Thermal Degradation Characteristic and Flame Retardancy of Polylactide-Based Nanobiocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
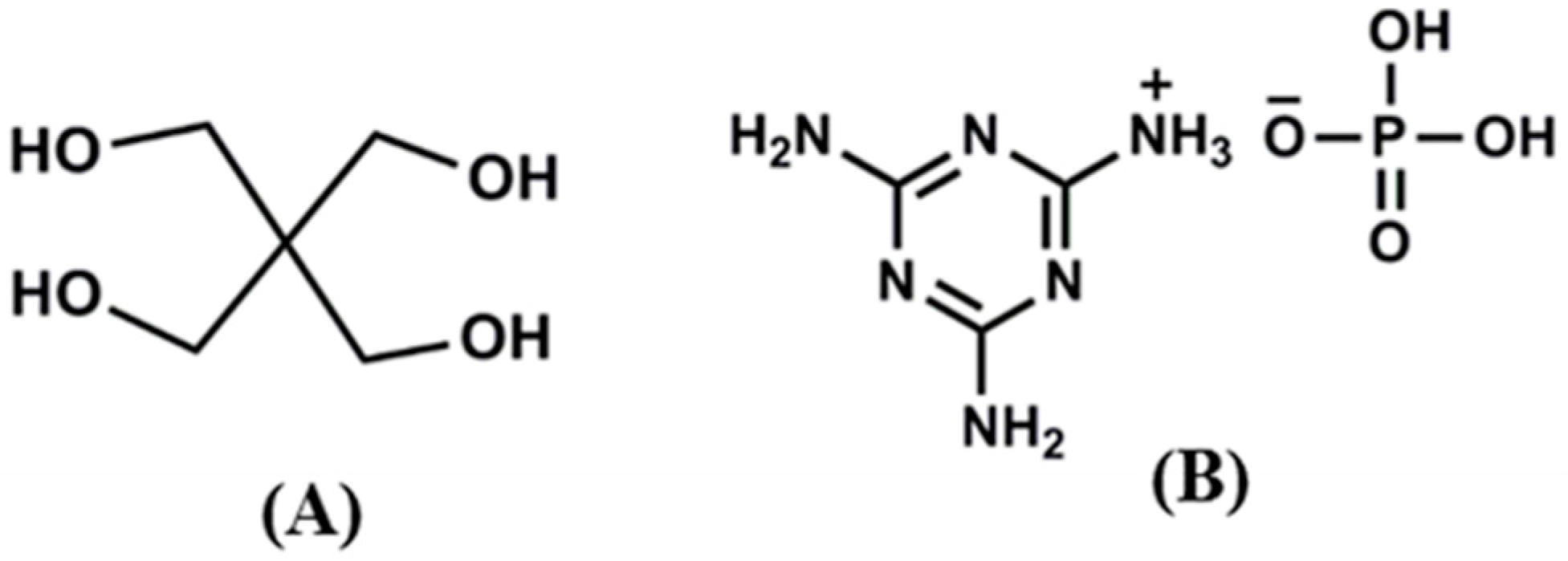
2.2. Synthesis of Melamine Phosphate
2.3. Surface Modification of MMT
2.4. Nanocomposite Processing
2.5. Characterization
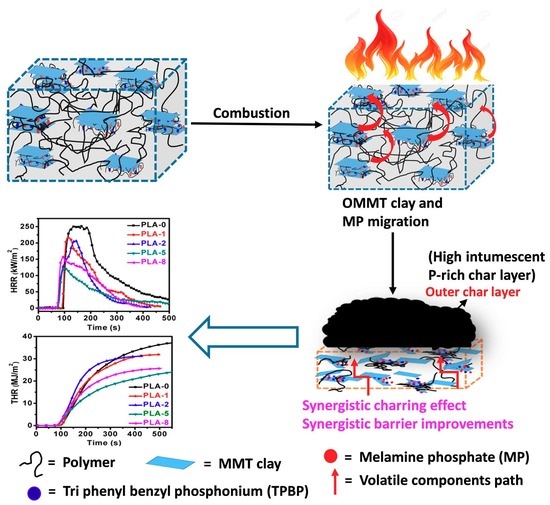
3. Results and Discussion
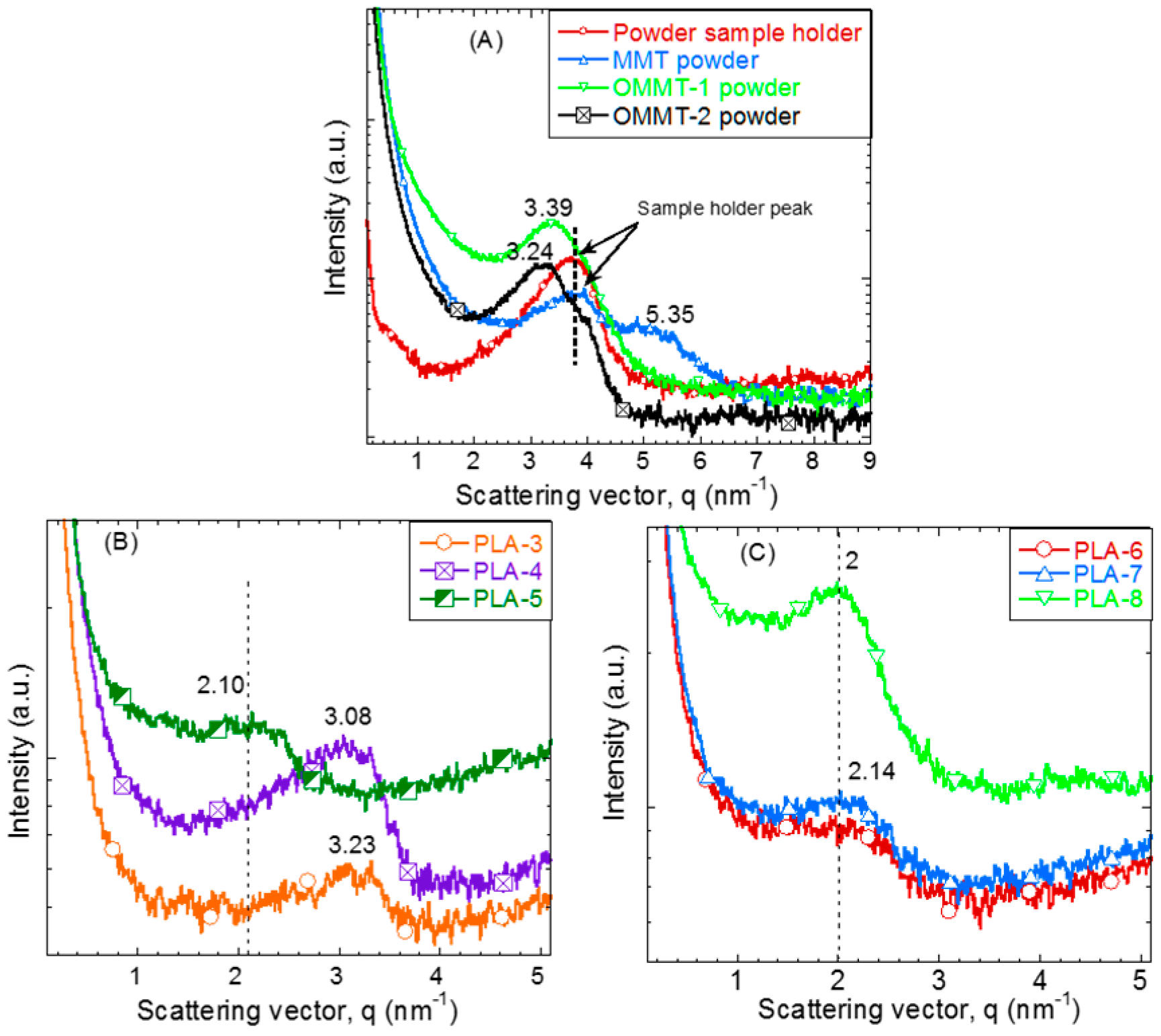
3.1. Structural and Morphological Analysis
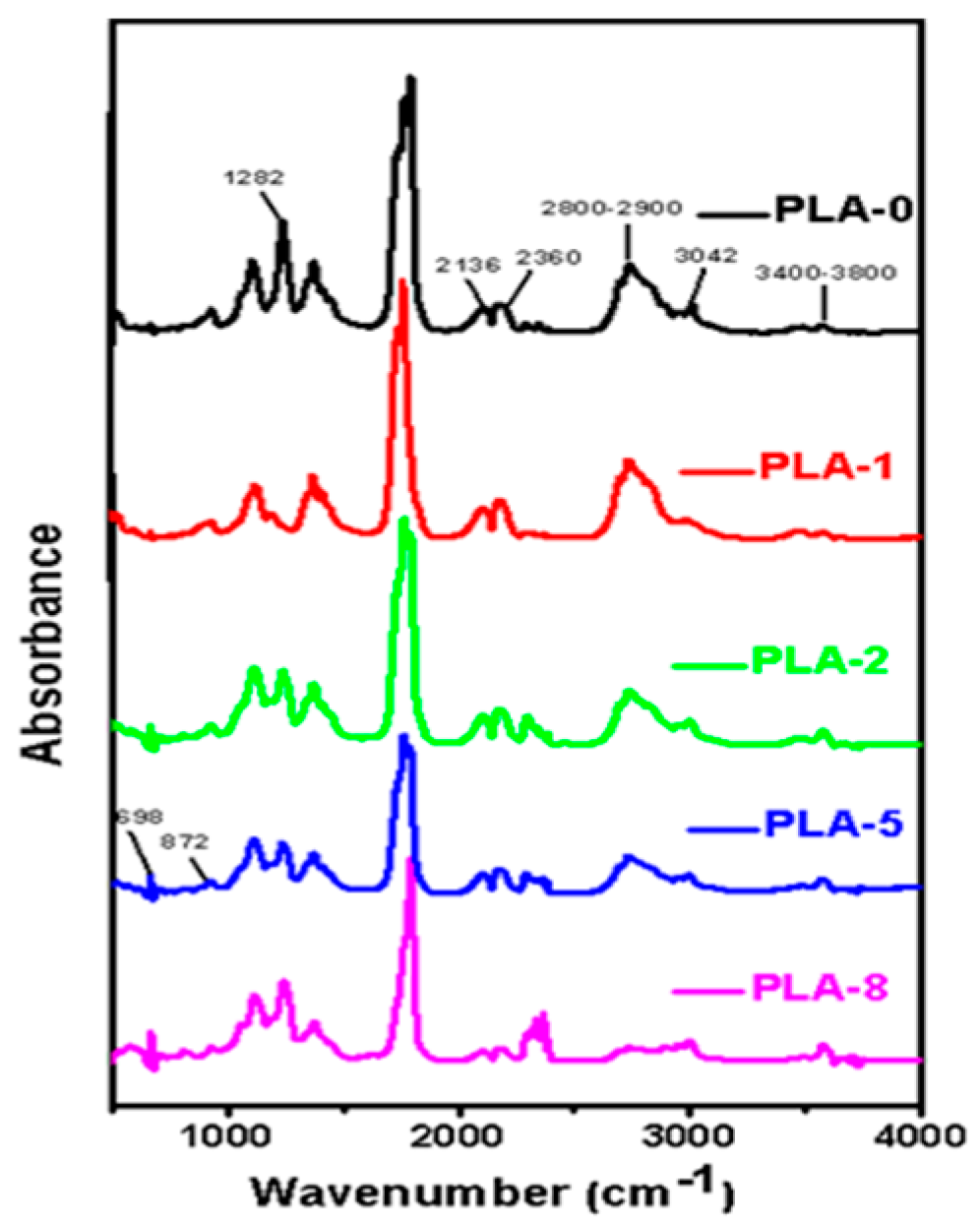
3.2. Thermal Degradation Characteristic
3.3. Volatile Gaseous Products of PLA Nanocomposites Analyzed by TG-FTIR
3.4. Cone Calorimetry Analysis
3.5. Analysis of Crosslinked Behavior of PLA Nanocomposites
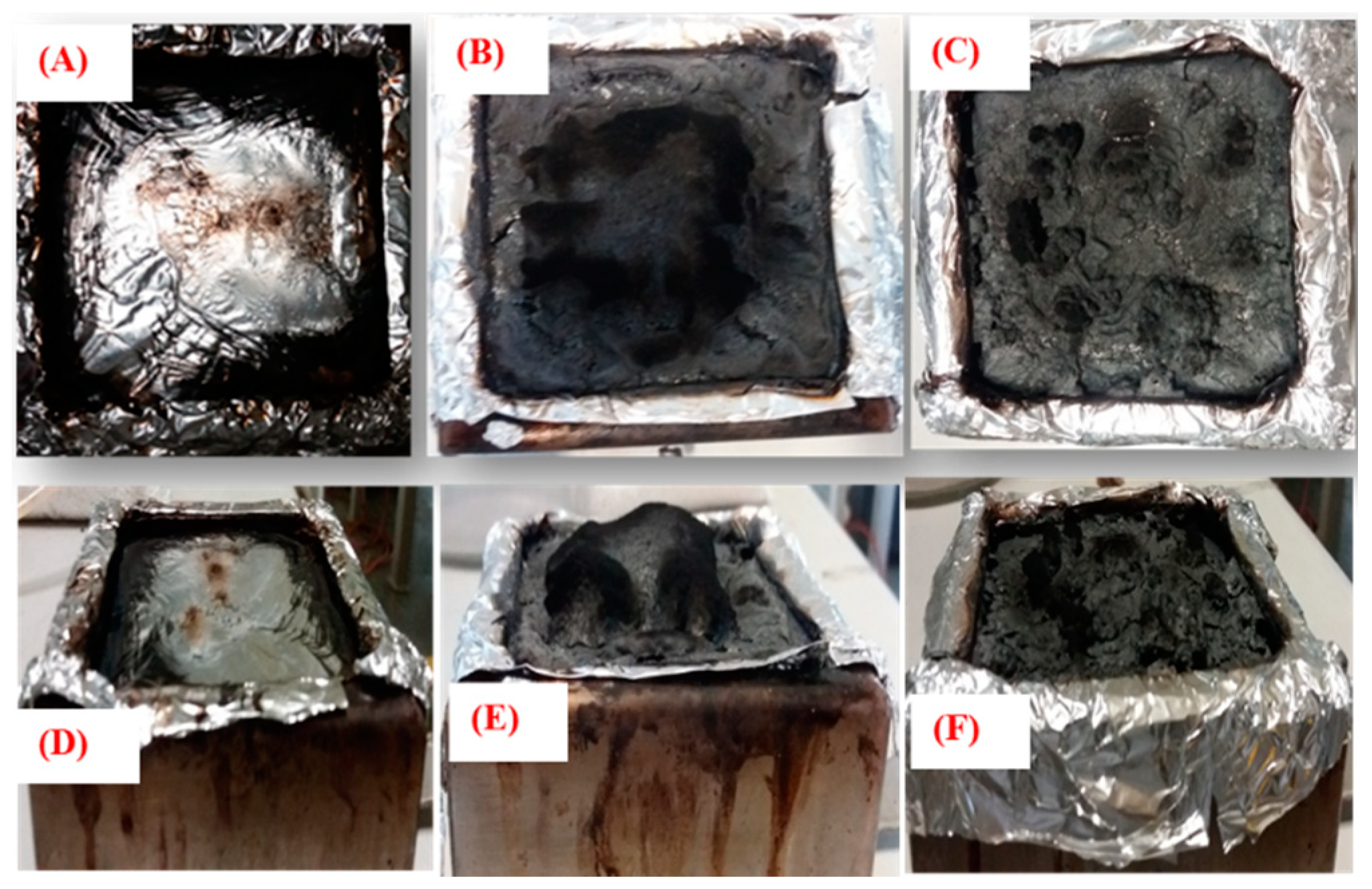
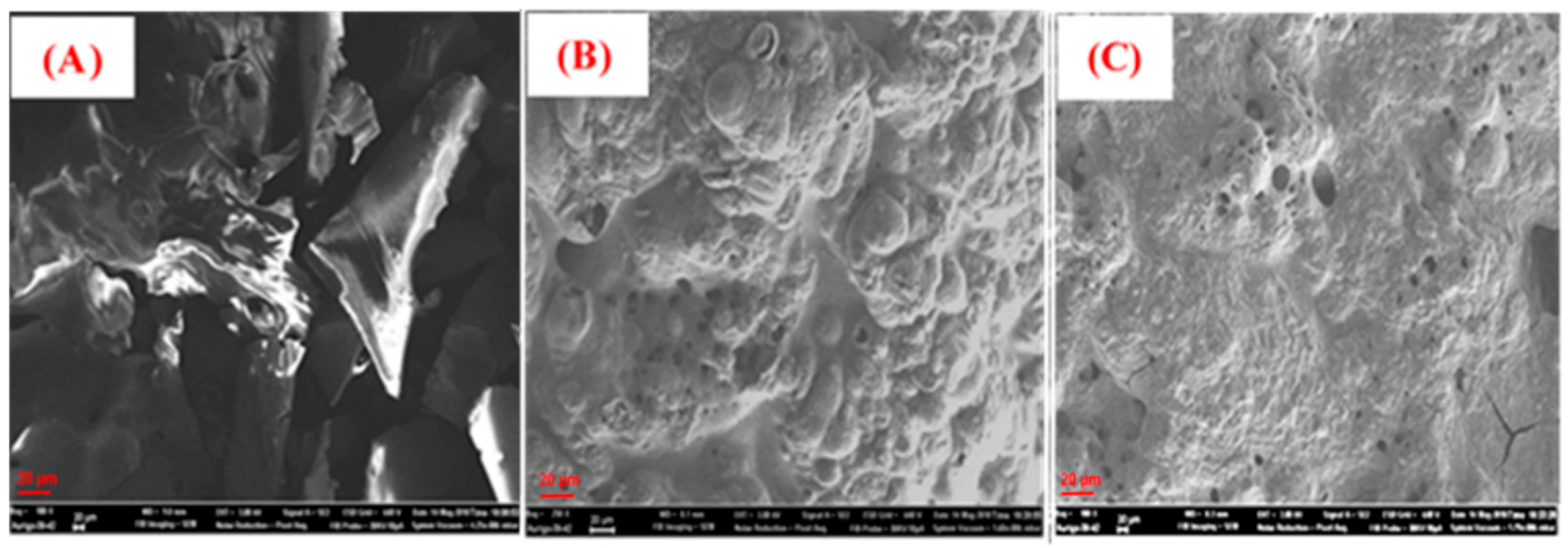
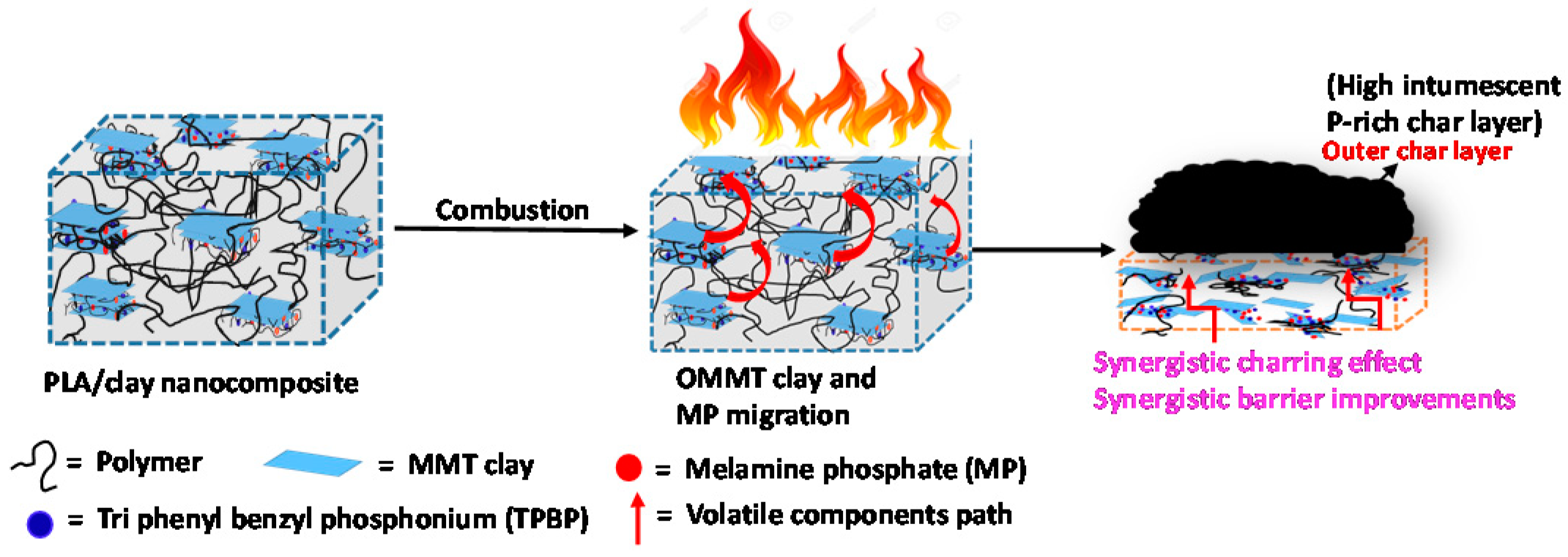
3.6. Char Residue Analysis of PLA Nanocomposites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ojijo, V.; Ray, S.S. Super toughned biodegradable polylactide blends with non-liner copolymer interfacial architecture obtained via facile in-situ reactive compatibilization. Polymer 2015, 80, 1–17. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Gohs, U.; Kang, N.-J.; Leuteritz, A.; Boldt, R.; Wagenknecht, U.; Hinrich, G. Method for simultaneously improving the thermal stability and mechanical properties of poly (lactic acid): Effect of high-energy electrons on the morphological, mechanical, and thermal properties of PLA/MMT nanocomposites. Langmuir 2012, 28, 12601–12608. [Google Scholar] [CrossRef] [PubMed]
