Synthesis of Binderless ZK-4 Zeolite Microspheres at High Temperature
Abstract
1. Introduction
2. Methodology
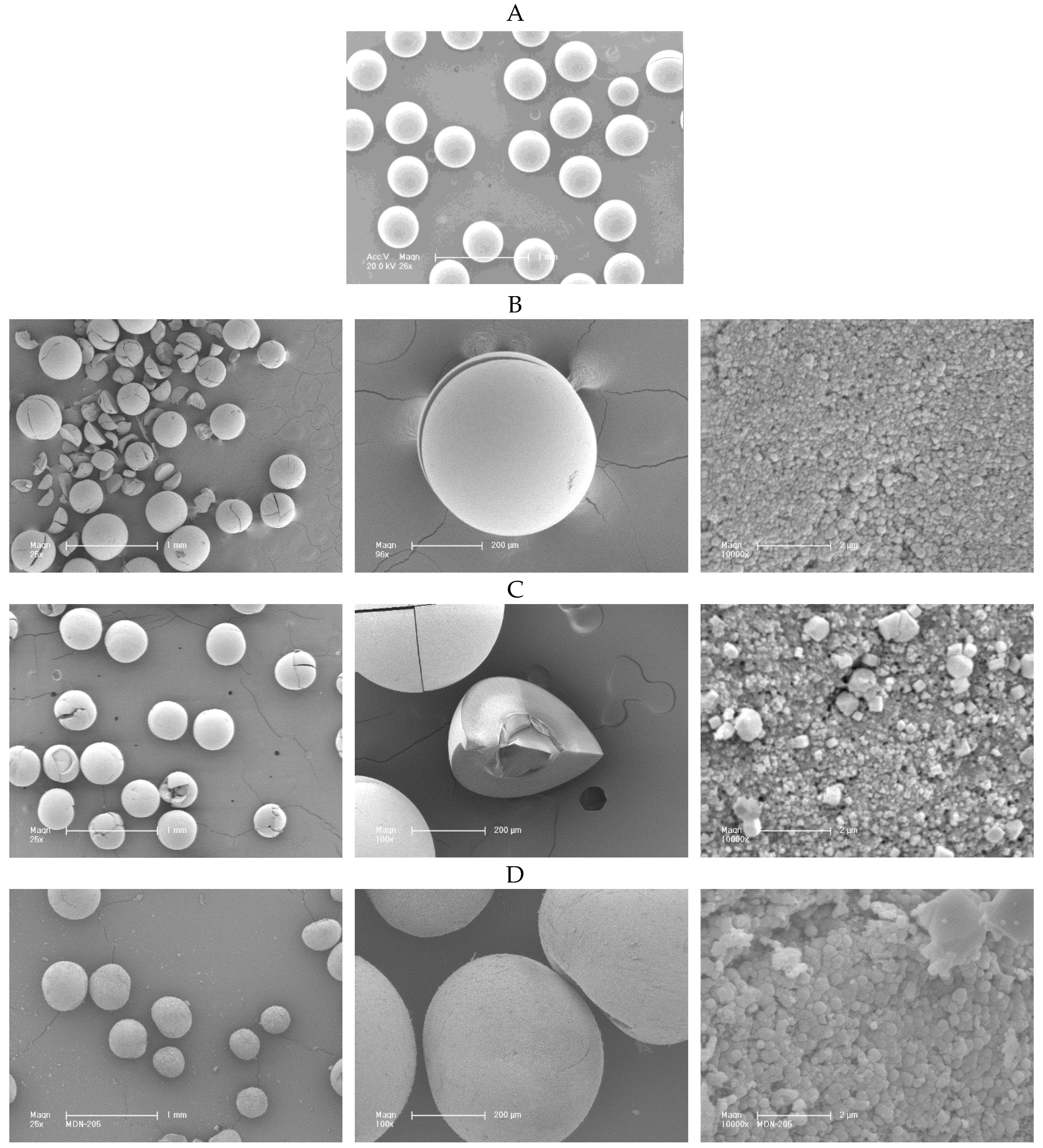
2.1. Preparation of the ZK-4 Zeolite Microspheres
2.2. Characterization of Zeosils
2.3. VOC Adsorption Measurements
2.3.1. Experimental Measurements
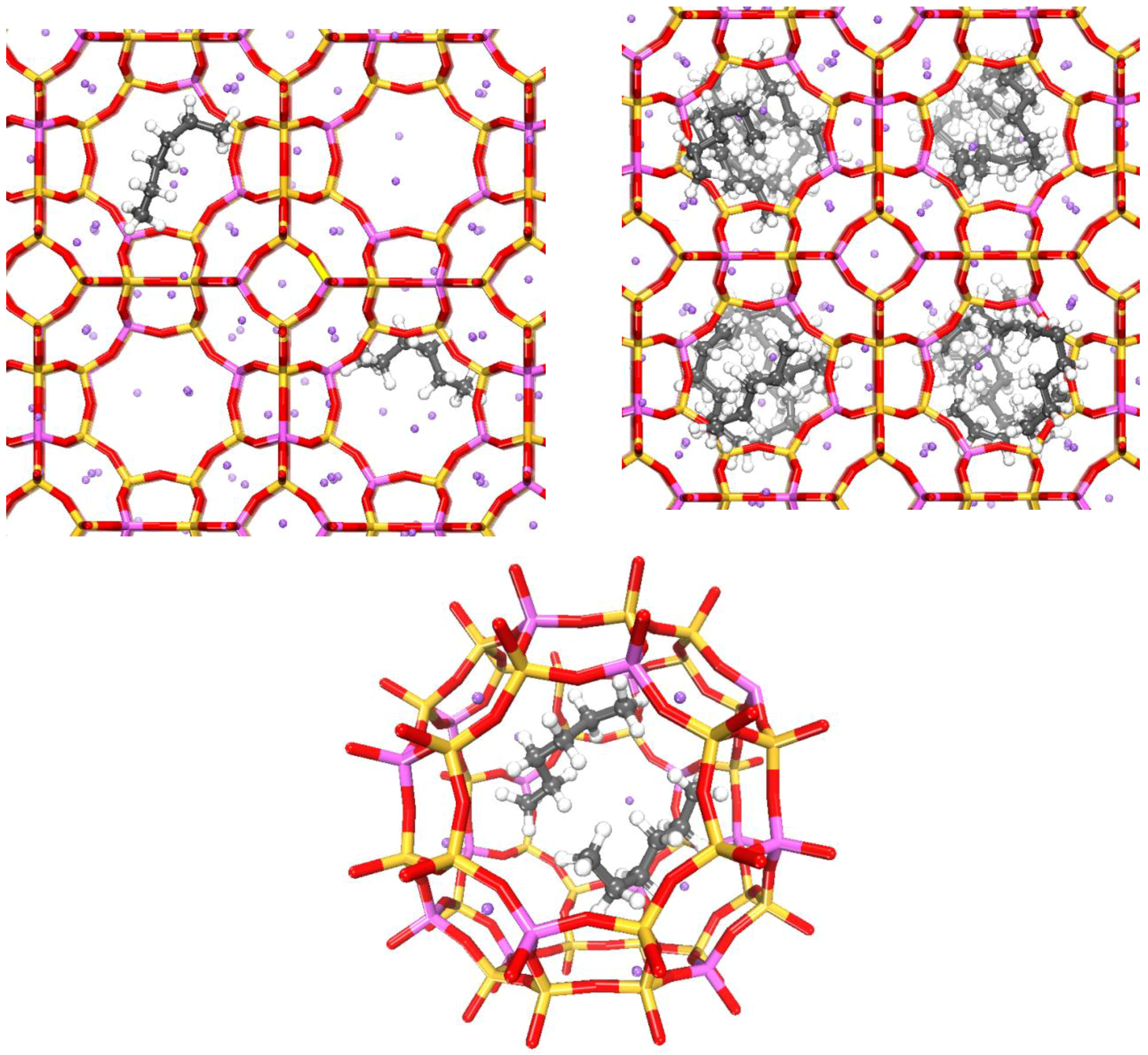
2.3.2. CB–GCMC Simulation
3. Results and Discussion
3.1. XRD Analysis
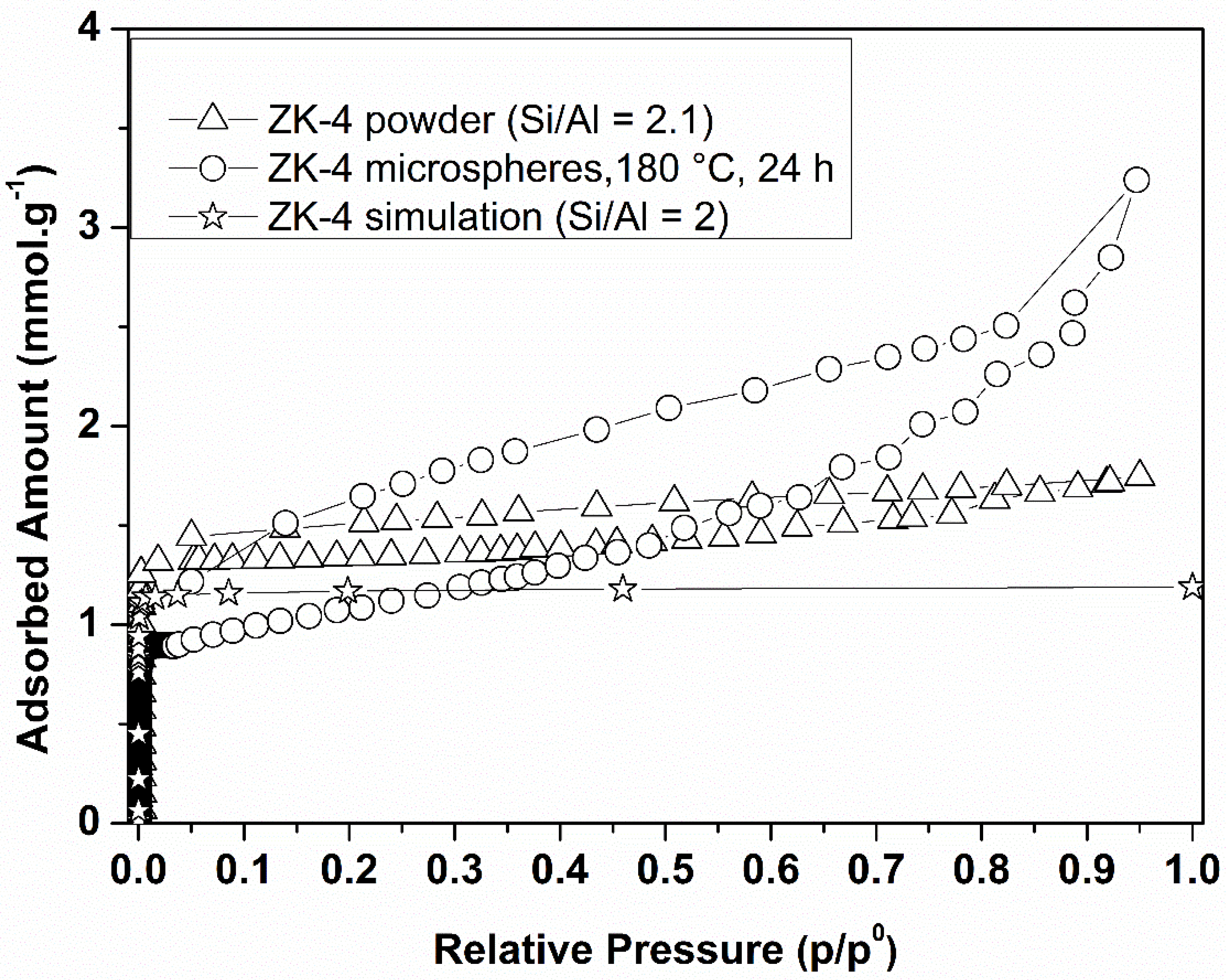
3.2. n-Hexane Adsorption Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cosseron, A.-F.; Daou, T.J.; Tzanis, L.; Nouali, H.; Deroche, I.; Coasne, B.; Tchamber, V. Adsorption of volatile organic compounds in pure silica CHA, *BEA, MFI and STT-type zeolites. Microporous Mesoporous Mater. 2013, 173, 147–154. [Google Scholar] [CrossRef]
- Kabalan, I.; Rioland, G.; Nouali, H.; Lebeau, B.; Rigolet, S.; Fadlallah, M.-B.; Daou, T.J. Synthesis of purely silica MFI-type nanosheets for molecular decontamination. RSC Adv. 2014, 4, 37353. [Google Scholar] [CrossRef]
- Kabalan, I.; Lebeau, B.; Fadlallah, M.-B.; Toufaily, J.; Hamieh, T.; Bellat, J.P.; Daou, T.J. Hierarchical Faujasite-Type Zeolite for Molecular Decontamination. J. Nanosci. Nanotechnol. 2016, 16, 9318–9322. [Google Scholar] [CrossRef]
- Kabalan, I.; Lebeau, B.; Nouali, H.; Toufaily, J.; Hamieh, T.; Koubaissy, B.; Daou, T.J. New generation of zeolite materials for environmental applications. J. Phys. Chem. C 2016, 120, 2688–2697. [Google Scholar] [CrossRef]
- den Exter, M.J.; Jansen, J.C.; van Bekkum, H.; Zikánova, A. Synthesis and characterization of the all-silica 8-ring Clathrasil DD3R comparison of adsorption properties with the hydrophilic zeolite A. Zeolites 1997, 19, 353–358. [Google Scholar] [CrossRef]
