Halogen and Hydrogen Bonding Interplay in the Crystal Packing of Halometallocenes
Abstract
:1. Introduction
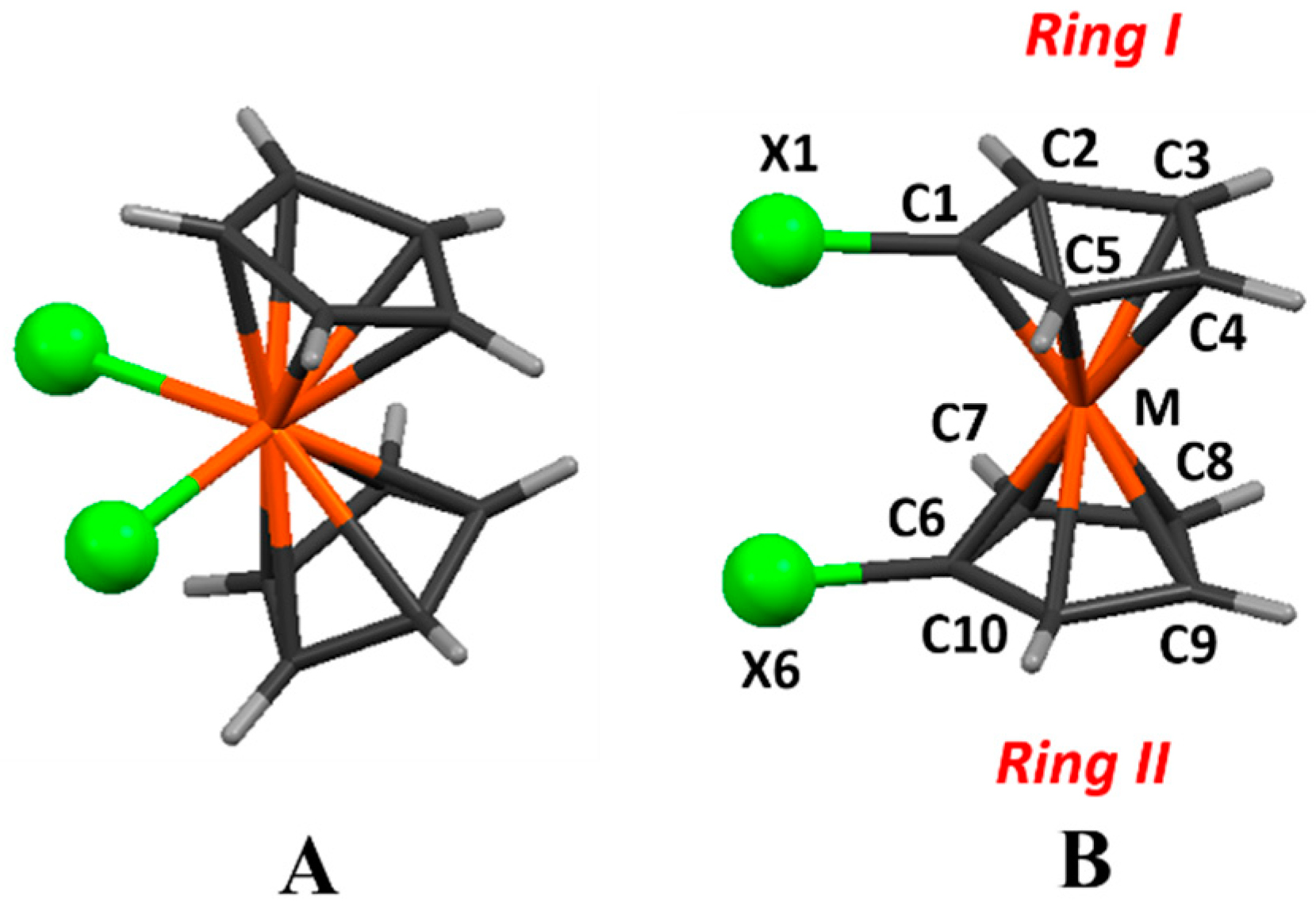
2. Results
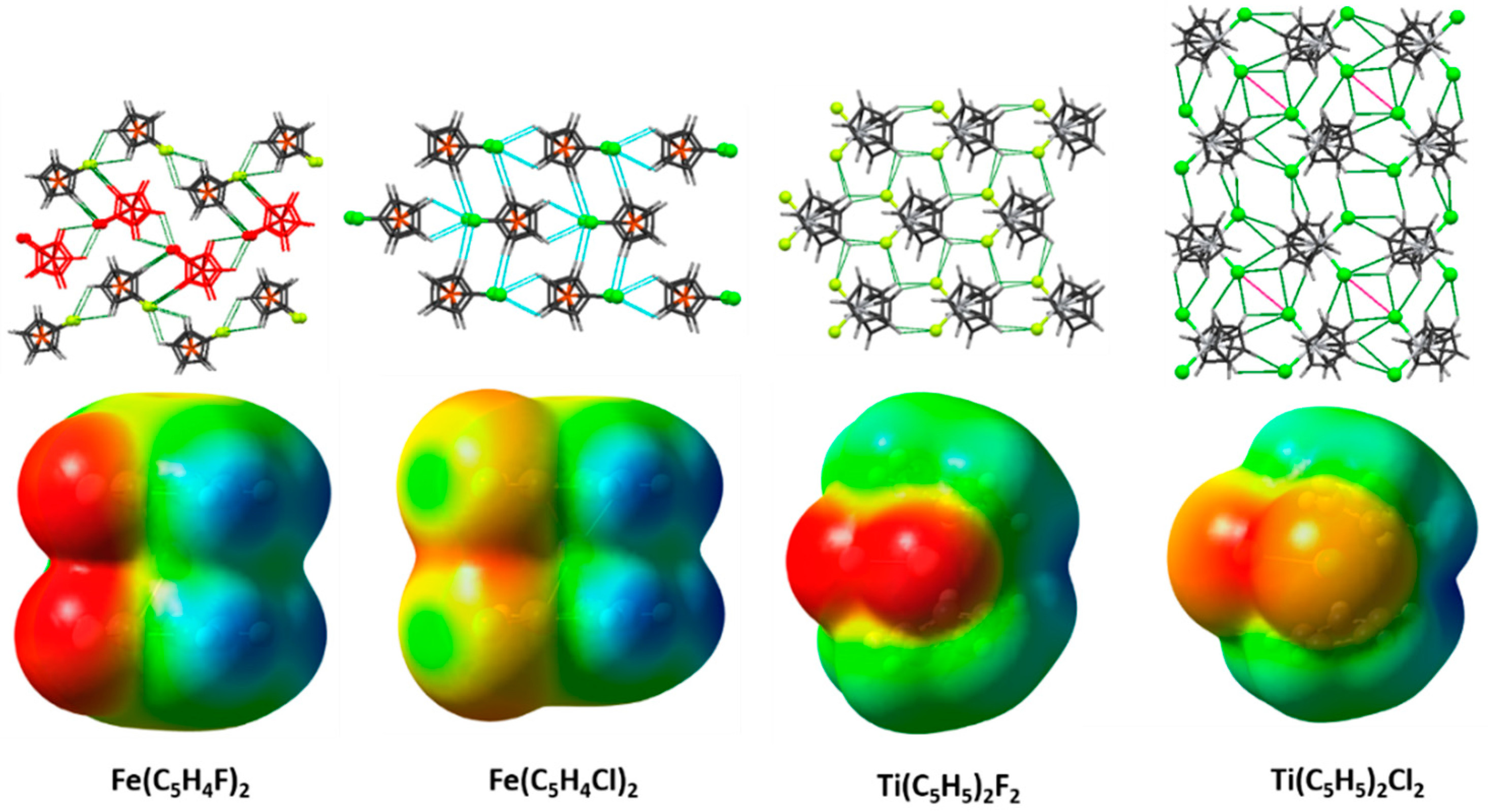
2.1. Electrostatic Potentials by DFT Calculations
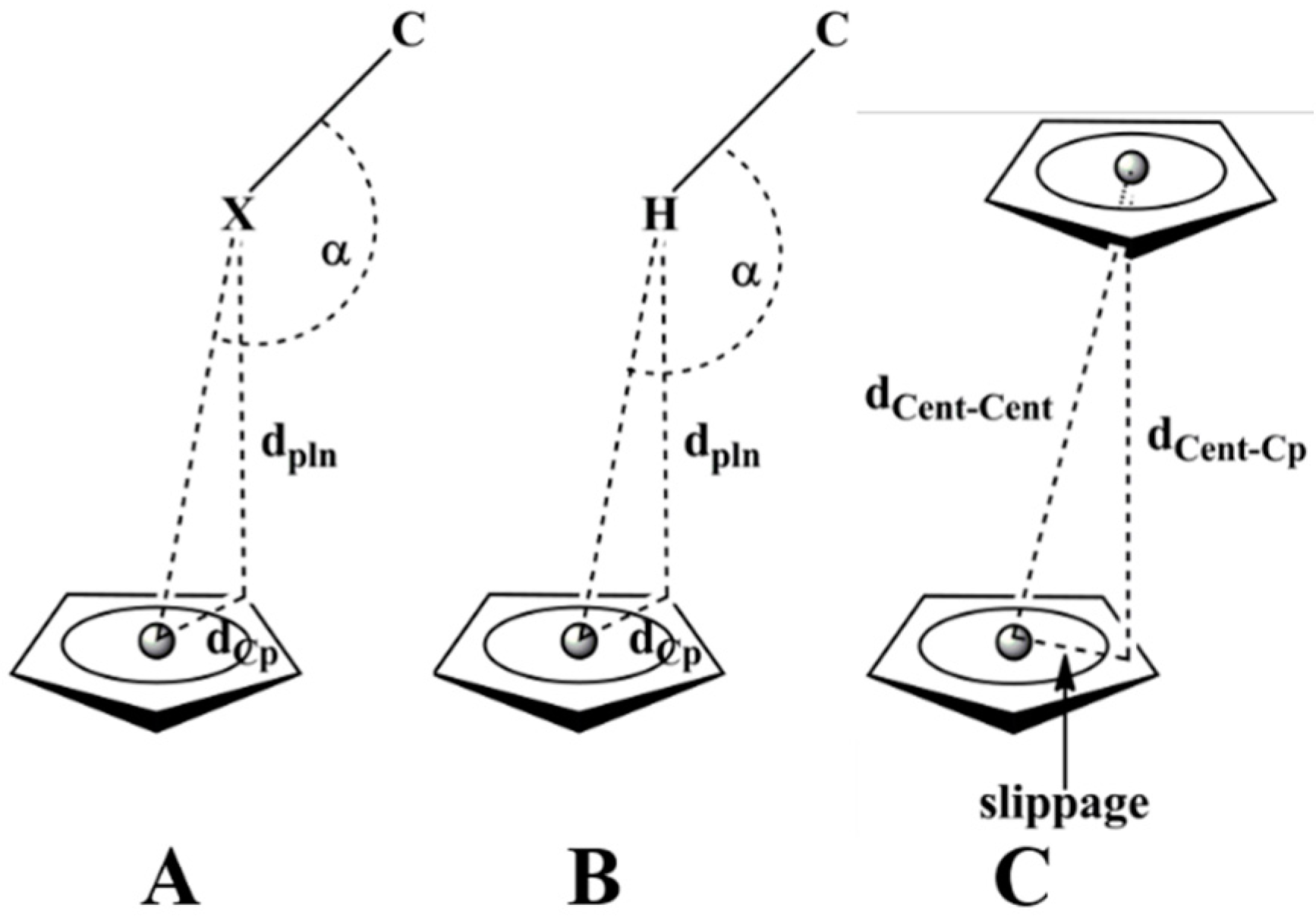
2.2. Analysis of Interactions Recurring to Hirshfeld Surfaces
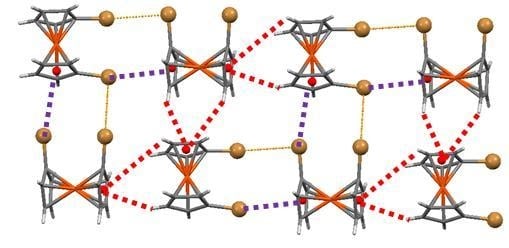
2.3. Supramolecular Arrangements
2.4. 1,1′-Difluoroferrocene and 1,1′-Dichloroferrocene
2.5. 1,1′-Dibromoferrocene
2.6. 1,1′-Diiodoferrocene
2.7. 1,1′-Diiodoruthenocene
3. Discussion
4. Methods
4.1. X-ray Crystallographic Analysis
4.2. Ab Initio Calculations
4.3. Hirshfeld Surface Calculations
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: Oxford, UK, 1997; pp. 1–6. ISBN 0195095499. [Google Scholar]
- Keesom, W.H. Van der Waals attractive force. Physik. Z. 1921, 22, 129–141. [Google Scholar]
- Debye, P. Van der Waals’ cohesion forces. Physik. Z. 1920, 21, 178–187. [Google Scholar]
- London, F. The general theory of molecular forces. Trans. Faraday Soc. 1937, 33, 8b. [Google Scholar] [CrossRef]
- Latimer, W.M.; Rodebush, W.H. Polarity and Ionization from the standpoint of the Lewis Theory of valence. J. Am. Chem. Soc. 1920, 42, 1419–1433. [Google Scholar] [CrossRef]
- Huggins, M.L. Electronic Structures of Atoms. J. Phys. Chem. 1922, 26, 601–625. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the chemical bond. II. The one-electron bond and the three-electron bond. J. Am. Chem. Soc. 1931, 53, 1367–1400. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry, 3rd ed.; Cornell University Press: Ithaca, NY, USA, 1939; p. 449. [Google Scholar]
- Pimentel, G.C.; McClellan, A.L. The Hydrogen Bond; W.H. Freeman: San Francisco, SF, USA, 1960; p. 3. [Google Scholar]
- Steiner, T.; Saenger, W. Role of C-H⋯O hydrogen bonds in the coordination of water molecules. Analysis of neutron diffraction data. J. Am. Chem. Soc. 1993, 115, 4540–4547. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; IUCR, Oxford University Press: Oxford, UK, 1999; pp. 1–19. ISBN 0198509707. [Google Scholar]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Defining the hydrogen bond: An account (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 1619–1636. [Google Scholar] [CrossRef] [Green Version]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef] [Green Version]
- Borissova, A.O.; Antipin, M.Y.; Perekalin, D.S.; Lyssenko, K.A. Crucial role of Ru⋯H interactions in the crystal packing of ruthenocene and its derivatives. CrystEngComm 2008, 10, 827–832. [Google Scholar] [CrossRef]