- Matuana, L.M.; Diaz, C.A. Strategy to produce microcellular foamed poly (lactic acid)/wood-flour composites in a continuous extrusion process. Ind. Eng. Chem. Res. 2013, 52, 12032–12040. [Google Scholar] [CrossRef]
- Persson, M.; Lorite, G.S.; Cho, S.-W.; Tuukkanen, J.; Skrifvars, M. Melt spinning of poly (lactic acid) and hydroxyapatite composite fibers: Influence of the filler content on the fiber properties. ACS Appl. Mater. Interfaces 2013, 5, 6864–6872. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xie, L.; Chen, Y.-H.; Huang, H.-D.; Xu, J.-Z.; Zhong, G.-J.; Hsiao, B.S.; Li, Z.-M. Strong shear flow-driven simultaneous formation of classic shish-kebab, hybrid shish-kebab, and transcrystallinity in poly (lactic acid)/natural fiber biocomposites. ACS Sustain. Chem. Eng. 2013, 1, 1619–1629. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.-Z.; Mai, Y.-W.; Liu, S. Flame retardancy of highly filled polyamide 6/clay nanocomposites. Nanotechnology 2007, 18, 445602. [Google Scholar] [CrossRef]
- Zhao, F.; Bao, X.; McLauchlin, A.R.; Gu, J.; Wan, C.; Kandasubramanian, B. Effect of POSS on morphology and mechanical properties of polyamide 12/montmorillonite nanocomposites. Appl. Clay Sci. 2010, 47, 249–256. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, J.; Shi, Y.; Jiang, S.; Wang, D.; Hu, Y.; Gui, Z. MoS2 nanolayers grown on carbon nanotubes: An advanced reinforcement for epoxy composites. ACS Appl. Mater. Interfaces 2015, 7, 6070–6081. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ju, Y.; Liao, F.; Yang, Y.; Wang, X. Improve the mechanical property and flame retardant efficiency of the composites of poly (lactic acid) and resorcinol di (phenyl phosphate)(RDP) with ZnO-coated kenaf. Fire Mater. 