- Schick, J.; Daou, T.J.; Caullet, P.; Paillaud, J.-L.; Patarin, J.; Mangold-Callarec, C. Surfactant-modified MFI nanosheets: A high capacity anion-exchanger. Chem. Commun. 2011, 47, 902–904. [Google Scholar] [CrossRef] [PubMed]
- Daou, T.J.; Boltz, M.; Tzanis, L.; Michelin, L.; Louis, B. Gas-phase chlorination of aromatics over FAU- and EMT-type zeolites. Catal. Commun. 2013, 39, 10–13. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, X.; Wannapakdee, W.; Pestman, R.; Goesten, M.G.; Gao, L.; Hensen, E.J.M. A dual-templating synthesis strategy to hierarchical ZSM-5 zeolites as efficient catalysts for the methanol-to-hydrocarbons reaction. J. Catal. 2018, 361, 135–142. [Google Scholar] [CrossRef]
- Mostowicz, R.; Sand, L.B. Morphological study of ZSM—5 grown in the 12na2O/4.5(tpa)2O system. Zeolites 1983, 3, 219–225. [Google Scholar] [CrossRef]
- Rioland, G.; Daou, T.J.; Faye, D.; Patarin, J. A new generation of MFI-type zeolite pellets with very high mechanical performance for space decontamination. Microporous Mesoporous Mater. 2016, 221, 167–174. [Google Scholar] [CrossRef]
- Rioland, G.; Bullot, L.; Daou, T.J.; Simon-Masseron, A.; Chaplais, G.; Faye, D.; Patarin, J. Elaboration of FAU-type zeolite beads with good mechanical performances for molecular decontamination. RSC Adv. 2016, 6, 2470–2478. [Google Scholar] [CrossRef]
- Lauridant, N.; Daou, T.J.; Arnold, G.; Soulard, M.; Nouali, H.; Patarin, J.; Faye, D. Key steps influencing the formation of ZSM-5 films on aluminum substrates. Microporous Mesoporous Mater. 2012, 152, 1–8. [Google Scholar] [CrossRef]
- Lauridant, N.; Daou, T.J.; Arnold, G.; Nouali, H.; Patarin, J.; Faye, D. Zeolite hybrid films for space decontamination. Microporous Mesoporous Mater. 2013, 172, 36–43. [Google Scholar] [CrossRef]
