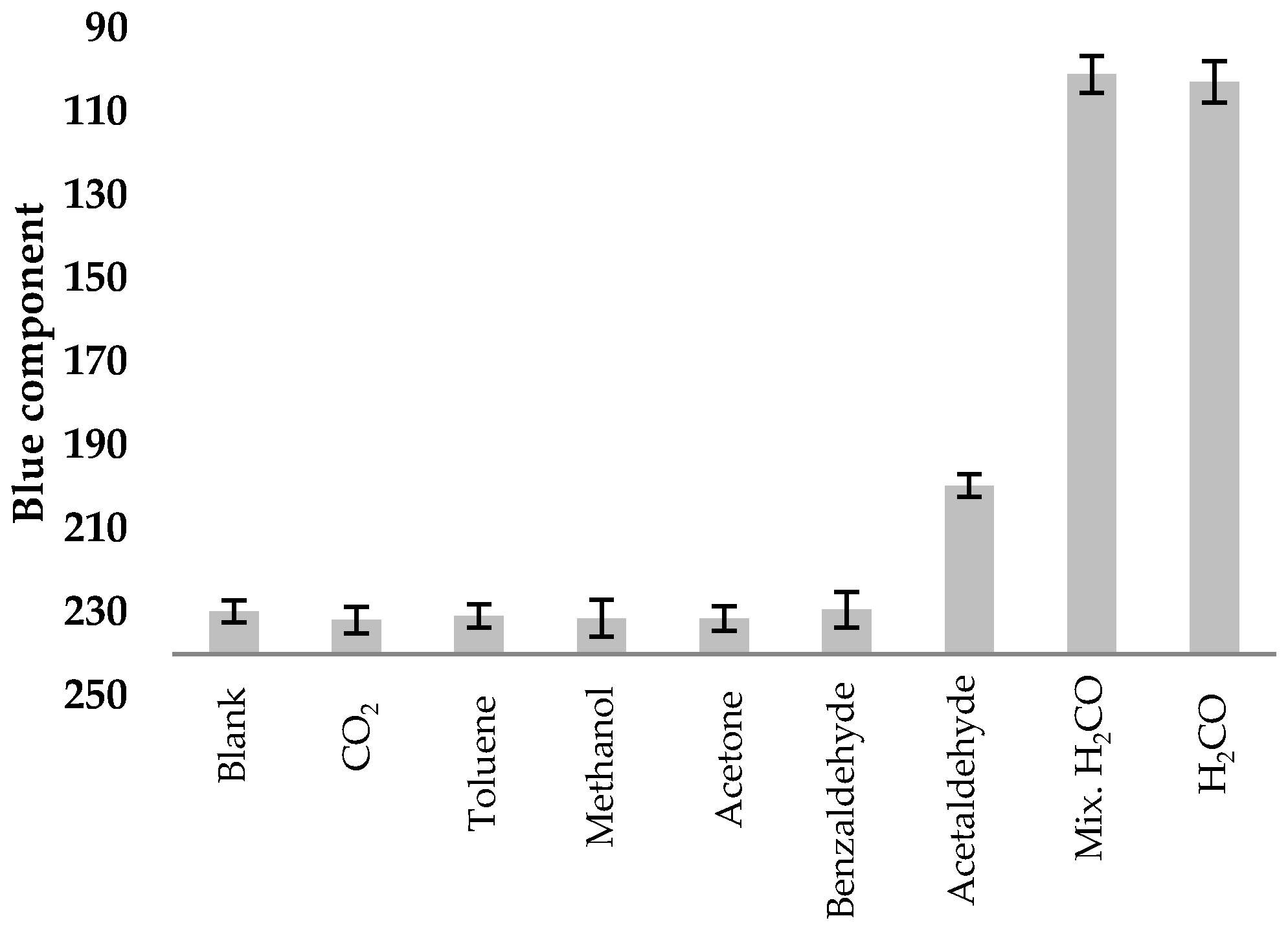
2. Results and Discussion
3. Materials and Methods
3.1. General Procedures
3.2. Preparation of Test Strips
3.3. Gaseous Formaldehyde Measurements
3.4. RGB Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Merchant Research and Consulting Ltd. Available online: https://mcgroup.co.uk/news/20140627/formaldehyde-production-exceed-52-mln-tonnes.html (accessed on 5 October 2018).