- Wilkinson, G.; Birmingham, J.M. Bis-cyclopentadienyl Compounds of Ti, Zr, V, Nb and Ta. J. Am. Chem. Soc. 1954, 76, 4281–4284. [Google Scholar] [CrossRef]
- Ferreira da Silva, J.L.; Shimizu, K.; Duarte, M.T. The role of halogen interactions in the crystal structure of biscyclopentadienyl dihalides. CrystEngComm 2017, 19, 2802. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Parthasarathy, R. The nature of halogen⋯halogen interactions: Are short halogen contacts due to specific attractive forces or due to close packing of nonspherical atoms? J. Am. Chem. Soc. 1989, 111, 8725–8726. [Google Scholar] [CrossRef]
- Metrangolo, P.; Neukirch, H.; Pilati, T.; Resnatti, G. Halogen Bonding Based Recognition Processes: A World Parallel to Hydrogen Bonding. Acc. Chem. Res. 2005, 38, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Panikkattu, S.; Chopade, P.D.; Desper, J. Competing hydrogen-bond and halogen-bond donors in crystal engineering. CrystEngComm 2013, 15, 3125–3136. [Google Scholar] [CrossRef] [Green Version]
- Dunitz, J.D.; Taylor, R. Organic Fluorine Hardly Ever Accepts Hydrogen Bonds. Chem. Eur. J. 1997, 3, 89–98. [Google Scholar] [CrossRef]
- Rathore, R.S.; Karthikeyan, N.S.; Alekhya, Y.; Sathiyanarayanan, K.; Aravindan, F.G. The role of weak intermolecular C-H⋯F interactions in supramolecular assembly: Structural investigations on 3,5-dibenzylidene-piperidin-4-one and database analysis. J. Chem. Sci. 2011, 123, 403–409. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond: In Structural Chemistry and Biology; IUCR, Oxford University Press: Oxford, UK, 1999; pp. 202–224. ISBN 0198509707. [Google Scholar]
- Sakurai, T.; Sundaralingam, M.; Jeffrey, G.A. A nuclear quadrupole resonance and X-ray study of the crystal structure of 2,5-di-chloroaniline. Acta Crystallogr. 1963, 16, 354–363. [Google Scholar] [CrossRef]
- Zefirov, Y.V. Characteristic features of various intermolecular contacts x⋯x in crystals. J. Struct. Chem. 1981, 22, 307–309. [Google Scholar] [CrossRef]
- Zefirov, Y.V.; Porai-Koshits, M.A. Geometry of halogen-halogen specific interactions in organic crystals. J. Struct. Chem. 1986, 27, 239–244. [Google Scholar] [CrossRef]
- Bui, T.T.T.; Dahaoui, S.; Lecomte, C.; Desiraju, G.R.; Espinosa, E. The Nature of Halogen⋯Halogen Interactions: A Model Derived from Experimental Charge-Density Analysis. Angew. Chem. 2009, 121, 3896–3899. [Google Scholar] [CrossRef]
- Saha, B.K.; Nangia, A.; Nicoud, J. Using Halogen⋯Halogen Interactions to Direct Noncentrosymmetric Crystal Packing in Dipolar Organic Molecules. Cryst. Growth Des. 2006, 6, 1278–1280. [Google Scholar] [CrossRef]