- Lauridant, N.; Daou, T.J.; Arnold, G.; Patarin, J.; Faye, D. MFI/*BEA hybrid coating on aluminum alloys. Microporous Mesoporous Mater. 2013, 166, 79–85. [Google Scholar] [CrossRef]
- Anbia, M.; Koohsaryan, E.; Borhani, A. Novel hydrothermal synthesis of hierarchically-structured zeolite LTA microspheres. Mater. Chem. Phys. 2017, 193, 380–390. [Google Scholar] [CrossRef]
- Song, J.; Ren, L.; Yin, C.; Ji, Y.; Wu, Z.; Li, J.; Xiao, F.-S. Stable, porous, and bulky particles with high external surface and large pore volume from self-assembly of zeolite nanocrystals with cationic polymer. J. Phys. Chem. C 2008, 112, 8609–8613. [Google Scholar] [CrossRef]
- Wang, D.; Li, X.; Liu, Z.; Zhang, Y.; Xie, Z.; Tang, Y. Hierarchical structured ZSM-5 zeolite of oriented nanorods and its performance in the alkylation of phenol with isopropanol. J. Colloid Interface. Sci. 2010, 350, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Chal, R.; Gérardin, C.; Bulut, M.; van Donk, S. Overview and industrial assessment of synthesis strategies towards zeolites with mesopores. ChemCatChem 2011, 3, 67–81. [Google Scholar] [CrossRef]
- Li, K.; Valla, J.; Garcia-Martinez, J. Realizing the Commercial potential of hierarchical zeolites: New opportunities in catalytic cracking. ChemCatChem 2014, 6, 46–66. [Google Scholar] [CrossRef]
- Yang, H.; Yang, P.; Liu, X.; Wang, Y. Space-confined synthesis of zeolite Beta microspheres via steam-assisted crystallization. Chem. Eng. J. 2016, 299, 112–119. [Google Scholar] [CrossRef]
- Tosheva, L.; Valtchev, V.; Sterte, J. Silicalite-1 containing microspheres prepared using shape-directing macro-templates. Microporous Mesoporous Mater. 2000, 35–36, 621–629. [Google Scholar] [CrossRef]