- NIOSH. Occupational and Safety and Health Guideline for Formaldehyde. Available online: https://www.cdc.gov/niosh/docs/81-123/pdfs/0293.pdf (accessed on 16 October 2018).
- Goris, J.A.; Ang, S.; Navarro, C. Laboratory safety: Minimizing the toxic effects of formaldehyde. Lab. Med. 1998, 29, 39–43. [Google Scholar] [CrossRef]
- IARC. Chemical Agents and Related Occupations Vol 100 F Review of Human Carcinogens; International Agency for Research on Cancer: Lyon, France, 2012; pp. 401–430. [Google Scholar]
- Luo, W.; Li, H.; Zhang, Y.; Ang, C.Y.W. Determination of formaldehyde in blood plasma by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B 2001, 2, 253–257. [Google Scholar] [CrossRef]
- Rocha, F.R.; Coelho, L.H.G.; Lopes, M.L.A.; Carvalho, L.R.F.; Fracassi da Silva, J.A.; do Lago, C.L.; Gutz, I.G.R. Environmental formaldehyde analysis by active diffusive sampling with a bundle of polypropylene porous capillaries followed by capillary zone electrophoretic separation and contactless conductivity detection. Talanta 2008, 76, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Korpan, Y.I.; Gonchar, M.V.; Sibirny, A.A.; Martelet, C.; Elskaya, A.V.; Gibson, T.D.; Soldatkin, A.P. Development of highly selective and stable potentiometric sensors for formaldehyde determination. Biosens. Bioelectron. 2000, 15, 77–83. [Google Scholar] [CrossRef]
- Dong, S.; Dasgupta, P.K. Solubility of gaseous formaldehyde in liquid water and generation of trace standard gaseous formaldehyde. Environ. Sci. Technol. 1986, 20, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Mitsubayashi, K.; Nishio, G.; Sawai, M.; Saito, T.; Kudo, H.; Saito, H.; Otsuka, K.; Noguer, T.; Marty, I.L. A bio-sniffer stick with FALDH (formaldehyde dehydrogenase) for convenient analysis of gaseous formaldehyde. Sens. Actuator B 2008, 130, 32–37. [Google Scholar] [CrossRef]
- Demkiv, O.; Smutok, O.; Paryzhak, S.; Gayda, G.; Sultanov, Y.; Guschin, D.; Shkil, H.; Schuhmann, W.; Gonchar, M. Reagentless amperometric formaldehyde-selective biosensors based on the recombinant yeast formaldehyde dehydrogenase. Talanta 2008, 76, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Dennison, M.J.; Hall, J.M.; Turner, A.P.F. Direct monitoring of formaldehyde vapour and detection of ethanol vapour using dehydrogenase-based biosensors. Analyst 1996, 121, 1769–1773. [Google Scholar] [CrossRef]
- Wang, X.; Si, Y.; Mao, X.; Li, Y.; Yu, J.; Wang, H.; Ding, B. Colorimetric sensor strips for formaldehyde assay utilizing fluoral-p decorated polyacrylonitrile nanofibrous membranes. Analyst 2013, 138, 5129–5136. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, H.L.C.; de Andrade, M.V.; de Paula Pereira, P.A.; de Andrade, J.B. Spectrofluorimetric determination of formaldehyde in air after collection onto silica cartridges coated with Fluoral P. Microchem. J. 2004, 78, 15–20. [Google Scholar] [CrossRef]
- Antwi-Boampong, S.; Peng, J.S.; Carlan, J.; BelBruno, J.J. A molecularly imprinted fluoral-P/polyaniline double layer sensor system for selective sensing of formaldehyde. IEEE Sens. J. 2014, 14, 1490–1498. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, J.; Hu, L.-L.; Tan, Y.; Liu, S.-H.; Yin, J. Recent advances in formaldehyde-responsive fluorescent probes. Chin. Chem. Lett. 2017, 28, 1935–1942. [Google Scholar] [CrossRef]