- Tothadi, S.; Joseph, S.; Desiraju, G.R. Synthon Modularity in Cocrystals of 4-Bromobenzamide with n-Alkanedicarboxylic Acids: Type I and Type II Halogen⋯Halogen Interactions. Cryst. Growth Des. 2013, 13, 3242–3254. [Google Scholar] [CrossRef]
- Mukherjee, A.; Desiraju, G.R. Halogen bonds in some dihalogenated phenols: Applications to crystal engineering. IUCrJ 2014, 1, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef] [Green Version]
- Metrangolo, P.; Resnati, G. Type II halogen···halogen contacts are halogen bonds. IUCrJ 2014, 1, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, P.; Resnati, G. Halogen Bonding: A Paradigm in Supramolecular Chemistry. Chem. Eur. J. 2001, 7, 2511–2519. [Google Scholar] [CrossRef]
- Clark, T.; Henneman, M.; Murray, J.S.; Politzer, P. Halogen bonding: The sigma-hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Organic fluorine compounds: A great opportunity for enhanced materials properties. Chem. Soc. Rev. 2011, 40, 3496–3508. [Google Scholar] [CrossRef] [PubMed]
- Marushima, Y.; Uchiumi, Y.; Ogu, K.; Hori, A. Intermolecular π-stacking and F⋯F interactions of fluorine-substituted meso-alkynylporphyrin. Acta Crystallogr. Sect. C 2010, 66, o406–o409. [Google Scholar] [CrossRef] [PubMed]
- Brinck, T.; Murray, J.S.; Politzer, P. Surface electrostatic potentials of halogenated methanes as indicators of directional intermolecular interactions. Int. J. Quantum Chem. Quantum Biol. Symp. 1992, 44, 57–64. [Google Scholar] [CrossRef]
- Wilcken, R.; Zimmermann, M.O.; Lange, A.; Joerger, A.C.; Boeckler, F.M. Principles and Applications of Halogen Bonding in Medicinal Chemistry and Chemical Biology. J. Med. Chem. 2013, 56, 1363–1388. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, P.; Resnati, G. Halogen Bonding: Fundamentals and Applications. Struct. Bond. 2008, 126, 17. [Google Scholar]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.S.; Lane, P.; Politzer, P. A predicted new type of directional noncovalent interaction. Int. J. Quantum Chem. 2007, 107, 3046–3052. [Google Scholar] [CrossRef]
- Murray, J.S.; Clark, T.; Lane, P.; Politzer, P. σ-hole bonding: Molecules containing group VI atoms. J. Mol. Model. 2007, 13, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Lane, P.; Politzer, P. Expansion of the σ-hole concept. J. Mol. Model. 2009, 15, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, P.; Meyer, F.; Pilati, T.; Resnati, G.; Terraneo, G. Halogen bonding in supramolecular chemistry. Angew. Chem. Int. Ed. 2008, 47, 6114–6127. [Google Scholar] [CrossRef] [PubMed]
- Auffinger, P.; Hays, F.A.; Westhof, E.; Ho, P.S. Halogen bonds in biological molecules. Proc. Natl. Acad. Sci. USA 2004, 101, 16789–16794. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Tothadi, S.; Desiraju, G.R. Halogen Bonds in Crystal Engineering: Like Hydrogen Bonds yet Different. Acc. Chem. Res. 2014, 47, 2514–2524. [Google Scholar] [CrossRef] [PubMed]