- Mintova, S.; Gilson, J.-P.; Valtchev, V. Advances in nanosized zeolites. Nanoscale 2013, 5, 6693. [Google Scholar] [CrossRef] [PubMed]
- Zaarour, M.; Dong, B.; Naydenova, I.; Retoux, R.; Mintova, S. Progress in zeolite synthesis promotes advanced applications. Microporous Mesoporous Mater. 2014, 189, 11–21. [Google Scholar] [CrossRef]
- Zhang, B.; Davis, S.A.; Mann, S.; Mendelson, N.H. Bacterial templating of zeolite fibres with hierarchical structure. Chem. Commun. 2000, 0, 781–782. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Luo, C.; Liu, J.; Chen, B. Direct synthesis of hierarchical zeolites with oriented nanocrystals without adding extra templates. RSC Adv. 2013, 3, 6295. [Google Scholar] [CrossRef]
- Yin, C.; Tian, D.; Xu, M.; Wei, Y.; Bao, X.; Chen, Y.; Wang, F. One-step synthesis of hierarchical mesoporous zeolite Beta microspheres from assembly of nanocrystals. J. Colloid Interface Sci. 2013, 397, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Yilmaz, B.; Müller, U.; Bein, T. Hierarchical zeolite beta via nanoparticle assembly with a cationic polymer. Chem. Mater. 2011, 23, 4301–4310. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Y.; Zhu, L.; Rigutto, M.; van der Made, A.; Yang, C.; Xiao, F.-S. Highly mesoporous single-crystalline zeolite beta synthesized using a nonsurfactant cationic polymer as a dual-function template. J. Am. Chem. Soc. 2014, 136, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dong, X.; Zhu, Y.; Emwas, A.-H.; Zhang, D.; Tian, Q.; Han, Y. Investigating the influence of mesoporosity in zeolite beta on its catalytic performance for the conversion of methanol to hydrocarbons. ACS Catal. 2015, 5, 5837–5845. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, J.; Zhang, K.; Feng, W.; Liu, S.; Ding, C.; Liu, P. Nanocrystallite self-assembled hierarchical ZSM-5 zeolite microsphere for methanol to aromatics. Microporous Mesoporous Mater. 2017, 247, 103–115. [Google Scholar] [CrossRef]