- Brewer, T.F.; Chang, C.J. An aza-cope reactivity-based fluorescent probe for imaging formaldehyde in living cells. J. Am. Chem. Soc. 2015, 137, 10886–10889. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.; Li, H.; Anorma, C.; Chan, J. A reaction-based fluorescent probe for imaging of formaldehyde in living cells. J. Am. Chem. Soc. 2015, 137, 10890–10893. [Google Scholar] [CrossRef] [PubMed]
- Li, J.B.; Wang, Q.Q.; Yuan, L.; Wu, Y.X.; Hu, X.X.; Zhang, X.B.; Tan, W. A two-photon fluorescent probe for bio-imaging of formaldehyde in living cells and tissues. Analyst 2016, 141, 3395–3402. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Kong, X.; Xu, A.; Dong, B.; Lin, W. Development of a two-photon fluorescent probe for imaging of endogenous formaldehyde in living tissues. Angew. Chem. Int. Ed. 2016, 55, 3356–3359. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, X.; Liu, Y.; Kong, X.; Lin, W. A ratiometric fluorescent formaldehyde probe for bioimaging applications. Chem. Commun. 2016, 52, 4029–4032. [Google Scholar] [CrossRef] [PubMed]
- Singha, S.; Jun, Y.W.; Bae, J.; Ahn, K.H. Ratiometric Imaging of Tissue by Two-Photon Microscopy: Observation of a High Level of Formaldehyde around Mouse Intestinal Crypts. Anal. Chem. 2017, 89, 3724–3731. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Rajendiran, S.; Kim, N.; Jeong, S.K.; Ko, E.; Park, R.; Thangadurai, T.D.; Yoon, S. A tailor designed fluorescent ‘turn-on’ sensor of formaldehyde based on the BODIPY motif. Tetrahedron Lett. 2012, 53, 4913–4916. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, J.; Zhang, N.; Li, D.; Xiao, S.; Zheng, K. A ratiometric fluorescent probe for formaldehyde in aqueous solution, serum and air using aza-cope reaction. Sens. Actuators B Chem. 2018, 258, 156–162. [Google Scholar] [CrossRef]
- Chaiendoo, K.; Sooksin, S.; Kulchat, S.; Promarak, V.; Tuntulani, T.; Ngeontae, W. A new formaldehyde sensor from silver nanoclusters modified Tollens’ reagent. Food Chem. 2018, 255, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Fauzia, V.; Imawan, C.; Kusuma, N.; Narayani, N.M.M.S.; Putri, A.E. A localized surface plasmon resonance enhanced dye-based biosensor for formaldehyde detection. Sens. Actuators B Chem. 2018, 257, 1128–1133. [Google Scholar] [CrossRef]
- El Sayed, S.; Pascual, L.; Licchelli, M.; Martínez-Máñez, R.; Gil, S.; Costero, A.M.; Sancenón, F. Chromogenic Detection of Aqueous Formaldehyde Using Functionalized Silica Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 14318–14322. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xue, Z.; Wu, X.; Han, J.; Han, S. Chromo-fluorogenic detection of aldehydes with a rhodamine based sensor featuring an intramolecular deoxylactam. Org. Biomol. Chem. 2011, 9, 7652–7654. [Google Scholar] [CrossRef] [PubMed]
- Guglielmino, M.; Allouch, A.; Serra, C.A.; Le Calvé, S. Development of microfluidic analytical method for on-line gaseous Formaldehyde detection. Sens. Actuators B Chem. 2017, 243, 963–970. [Google Scholar] [CrossRef]