2016, 40, 129–140. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, J.; Wen, P.; Hu, Y.; Gui, Z. A noncovalent functionalization approach to improve the dispersibility and properties of polymer/MoS2 composites. Appl. Surf. Sci. 2014, 316, 237–244. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, S.; Xing, W.; Yu, B.; Feng, X.; Song, L.; Hu, Y. Self-assembly of Ni–Fe layered double hydroxide/graphene hybrids for reducing fire hazard in epoxy composites. J. Mater. Chem. A 2013, 1, 4383–4390. [Google Scholar] [CrossRef]
- Wang, X.; Xuan, S.; Song, L.; Yang, H.; Lu, H.; Hu, Y. Synergistic effect of POSS on mechanical properties, flammability, and thermal degradation of intumescent flame retardant polylactide composites. J. Macromol. Sci. 2012, 51, 255–268. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, Q.; Li, J.; Tao, K.; Xue, L.; Yan, Q. Synergistic effect between expandable graphite and ammonium polyphosphate on flame retarded polylactide. Polym. Degrad. Stab. 2011, 96, 183–189. [Google Scholar] [CrossRef]
- Zanetti, M.; Camino, G.; Canavese, D.; Morgan, A.B.; Lamelas, F.J.; Wilkie, C.A. Fire retardant halogen- antimony- clay synergism in polypropylene layered silicate nanocomposites. Chem. Mater. 2002, 14, 189–193. [Google Scholar] [CrossRef]
- Jang, B.N.; Costache, M.; Wilkie, C.A. The relationship between thermal degradation behavior of polymer and the fire retardancy of polymer/clay nanocomposites. Polymer 2005, 46, 10678–10687. [Google Scholar] [CrossRef]