- Chen, H.; Wydra, J.; Zhang, X.; Lee, P.S.; Wang, Z.; Fan, W.; Tsapatsis, M. Hydrothermal synthesis of zeolites with three-dimensionally ordered mesoporous-imprinted structure. J. Am. Chem. Soc. 2011, 133, 12390–12393. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Yang, H.; Zhang, Y.; Ren, J.; Liu, X.; Wang, Y.; Lu, G. Space-confined synthesis of nanorod oriented-assembled hierarchical MFI zeolite microspheres. J. Mater. Chem. A 2013, 1, 13821. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Shi, B.; Ren, J.; Liu, X.; Wang, Y.; Lu, G. Synthesis of hierarchical MFI zeolite microspheres with stacking nanocrystals. Microporous Mesoporous Mater. 2009, 117, 104–110. [Google Scholar] [CrossRef]
- Mańko, M.; Vittenet, J.; Rodriguez, J.; Cot, D.; Mendret, J.; Brosillon, S.; Galarneau, A. Synthesis of binderless zeolite aggregates (SOD, LTA, FAU) beads of 10, 70 μm and 1 mm by direct pseudomorphic transformation. Microporous Mesoporous Mater. 2013, 176, 145–154. [Google Scholar] [CrossRef]
- Yue, N.; Xue, M.; Qiu, S. Fabrication of hollow zeolite spheres using oil/water emulsions as templates. Inorg. Chem. Commun. 2011, 14, 1233–1236. [Google Scholar] [CrossRef]
- Chen, H.; Liu, X.; Ma, X. Confined synthesis of silicalite-1 hollow spheres with a lamellar shell. Scr. Mater. 2015, 95, 31–34. [Google Scholar] [CrossRef]
- Pashkova, V.; Tokarova, V.; Brabec, L.; Dedecek, J. Self-templating synthesis of hollow spheres of zeolite ZSM-5 from spray-dried aluminosilicate precursor. Microporous Mesoporous Mater. 2016, 228, 59–63. [Google Scholar] [CrossRef]