- Xia, H.; Hu, J.; Tang, J.; Xu, K.; Hou, X.; Wu, P. A RGB-Type Quantum Dot-based Sensor Array for Sensitive Visual Detection of Trace Formaldehyde in Air. Sci. Rep. 2016, 6, 36794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, L.; Musto, C.J.; Suslick, D.S. A Simple and Highly Sensitive Colorimetric Detection Method for Gaseous Formaldehyde. J. Am. Chem. Soc. 2010, 132, 4046–4047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.-L.; Chen, Y.; Jiang, H.-L.; Qiu, X.-B.; Yu, D.-Y. Smartphone-Based Microfluidic Colorimetric Sensor for Gaseous Formaldehyde Determination with High Sensitivity and Selectivity. Sensors 2018, 18, 3141. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, X.; Ren, M.; Kong, X.; Liu, Y.; Lin, W. An ultra-fast illuminating fluorescent probe for monitoring formaldehyde in living cells, shiitake mushrooms, and indoors. Chem. Commun. 2016, 52, 9582–9585. [Google Scholar] [CrossRef] [PubMed]
- Gangopadhyay, A.; Maiti, K.; Ali, S.S.; Pramanik, A.K.; Guria, U.N.; Samanta, S.K.; Sarkar, R.; Datta, P.; Mahapatra, A.K. A PET based fluorescent chemosensor with real time application in monitoring formaldehyde emissions from plywood. Anal. Methods 2018, 10, 2888–2894. [Google Scholar] [CrossRef]
- Lin, Q.; Fan, Y.-Q.; Gong, F.-F.; Mao, P.P.; Wang, J.; Guan, X.-X.; Liu, J.; Zhang, Y.-M.; Yao, H.; Wei, T.-B. Ultrasensitive Detection of Formaldehyde in Gas and Solutions by a Catalyst Preplaced Sensor Based on a Pillar[5]arene Derivative. ACS Sustain. Chem. Eng. 2018, 6, 8775–8781. [Google Scholar] [CrossRef]
- Cox, E.D.; Cook, J.M. The Pictet-Spengler condensation: A new direction for an old reaction. Chem. Rev. 1995, 95, 1797–1842. [Google Scholar] [CrossRef]
- Jonsson, G. Fluorescence studies on some 6,7-substituted 3,4-dihydroisoquinolines formed from 3-hydroxytyramine (dopamine) and formaldehyde. Acta Chem. Scand. 1966, 20, 2755–2762. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, A.; Ehinger, B.; Falck, B. A method for differentiating dopamine from noradrenaline in tissue sections by microspectrofluorometry. J. Histochem. Cytochem. 1968, 16, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Stockigt, J.; Antonchick, A.P.; Wu, F.; Waldmann, H. The Pictet-Spengler reaction in nature and in organic chemistry. Angew. Chem. Int. Ed. 2011, 50, 8538–8564. [Google Scholar] [CrossRef] [PubMed]
- Allou, L.; El Maimouni, L.; Le Calvé, S. Henry’s law constant measurements for formaldehyde and benzaldehyde as a function of temperature and water composition. Atmos. Environ. 2011, 45, 2991–2998. [Google Scholar] [CrossRef]
Sample Availability: Samples of the strips are available from the authors. |

| Hours | Without Corpses % Variation | With Corpses % Variation |
|---|---|---|
| 24 | 2 ± 1 | 3 ± 1 |
| 48 |  7 ± 2 7 ± 2 |  10 ± 2 10 ± 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Aquino, C.; Costero, A.M.; Gil, S.; Gaviña, P. A New Environmentally-Friendly Colorimetric Probe for Formaldehyde Gas Detection under Real Conditions. Molecules 2018, 23, 2646. https://doi.org/10.3390/molecules23102646
Martínez-Aquino C, Costero AM, Gil S, Gaviña P. A New Environmentally-Friendly Colorimetric Probe for Formaldehyde Gas Detection under Real Conditions. Molecules. 2018; 23(10):2646. https://doi.org/10.3390/molecules23102646
Chicago/Turabian StyleMartínez-Aquino, Carlos, Ana M. Costero, Salvador Gil, and Pablo Gaviña. 2018. "A New Environmentally-Friendly Colorimetric Probe for Formaldehyde Gas Detection under Real Conditions" Molecules 23, no. 10: 2646. https://doi.org/10.3390/molecules23102646