- He, X.; Zhang, W.; Yi, D.; Yang, R. Flame retardancy of ammonium polyphosphate–montmorillonite nanocompounds on epoxy resin. J. Fire Sci. 2016, 34, 212–225. [Google Scholar] [CrossRef]
- Bee, S.-T.; Hassan, A.; Ratnam, C.; Tee, T.-T.; Sin, L.T.; Hui, D. Dispersion and roles of montmorillonite on structural, flammability, thermal and mechanical behaviours of electron beam irradiated flame retarded nanocomposite. Compos. Part B 2014, 61, 41–48. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Duquesne, S.; Rochery, M. Recent advances for intumescent polymers. Macromol. Mater. Eng. 2004, 289, 499–511. [Google Scholar] [CrossRef]
- Levchik, S.; Balabanovich, A.; Levchik, G.; Costa, L. Effect of melamine and its salts on combustion and thermal decomposition of polyamide 6. Fire Mater. 1997, 21, 75–83. [Google Scholar] [CrossRef]
- Reti, C.; Casetta, M.; Duquesne, S.; Bourbigot, S.; Delobel, R. Flammability properties of intumescent PLA including starch and lignin. Polym. Adv. Technol. 2008, 19, 628–635. [Google Scholar] [CrossRef]
- Zhang, R.; Xiao, X.; Tai, Q.; Huang, H.; Yang, J.; Hu, Y. The effect of different organic modified montmorillonites (OMMTs) on the thermal properties and flammability of PLA/MCAPP/lignin systems. J. Appl. Polym. Sci. 2013, 127, 4967–4973. [Google Scholar] [CrossRef]
- Li, S.; Yuan, H.; Yu, T.; Yuan, W.; Ren, J. Flame-retardancy and anti-dripping effects of intumescent flame retardant incorporating montmorillonite on poly (lactic acid). Polym. Adv. Technol. 2009, 20, 1114–1120. [Google Scholar] [CrossRef]
- Lv, P.; Wang, Z.; Hu, K.; Fan, W. Flammability and thermal degradation of flame retarded polypropylene composites containing melamine phosphate and pentaerythritol derivatives. Polym. Degrad. Stab. 2005, 90, 523–534. [Google Scholar] [CrossRef]