- Yan, B.; Zeng, C.; Yu, L.; Wang, C.; Zhang, L. Preparation of hollow zeolite NaA/chitosan composite microspheres via in situ hydrolysis-gelation-hydrothermal synthesis of TEOS. Microporous Mesoporous Mater. 2018, 257, 262–271. [Google Scholar] [CrossRef]
- Wang, Z.; Guan, J.; Wu, S.; Xu, C.; Ma, Y.; Lei, J.; Kan, Q. Preparation of uniform silicalite-1 microspheres with large secondary pore architecture using monodisperse porous polystyrene particles as template. Mater. Lett. 2010, 64, 1325–1327. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Ren, F.; Du, J.; Li, R. Hierarchical architectures of ZSM-5 nanocrystalline aggregates with particular catalysis for lager molecule reaction. Microporous Mesoporous Mater. 2017, 240, 22–30. [Google Scholar] [CrossRef]
- Sashkina, K.A.; Gurikov, P.A.; Ayupov, A.B.; Smirnova, I.; Parkhomchuk, E.V. Zeolite/silica aerogel composite monoliths and microspheres. Microporous Mesoporous Mater. 2018, 263, 106–112. [Google Scholar] [CrossRef]
- Tosheva, L.; Mihailova, B.; Valtchev, V.; Sterte, J. Zeolite beta spheres. Microporous Mesoporous Mater. 2001, 48, 31–37. [Google Scholar] [CrossRef]
- Yin, C.; Wei, Y.; Wang, F.; Chen, Y. Introduction of mesopority in zeolite ZSM-5 using resin as templates. Mater. Lett. 2013, 98, 194–196. [Google Scholar] [CrossRef]
- Engelhardt, G.; Michel, D. High-Resolution Solid-State NMR of Silicates and Zeolites; Wiley: New York, NY, USA, 1988. [Google Scholar]
- Massiot, D.; Fayon, F.; Capron, M.; King, I.; Le Calvé, S.; Alonso, B.; Hoatson, G. Modelling one- and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]
- Linstrom, P.J., Ed.; NIST Standard Reference Database Number 69. NIST Chem. WebBook. 2003. Available online: https://ci.nii.ac.jp/naid/10015264409/ (accessed on 16 May 2018).
