Extraction, Purification, and Hydrolysis Behavior of Apigenin-7-O-Glucoside from Chrysanthemum Morifolium Tea
Abstract
:1. Introduction
2. Results and Discussion
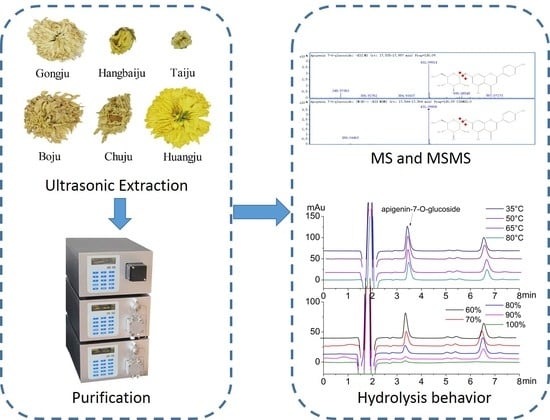
2.1. Screening the Appropriate Chrysanthemum Morifolium Cultivar
2.2. Optimization of Ultrasound Conditions
2.3. Analysis and Purification Of Apigenin-7-O-Glucoside from C. Morifolium ‘Huangju’
2.4. Hydrolysis Behavior
2.5. Antioxidant Activities
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. Optimization of Ultrasound Conditions
3.4. Analysis and Purification of Apigenin-7-O-Glucoside by Preparative HPLC
3.5. Analysis with Q-TOF/MS
3.6. Hydrolysis Behavior of Apigenin-7-O-Glucoside
3.7. In-Vitro Antioxidant Activities
3.7.1. ABTS Radical Scavenging Activity
3.7.2. DPPH Radical Scavenging Activity
3.7.3. Metal Chelating Activity
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, F.; Ong, E.S.; Li, S.F. A green and effective approach for characterisation and quality control of chrysanthemum by pressurized hot water extraction in combination with HPLC with UV absorbance detection. Food Chem. 2013, 141, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Guzelmeric, E.; Vovk, I.; Yesilada, E. Development and validation of an HPTLC method for apigenin 7-O-glucoside in chamomile flowers and its application for fingerprint discrimination of chamomile-like materials. J. Pharm. Biomed. Anal. 2015, 107, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Beninger, C.W.; Hall, J.C. Allelopathic activity of luteolin 7-O-β-glucuronide isolated from Chrysanthemum morifolium L. Biochem. Syst. Ecol. 2005, 33, 103–111. [Google Scholar] [CrossRef]
- Avula, B.; Wang, Y.H.; Wang, M.; Avonto, C.; Zhao, J.; Smillie, T.J.; Rua, D.; Khan, I.A. Quantitative determination of phenolic compounds by UHPLC-UV-MS and use of partial least-square discriminant analysis to differentiate chemo-types of Chamomile/Chrysanthemum flower heads. J. Pharm. Biomed. 2014, 88, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Bolzon, L.B.; Dos Santos, J.S.; Silva, D.B.; Crevelin, E.J.; Moraes, L.A.; Lopes, N.P.; Assis, M.D. Apigenin-7-O-glucoside oxidation catalyzed by P450-bioinspired systems. J. Inorg. Biochem. 2017, 170, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhou, M.; Wang, L.; Ying, X.; Peng, J.; Jiang, M.; Bai, G.; Luo, G. Comparative evaluation of different cultivars of Flos Chrysanthemi by an anti-inflammatory-based NF-kappaB reporter gene assay coupled to UPLC-Q/TOF MS with PCA and ANN. J. Ethnopharmacol. 2015, 174, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Nasr Bouzaiene, N.; Chaabane, F.; Sassi, A.; Chekir-Ghedira, L.; Ghedira, K. Effect of apigenin-7-glucoside, genkwanin and naringenin on tyrosinase activity and melanin synthesis in B16F10 melanoma cells. Life Sci. 2016, 144, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Samet, I.; Villareal, M.O.; Motojima, H.; Han, J.; Sayadi, S.; Isoda, H. Olive leaf components apigenin 7-glucoside and luteolin 7-glucoside direct human hematopoietic stem cell differentiation towards erythroid lineage. Differentiation 2015, 89, 146–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmastas, M.; Demir, A.; Genc, N.; Dolek, U.; Gunes, M. Changes in flavonoid and phenolic acid contents in some Rosa species during ripening. Food Chem. 2017, 235, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Uehara, A.; Nakata, M.; Uchida, A.; Iwashina, T. Chemotaxonomic consideration of flavonoids from the leaves of Chrysanthemum arcticum subsp. arcticum and yezoense, and related species. Biochem. Syst. Ecol. 2017, 73, 11–15. [Google Scholar] [CrossRef]
- Wang, M.; Avula, B.; Wang, Y.H.; Zhao, J.; Avonto, C.; Parcher, J.F.; Raman, V.; Zweigenbaum, J.A.; Wylie, P.L.; Khan, I.A. An integrated approach utilising chemometrics and GC/MS for classification of chamomile flowers, essential oils and commercial products. Food Chem. 2014, 152, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Qu, W.; Ma, H.; Atungulu, G.G.; McHugh, T.H. Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason. Sonochem. 2012, 19, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Z.; Harnly, J.M. Identification of the phenolic components of chrysanthemum flower (Chrysanthemum morifolium Ramat). Food Chem. 2010, 120, 319–326. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, X.; Li, J.; Liu, P.; Yang, Y.; Ni, Y. Preparative isolation and purification of cuminaldehyde and p-menta-1,4-dien-7-al from the essential oil of Cuminum cyminum L. by high-speed counter-current chromatography. Anal. Chim. Acta 2011, 689, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Guzelmeric, E.; Ristivojevic, P.; Vovk, I.; Milojkovic-Opsenica, D.; Yesilada, E. Quality assessment of marketed chamomile tea products by a validated HPTLC method combined with multivariate analysis. J. Pharm Biomed. Anal. 2017, 132, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Pereira, O.R.; Silva, A.M.S.; Domingues, M.R.M.; Cardoso, S.M. Identification of phenolic constituents of Cytisus multiflorus. Food Chem. 2012, 131, 652–659. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, A.; Zhou, X.; Liu, Q.; Nan, Y.; Guan, Y.; Kong, L.; Han, Y.; Sun, H.; Yan, G. An integrated chinmedomics strategy for discovery of effective constituents from traditional herbal medicine. Sci. Rep. 2016, 6, 18997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, B.; Hu, Z.; Li, H.; Yan, C.; Zhang, L. Simultaneous determination of six flavonoids from Paulownia tomentosa flower extract in rat plasma by LC-MS/MS and its application to a pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 978–979, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Bergantin, C.; Maietti, A.; Cavazzini, A.; Pasti, L.; Tedeschi, P.; Brandolini, V.; Marchetti, N. Bioaccessibility and HPLC-MS/MS chemical characterization of phenolic antioxidants in Red Chicory (Cichorium intybus). J. Funct. Foods 2017, 33, 94–102. [Google Scholar] [CrossRef]
- Chen, L.; Wu, X.; Chen, X.D. Comparison between the digestive behaviors of a new in vitro rat soft stomach model with that of the in vivo experimentation on living rats—Motility and morphological influences. J. Food Eng. 2013, 117, 183–192. [Google Scholar] [CrossRef]
- Li, Y.; Bao, T.; Chen, W. Comparison of the protective effect of black and white mulberry against ethyl carbamate-induced cytotoxicity and oxidative damage. Food Chem. 2018, 243, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, Y.; Duan, X.; Tang, T.; Shen, Y.; Hu, B.; Liu, A.; Chen, H.; Li, C.; Liu, Y. Characterization and antioxidant activities of polysaccharides from thirteen boletus mushrooms. Int. J. Biol. Macromol. 2018, 113, 1–7. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds apigenin-7-O-glucoside and apigenin are available from the authors. |




| No. | RT | Formula | [M – H]− | Score | MS/MS | Identification |
|---|---|---|---|---|---|---|
| 1 | 4.833 | C16H18O9 | 353.08785 | 97.69 | 191.05644, 248.97382, 112.98560 | Chlorogenic acid |
| 2 | 11.442 | C21H20O11 | 447.09376 | 87.93 | 285.03972 | Luteolin-7-O-glucoside |
| 3 | 17.242 | C25H24O12 | 515.12035 | 95.93 | 353.08736, 179.03450, 173.04544, 135.04501, 191.05577 | 3,5-dicaffeoylquinic acid |
| 4 | 19.575 | C21H20O10 | 431.09920 | 95.44 | 268.03810, 269.04341 | Apigenin-7-O-Glucoside |
| 5 | 21.275 | C21H18O11 | 445.07743 | 79.28 | 269.04514, 113.02422 | Apigenin-7-O-glucuronide |
| 6 | 22.208 | C24H22O14 | 533.09396 | 94.85 | 489.10472, 285.04009 | Luteolin-7-O-6”-malonylglucoside |
| 7 | 32.767 | C15H10O5 | 269.04627 | 94.96 | 117.03469, 151.00383, 149.02439 | Apigenin |
| ± | ABTS (EC50) | DPPH (EC50) | FI (EC50) |
|---|---|---|---|
| Apigenin-7-O-glucoside | 5.49 ± 0.74 a | / | / |
| Apigenin | 0.68 ± 0.01 b | / | / |
| Glucose | / | / | / |
| BHT | 0.17 ± 0.00 b | 0.41 ± 0.01 a | / |
| Ascorbic acid | 0.12 ± 0.00 b | 0.11 ± 0.00 b | / |
| Rutin | 0.52 ± 0.10 b | 0.52 ± 0.07 a | / |
| EDTA | / | / | 0.32 ± 0.03 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xu, Z.; Huang, Y.; Wen, X.; Wu, Y.; Zhao, Y.; Ni, Y. Extraction, Purification, and Hydrolysis Behavior of Apigenin-7-O-Glucoside from Chrysanthemum Morifolium Tea. Molecules 2018, 23, 2933. https://doi.org/10.3390/molecules23112933
Wang Y, Xu Z, Huang Y, Wen X, Wu Y, Zhao Y, Ni Y. Extraction, Purification, and Hydrolysis Behavior of Apigenin-7-O-Glucoside from Chrysanthemum Morifolium Tea. Molecules. 2018; 23(11):2933. https://doi.org/10.3390/molecules23112933
Chicago/Turabian StyleWang, Yuxiao, Zhenzhen Xu, Yuqi Huang, Xin Wen, Yue Wu, Yuhan Zhao, and Yuanying Ni. 2018. "Extraction, Purification, and Hydrolysis Behavior of Apigenin-7-O-Glucoside from Chrysanthemum Morifolium Tea" Molecules 23, no. 11: 2933. https://doi.org/10.3390/molecules23112933