- Pang, X.Y.; Tian, Y.; Weng, M.Q. Preparation of expandable graphite with silicate assistant intercalation and its effect on flame retardancy of ethylene vinyl acetate composite. Polym. Compos. 2015, 36, 1407–1416. [Google Scholar] [CrossRef]
- Jahromi, S.; Gabriëlse, W.; Braam, A. Effect of melamine polyphosphate on thermal degradation of polyamides: A combined X-ray diffraction and solid-state NMR study. Polymer 2003, 44, 25–37. [Google Scholar] [CrossRef]
- Zhu, J.; Morgan, A.B.; Lamelas, F.J.; Wilkie, C.A. Fire properties of polystyrene–clay nanocomposites. Chem. Mater. 2001, 13, 3774–3780. [Google Scholar] [CrossRef]
- Hato, M.J.; Choi, H.J.; Sim, H.H.; Park, B.O.; Ray, S.S. Magnetic carbonyl iron suspension with organoclay additive and its magnetorheological properties. Colloids Surf. A 2011, 377, 103–109. [Google Scholar] [CrossRef]
- Malkappa, K.; Rao, B.N.; Jana, T. Functionalized polybutadiene diol based hydrophobic, water dispersible polyurethane nanocomposites: Role of organo-clay structure. Polymer 2016, 99, 404–416. [Google Scholar] [CrossRef]
- Lai, S.-M.; Wu, S.-H.; Lin, G.-G.; Don, T.-M. Unusual mechanical properties of melt-blended poly (lactic acid)(PLA)/clay nanocomposites. Eur. Polym. J. 2014, 52, 193–206. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, P.; Hu, Y.; Hu, K. Thermal degradation study of intumescent flame retardants by TG and FTIR: Melamine phosphate and its mixture with pentaerythritol. J. Anal. Appl. Pyrolysis 2009, 86, 207–214. [Google Scholar] [CrossRef]