- Bertani, R.; Sgarbossa, P.; Venzo, A.; Lelj, F.; Amati, M.; Resnati, G.; Pilati, T.; Metrangolo, P.; Terraneo, G. Halogen bonding in metal–organic–supramolecular networks. Coord. Chem. Rev. 2010, 254, 677–695. [Google Scholar] [CrossRef]
- Pennington, W.T.; Resnati, G.; Taylor, M.S. Halogen bonding: From self-assembly to materials and biomolecules. CrystEngComm 2013, 15, 3057. [Google Scholar] [CrossRef]
- Metrangolo, P.; Pilati, T.; Resnati, G. Halogen bonding and other noncovalent interactions involving halogens: A terminology issue. CrystEngComm 2006, 8, 946–947. [Google Scholar] [CrossRef]
- Dumele, O.; Trapp, N.; Diederich, F. Halogen Bonding Molecular Capsules. Angew. Chem. Int. Ed. 2015, 54, 12339–12344. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zang, S.-Q.; Wang, L.-Y.; Mak, T.C.W. Halogen bonding: A. powerful, emerging tool for constructing high-dimensional metal-containing supramolecular networks. Coord. Chem. Rev. 2016, 308, 1–21. [Google Scholar] [CrossRef]
- Imai, Y.N.; Inoue, Y.; Yamamoto, Y. Propensities of Polar and Aromatic Amino Acids in Noncanonical Interactions: Nonbonded Contacts Analysis of Protein−Ligand Complexes in Crystal Structures. J. Med. Chem. 2007, 50, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Matter, H.; Nazare, M.; Gussregen, S.; Will, D.; Schreuder, H.; Bauer, A.; Urmann, M.; Ritter, K.; Wagner, M.; Wehner, V. Evidence for C-Cl/C-Br⋯π Interactions as an Important Contribution to Protein–Ligand Binding Affinity. Angew. Chem. Int. Ed. 2009, 48, 2911–2916. [Google Scholar] [CrossRef] [PubMed]
- Girão, E.C.; Liebold-Ribeiro, Y.; Batista, J.A.; Barros, E.B.; Fagan, S.B.; Mendes Filho, J.; Dresselhaus, M.S.; Souza Filho, A.G. Functionalization of single-wall carbon nanotubes through chloroform adsorption: Theory and experiment. Phys. Chem. Chem. Phys. 2010, 12, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Brown, P.A.; Lu, J.; Shuford, K.L. Electronic Properties of Halogen-Adsorbed Graphene. J. Phys. Chem. C 2015, 119, 17271–17277. [Google Scholar] [CrossRef]
- Riley, K.E.; Hobza, P. Investigations into the Nature of Halogen Bonding Including Symmetry Adapted Perturbation Theory Analyses. J. Chem. Theory Comput. 2008, 4, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Wallnoefer, H.; Fox, T.; Liedl, K.; Tautermann, C.S. Dispersion dominated halogen–π interactions: Energies and locations of mínima. Phys. Chem. Chem. Phys. 2010, 12, 14941–14949. [Google Scholar] [CrossRef] [PubMed]