- Guth, J.L.; Delmotte, L.; Soulard, M.; Brunard, B.; Joly, J.F.; Espinat, D. Synthesis of AI, Si-MFI-type zeolites in the presence of F− anions: Structural and physicochemical characteristics. Zeolites 1992, 12, 929–935. [Google Scholar] [CrossRef]
- Borel, M.; Dodin, M.; Daou, T.J.; Bats, N.; Harbuzaru, B.; Patarin, J. SDA-free hydrothermal synthesis of ultra-nanosized zeolite Y. Cryst. Growth Des. 2017, 17, 1173–1179. [Google Scholar] [CrossRef]
- Engelhardt, G.; Radeglia, R. A semi-empirical quantum-chemical rationalization of the correlation between SiOSi angles and 29Si NMR chemical shifts of silica polymorphs and framework aluminosilicates (zeolites). Chem. Phys. Lett. 1984, 108, 271–274. [Google Scholar] [CrossRef]
- Borel, M.; Dodin, M.; Daou, T.J.; Bats, N.; Patarin, J. Formation domain of SDA-free Y faujasite small crystals. New J. Chem. 2017, 41, 13260–13267. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds of ZK-4 beads are available from the authors. |





| Boiling Point (°C) | Vapor Pressure at 25 °C a (KPa) | Molar Volume at 25 °C (L·mol−1) | Kinetic Diameter b (Å) | |
|---|---|---|---|---|
| n-Hexane | 69 | ≈ 20.0 | 0.13198 | 4.3 |
| 150 °C, 24 h | 160 °C, 24 h | 170 °C, 24 h | 180 °C, 24 h | |
|---|---|---|---|---|
| SBET (m²·g−1) | 766 | 773 | 805 | 795 |
| Vmicro (cm3·g−1) | 0.28 | 0.28 | 0.29 | 0.29 |
| Vmeso (cm3·g−1) | 0.09 | 0.16 | 0.29 | 0.31 |
| Mesoporous diameter (nm) | 40–50 | 40–50 | 40–50 | 40–50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fawaz, E.G.; Salam, D.A.; Nouali, H.; Deroche, I.; Rigolet, S.; Lebeau, B.; Daou, T.J. Synthesis of Binderless ZK-4 Zeolite Microspheres at High Temperature. Molecules 2018, 23, 2647. https://doi.org/10.3390/molecules23102647
Fawaz EG, Salam DA, Nouali H, Deroche I, Rigolet S, Lebeau B, Daou TJ. Synthesis of Binderless ZK-4 Zeolite Microspheres at High Temperature. Molecules. 2018; 23(10):2647. https://doi.org/10.3390/molecules23102647
Chicago/Turabian StyleFawaz, Elyssa G., Darine A. Salam, Habiba Nouali, Irena Deroche, Severinne Rigolet, Benedicte Lebeau, and T. Jean Daou. 2018. "Synthesis of Binderless ZK-4 Zeolite Microspheres at High Temperature" Molecules 23, no. 10: 2647. https://doi.org/10.3390/molecules23102647
APA StyleFawaz, E. G., Salam, D. A., Nouali, H., Deroche, I., Rigolet, S., Lebeau, B., & Daou, T. J. (2018). Synthesis of Binderless ZK-4 Zeolite Microspheres at High Temperature. Molecules, 23(10), 2647. https://doi.org/10.3390/molecules23102647