- Cicero, J.A.; Dorgan, J.R.; Dec, S.F.; Knauss, D.M. Phosphite stabilization effects on two-step melt-spun fibers of polylactide. Polym. Degrad. Stab. 2002, 78, 95–105. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.-R.; Peng, Y.-L.; Wang, D.; Yang, L.; Peng, H. Effect of reactive time on flame retardancy and thermal degradation behavior of bio-based zinc alginate film. Polym. Degrad. Stab. 2016, 127, 20–31. [Google Scholar] [CrossRef]
- Zhang, W.; He, X.; Song, T.; Jiao, Q.; Yang, R. The influence of the phosphorus-based flame retardant on the flame retardancy of the epoxy resins. Polym. Degrad. Stab. 2014, 109, 209–217. [Google Scholar] [CrossRef]
- Dong, Y.; Gui, Z.; Hu, Y.; Wu, Y.; Jiang, S. The influence of titanate nanotube on the improved thermal properties and the smoke suppression in poly (methyl methacrylate). J. Hazard. Mater. 2012, 209, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Price, D.; Liu, Y.; Hull, T.R.; Milnes, G.J.; Kandola, B.K.; Horrocks, A.R. Burning behaviour of foam/cotton fabric combinations in the cone calorimeter. Polym. Degrad. Stab. 2002, 77, 213–220. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Gentiluomo, S.; Veca, A.D.; Monti, M.; Zaccone, M.; Zanetti, M. Fire behavior of polyamide 12 nanocomposites containing POSS and CNT. Polym. Degrad. Stab. 2016, 134, 151–156. [Google Scholar] [CrossRef]
- Salehiyan, R.; Malwela, T.; Ray, S.S. Thermo-oxidative degradation study of melt-processed polyethylene and its blend with polyamide using time-resolved rheometry. Polym. Degrad. Stab. 2017, 139, 130–137. [Google Scholar] [CrossRef]