- Forni, A.; Pieraccini, S.; Rendine, S.; Gabas, F.; Sironi, M. Halogen-Bonding Interactions with π Systems: CCSD(T), MP2, and DFT Calculations. ChemPhysChem 2012, 13, 4224–4234. [Google Scholar] [CrossRef] [PubMed]
- Forni, A.; Pieraccini, S.; Rendine, S.; Sironi, M. Halogen bonds with benzene: An assessement of DFT functionals. J. Comput. Chem. 2014, 35, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Youn, I.S.; Kim, D.Y.; Cho, W.J.; Madridejos, J.M.L.; Lee, H.M.; Kolaski, M.; Lee, J.; Baig, C.; Shin, S.K.; Filatov, M.; et al. Halogen-π Interactions between Benzene and X2/CX4 (X = Cl, Br): Assessment of Various Density Functionals with Respect to CCSD(T). J. Phys. Chem. A 2016, 120, 9305–9314. [Google Scholar] [CrossRef] [PubMed]
- Kodama, Y.; Nishihata, K.; Nishio, M.; Nakagawa, N. Attractive interaction between aliphatic and aromatic systems. Tetrahedron Lett. 1977, 2105–2108. [Google Scholar] [CrossRef]
- Tamres, M. Aromatic Compounds as Donor Molecules in Hydrogen Bonding. J. Am. Chem. Soc. 1952, 74, 3375–3378. [Google Scholar] [CrossRef]
- Nishio, M.; Umezawa, Y.; Honda, K.; Tsuboyama, S.; Suezawa, H. CH/π hydrogen bonds in organic and organometallic chemistry. CrystEngComm 2009, 11, 1757–1788. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; Tedesco, E. X-H⋯π (X = O, N, C) Hydrogen Bonds in Organometallic Crystals. Organometallics 1998, 17, 2669–2672. [Google Scholar] [CrossRef]
- Sakaki, S.; Kato, K.; Miyazaki, T.; Musashi, Y.; Ohkubo, K.; Ihara, H.; Hirayama, C. Structures and binding energies of benzene—Methane and benzene—Benzene complexes. An ab initio SCF/MP2 study. J. Chem. Soc. Faraday Trans. 1993, 89, 659–664. [Google Scholar] [CrossRef]
- Akazome, M.; Hirabayashi, A.; Senda, K.; Ogura, K. Inclusion compounds of l,d-dipeptide with small sulfoxides: Flexible sheet structure of (S)-phenylglycyl-(R)-phenylglycine. Tetrahedron 2007, 63, 9933–9938. [Google Scholar] [CrossRef]
- Anderson, C.D.; Dudding, T.; Gordillo, R.; Houk, K.N. Origin of Enantioselection in Hetero-Diels−Alder Reactions Catalyzed by Naphthyl-TADDOL. Org. Lett. 2008, 10, 2749–2752. [Google Scholar] [CrossRef] [PubMed]
- Harigai, M.; Kataoka, M.; Imamoto, Y. A Single CH/π Weak Hydrogen Bond Governs Stability and the Photocycle of the Photoactive Yellow Protein. J. Am. Chem. Soc. 2006, 128, 10646–10647. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M. The CH/π hydrogen bond in chemistry. Conformation, supramolecules, optical resolution and interactions involving carbohydrates. Phys. Chem. Chem. Phys. 2011, 13, 13873–13900. [Google Scholar] [CrossRef] [PubMed]