- Filippone, G.; Carroccio, S.; Curcuruto, G.; Passaglia, E.; Gambarotti, C.; Dintcheva, N.T. Time-resolved rheology as a tool to monitor the progress of polymer degradation in the melt state–Part II: Thermal and thermo-oxidative degradation of polyamide 11/organo-clay nanocomposites. Polymer 2015, 73, 102–110. [Google Scholar] [CrossRef]
Sample Availability: Samples of the nanocomposites are available from the authors. |






| Sample Name | Composition | PLA | IFR (MP:PER/1:1) | Pristine Clay | OMMT-1 | OMMT-2 |
|---|---|---|---|---|---|---|
| PLA-0 | PLA | 100 | - | - | - | - |
| PLA-1 | PLA/IFR | 86 | 14 | - | - | - |
| PLA-2 | PLA/IFR/MMT | 86 | 9 | 5% | - | - |
| PLA-3 | PLA/IFR/OMMT-1 | 86 | 13 | - | 1% | - |
| PLA-4 | PLA/IFR/OMMT-1 | 86 | 11 | - | 3% | - |
| PLA-5 | PLA/IFR/OMMT-1 | 86 | 9 | - | 5% | - |
| PLA-6 | PLA/IFR/OMMT-2 | 86 | 13 | - | - | 1% |
| PLA-7 | PLA/IFR/OMMT-2 | 86 | 11 | - | - | 3% |
| PLA-8 | PLA/IFR/OMMT-2 | 86 | 9 | - | - | 5% |
| Samples | N2 Atmosphere | O2 Atmosphere | ||||||
|---|---|---|---|---|---|---|---|---|
| Tdeg (°C) | TMax (°C) | Char at 800 °C (%) | αmax (%/°C) | Tdeg (°C) | TMax (°C) | Char at 800 °C (%) | αmax (%/°C) | |
| PLA-0 | 301.7 | 380.5 | 0.5 | 3.0 | 304.7 | 378.7 | 1.7 | 2.6 |
| PLA-1 | 195.7 | 401.2 | 4.3 | 1.6 | 192.3 | 395.7 | 4.5 | 1.8 |
| PLA-2 | 190.7 | 395.0 | 5.8 | 1.8 | 196.5 | 398.9 | 2.4 | 1.9 |
| PLA-4 | 192.4 | 392.7 | 8.6 | 1.2 | 194.4 | 389.9 | 11.2 | 1.8 |
| PLA-5 | 191.5 | 387.5 | 9.9 | 1.0 | 193.5 | 381.5 | 18.2 | 1.0 |
| PLA-7 | 194.7 | 383.6 | 7.1 | 1.8 | 191.3 | 378.5 | 2.6 | 2.1 |
| PLA-8 | 193.5 | 371.4 | 10.4 | 1.0, 0.0 | 190.8 | 379.8 | 6.0 | 1.0, 0.8 |
| Samples | pHRR (kW·m−2) | TTI (s) | tPHRR (s) | THR (MJ·m−2) | FGI (kW/(m2·s) | FPI (m2s/kW) | TSR (m2/m2) | TSP (m2) | Residue (wt.%) |
|---|---|---|---|---|---|---|---|---|---|
| PLA-0 | 253.7 | 92.7 | 142.2 | 37.2 | 1.8 | 0.4 | 295.9 | 37.3 | 1.3 |
| PLA-1 | 216.7 | 88.6 | 126.9 | 31.9 | 1.7 | 0.4 | 204.9 | 31.9 | 11.2 |
| PLA-2 | 205.5 | 72.5 | 139.1 | 31.1 | 1.5 | 0.4 | 118.2 | 31.2 | 16.2 |
| PLA-3 | 161.2 | 75.9 | 121.9 | 28.1 | 1.3 | 0.5 | 24.1 | 27.0 | 11.2 |
| PLA-4 | 154.2 | 76.7 | 118.2 | 26.9 | 1.3 | 0.5 | 21.1 | 26.5 | 16.8 |
| PLA-5 | 120.6 | 73.2 | 109.5 | 25.6 | 1.1 | 0.6 | 09.4 | 21.4 | 22.9 |
| PLA-6 | 181.7 | 77.0 | 115.6 | 26.5 | 1.6 | 0.4 | 125.6 | 29.8 | 09.8 |
| PLA-7 | 164.1 | 70.9 | 84.1 | 25.9 | 1.9 | 0.4 | 98.3 | 25.1 | 13.8 |
| PLA-8 | 163.4 | 69.9 | 89.4 | 24.0 | 1.8 | 0.4 | 102.4 | 27.5 | 15.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malkappa, K.; Bandyopadhyay, J.; Ray, S.S. Thermal Degradation Characteristic and Flame Retardancy of Polylactide-Based Nanobiocomposites. Molecules 2018, 23, 2648. https://doi.org/10.3390/molecules23102648
Malkappa K, Bandyopadhyay J, Ray SS. Thermal Degradation Characteristic and Flame Retardancy of Polylactide-Based Nanobiocomposites. Molecules. 2018; 23(10):2648. https://doi.org/10.3390/molecules23102648
Chicago/Turabian StyleMalkappa, Kuruma, Jayita Bandyopadhyay, and Suprakas Sinha Ray. 2018. "Thermal Degradation Characteristic and Flame Retardancy of Polylactide-Based Nanobiocomposites" Molecules 23, no. 10: 2648. https://doi.org/10.3390/molecules23102648