- Ferreira da Silva, J.L.; Harjivan, S.G.; Ferreira, A.P.; Shimizu, K.; Marques, M.M.; Duarte, M.T. Effect of substituents in the molecular and supramolecular architectures of 1-ferrocenyl-2-(aryl)thioethanones. CrystEngComm 2015, 17, 3089. [Google Scholar] [CrossRef]
- Choudhury, R.R.; Chitra, R. Stacking interaction between homostacks of simple aromatics and the factors influencing these interactions. CrystEngComm 2010, 12, 2113–2121. [Google Scholar] [CrossRef]
- Mignon, P.; Loverix, S.; Geerlings, P. Interplay between π-π interactions and the H-bonding ability of aromatic nitrogen bases. Chem. Phys. Lett. 2005, 401, 40–46. [Google Scholar] [CrossRef]
- Trifan, D.S.; Bacskai, R. Metal/hydrogen bonding in metallocene conompounds. J. Am. Chem. Soc. 1960, 82, 5010. [Google Scholar] [CrossRef]
- Roe, D.M.; Bailey, P.M.; Mosely, K.; Maitlis, P.M. Structure of bromobis(triphenylphosphine)-(1,2,3,4-tetrakismethoxycarbonylbuta-1,3-dienyl)palladium and evidence for a C–H⋯Pd interaction. J. Chem. Soc. Chem. Commun. 1972, 1273. [Google Scholar] [CrossRef]
- Cerichelli, G.; Illuminati, G.; Ortaggi, G.; Giuliani, A. The behaviour of ferrocene and ruthenocene in weakly to strongly protic media. Implications on the mechanism of substitutions involving proton as the electrophile. J. Organomet. Chem. 1977, 127, 357. [Google Scholar] [CrossRef]
- Drago, R.S.; Norazi, M.S.; Klinger, R.J.; Chamberlain, C. Quantitative data on some oxidative addition reactions and on the Lewis basicity of bis(triphenylphosphine)carbonylchloroiridium(I) Ir(I)[(C6H5)3P]2(CO)Cl. Inorg. Chem. 1979, 18, 1254. [Google Scholar] [CrossRef]
- Yoshida, T.; Tani, K.; Yamagata, T.; Tatsuno, Y.; Saito, T. Preparation and structure of [Rh{(η5-C5H4(2-C5H4N))(η5-C5H4PPh2)}(cod)]PF6 and [Ir(H){Fe[η5-C5H3(2-C5H4N)](η5-C5H4PPh2)}(cod)]PF6; a RhI complex having a C–H⋯RhI interaction and a hydrido IrIII complex (where cod = cyclo-octa-1,5-diene). J. Chem. Soc. Chem. Comm. 1990, 292–294. [Google Scholar] [CrossRef]
- Brammer, L. Metals and hydrogen bonds. DaltonTrans. 2003, 16, 3145–3157. [Google Scholar] [CrossRef]
- Shubina, E.S.; Epstein, L.M. Regularities in formation of intramolecular hydrogen bonds with the metal atom Part I. α-Metallocenylcarbinols of the iron subgroup. J. Mol. Struct. 1992, 265, 367–384. [Google Scholar] [CrossRef]
- Shubina, E.S.; Krylov, A.N.; Kreindlin, A.Z.; Rybinskaya, M.I.; Epstein, L. Intermolecular hydrogen bonds with d-electrons of transition metal atoms. H-complexes with metallocenes of the iron subgroup. J. Mol. Struct. 1993, 301, 1–5. [Google Scholar] [CrossRef]
- Epstein, L.M.; Krylov, A.N.; Shubina, E.S. Novel types of hydrogen bonds involving transition metal atoms and proton transfer (XH⋯M, [MH]+⋯B, [MH]+⋯A−). J. Mol. Struct. 1994, 322, 345–352. [Google Scholar] [CrossRef]
- Bruno, G.; Lanza, S.; Nicolò, F. Structure of [Pt(C6H5)2(btz-N,N′)].CHCl3, btz = 2,2′-bi-5,6-dihydro-4H-1,3-thiazine. Acta Crystallogr. Sect. C 1990, 46, 765–767. [Google Scholar] [CrossRef]
- Brammer, L.; Charnock, J.M.; Goggin, P.L.; Goodfellow, R.J.; Orpen, A.G.; Koetzle, T. The role of transition metal atoms as hydrogen bond acceptors: A neutron diffraction study of [NPrn4]2[PtCl4]·cis-[PtCl2(NH2Me)2] at 20 K. J. Chem. Soc. Dalton Trans. 1991, 7, 1789–1798. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; Tedesco, E. Hydrogen Bonding in Organometallic Crystals. 6. X−H⋯M Hydrogen Bonds and M⋯(H−X) Pseudo-Agostic Bonds. Organometallics 1997, 16, 1846–1856. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inkpen, M.S.; Du, S.; Hildebrand, M.; White, A.J.P.; Harrison, N.M.; Albrecht, T.; Long, N.J. The Unusual Redox Properties of Fluoroferrocenes Revealed through a Comprehensive Study of the Haloferrocenes. Organometallics 2015, 34, 5461–5469. [Google Scholar] [CrossRef] [Green Version]
- Hnetinka, C.A.; Hunter, A.D.; Zeller, M.; Lesley, M.J.G. 1,1′-Di-bromo-ferrocene. Acta Crystallogr. Sect. E 2004, 60, m1806. [Google Scholar] [CrossRef]
- Roemer, M.; Nijhuis, C.A. Syntheses and purification of the versatile synthons iodoferrocene and 1,1′-diiodoferrocene. Dalton Trans. 2014, 43, 11815–11818. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.; Konatschnig, S.; Morard, S.; Pierroz, V.; Ferrari, S.; Spingler, B.; Gasser, G. Novel, Mercury-Free Synthetic Pathway for Trifluoromethylthio-Substituted Metallocenes. Inorg. Chem. 2014, 53, 3662–3667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dance, I. Distance criteria for crystal packing analysis of supramolecular motifs. New J. Chem. 2003, 27, 22–27. [Google Scholar] [CrossRef]
- Glaser, R.; Murphy, R.F.; Sui, Y.; Barnes, C.L.; Kim, S.H. Multifurcated halogen bonding involving Ph–Cl⋯H-CPh=N-R′ interactions and its relation to idioteloamphiphile layer architecture. CrystEngComm 2006, 8, 372–376. [Google Scholar] [CrossRef]
- Anthony, A.; Desiraju, G.R.; Jetti, R.K.R.; Kuduva, S.S.; Madhavi, N.N.L.; Nangia, A.; Thaimattam, R.; Thalladi, V.R. Crystal Engineering: Some Further Strategies. Cryst. Eng. 1998, 1, 1–18. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03; revision C.05; Gaussian Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef]
- Wedig, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-Adjusted Pseudopotentials for Transition-Metal Elements. In Quantum Chemistry: The Challenge of Transition Metals and Coordination Chemistry; Veillard, A., Ed.; Springer: Dordrecht, The Netherlands, 1986; Volume 176, pp. 79–89. ISBN 978-90-277-2237-9. [Google Scholar]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row atoms. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Petersson, G.A.; Al-Laham, M.A. A complete basis set model chemistry. II. Open-shell systems and the total energies of the first-row atoms. J. Chem. Phys. 1991, 94, 6081–6090. [Google Scholar] [CrossRef]
- Breneman, C.M.; Wiberg, K.B. Determining atom-centered monopoles from molecular electrostatic potentials. The need for high sampling density in formamide conformational analysis. J. Comput. Chem. 1990, 11, 361–373. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Crawley, Australia, 2017. [Google Scholar]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds not are available from the authors. |










| Compound | Ref.-Year of Publication | CSD Refcode | Space Group | Z | Z′ | Temp. (K) | R-Factor (%) | |
|---|---|---|---|---|---|---|---|---|
| (CpF)2Fe | [85]-2015 | RACROF | Monoclinic | P21/n | 4 | 1 | 173 | 2.96 |
| (CpCl)2Fe | [84]-1986 | DUTSUH | Monoclinic | C2/c | 4 | 0 a | 295 | 4.60 a |
| (CpCl)2Fe | [85]-2015 | DUTSUH01 | Monoclinic | C2/c | 4 | 1 | 173 | 2.00 |
| (CpBr)2Fe | [86]-2004 | BIPDOU | Monoclinic | P21 | 2 | 1 | 100 | 3.67 |
| (CpI)2Fe | [87]-2014 | KOPFAY | Monoclinic | C2/c | 12 | 3 | 100 | 2.20 |
| (CpI)2Ru | [88]-2014 | JODWOQ | Triclinic | P-1 | 8 | 4 | 183 | 4.1 |
| Contact | (CpF)2Fe | (CpCl)2Fe | (CpBr)2Fe | (CpI)2Fe (A) | (CpI)2Fe (B) | (CpI)2Fe (C) | (CpI)2Ru (Average) |
|---|---|---|---|---|---|---|---|
| X-X | 1 | 0.6 | 2.3 | 11.3 | 13.7 | 8.9 | 7.2 |
| X-C | 0.2 | 1.9 | 3.7 | 0.8 | 1.1 | 0 | 8.7 |
| X-H | 36.8 | 40.6 | 39.6 | 36 | 33.3 | 29.3 | 33.7 |
| X-M | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C-C | 9.1 | 8.5 | 0 | 2.9 | 2.9 | 0 | 0.9 |
| C-H | 3.6 | 3.2 | 17.1 | 25.5 | 8.6 | 13.7 | 13.4 |
| C-M | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H-H | 49.3 | 45.2 | 37.3 | 23.5 | 40.4 | 48.1 | 35.8 |
| H-M | 0 | 0 | 0 | 0 | 0 | 0 | 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, K.; Ferreira da Silva, J. Halogen and Hydrogen Bonding Interplay in the Crystal Packing of Halometallocenes. Molecules 2018, 23, 2959. https://doi.org/10.3390/molecules23112959
Shimizu K, Ferreira da Silva J. Halogen and Hydrogen Bonding Interplay in the Crystal Packing of Halometallocenes. Molecules. 2018; 23(11):2959. https://doi.org/10.3390/molecules23112959
Chicago/Turabian StyleShimizu, Karina, and João Ferreira da Silva. 2018. "Halogen and Hydrogen Bonding Interplay in the Crystal Packing of Halometallocenes" Molecules 23, no. 11: 2959. https://doi.org/10.3390/molecules23112959