Immobilized Gold Nanoparticles Prepared from Gold(III)-Containing Ionic Liquids on Silica: Application to the Sustainable Synthesis of Propargylamines
Abstract
:1. Introduction
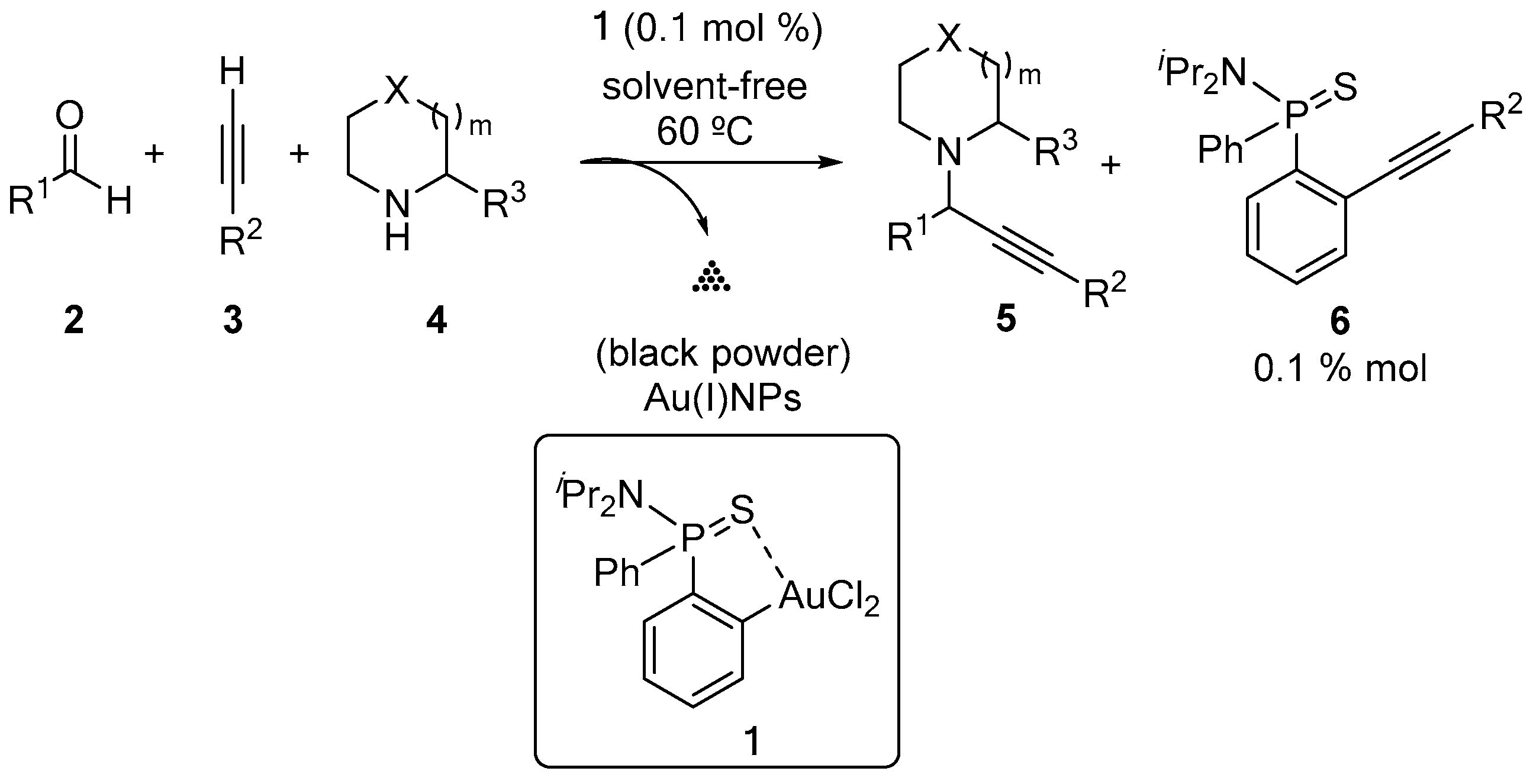
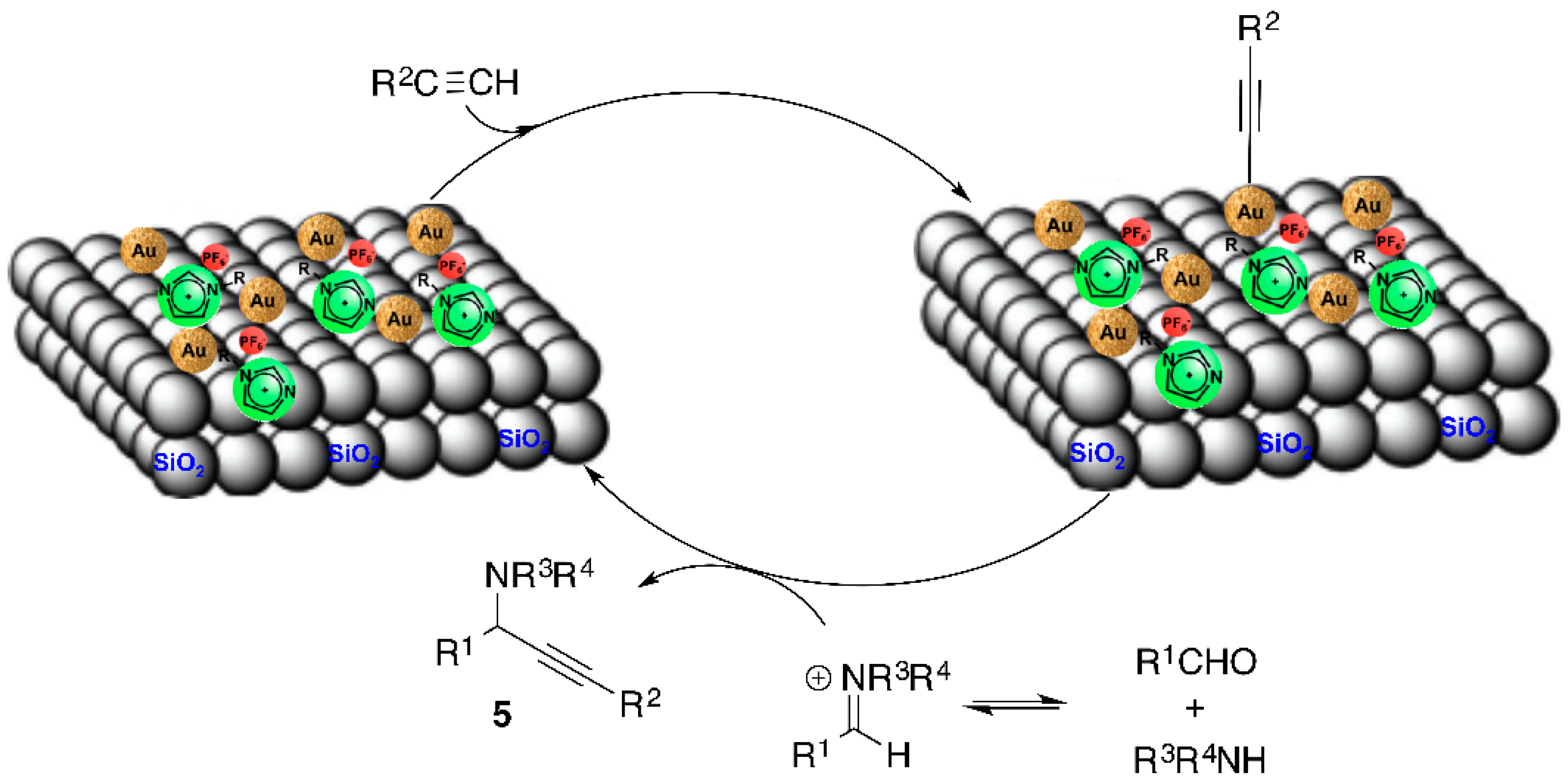
2. Results and Discussion
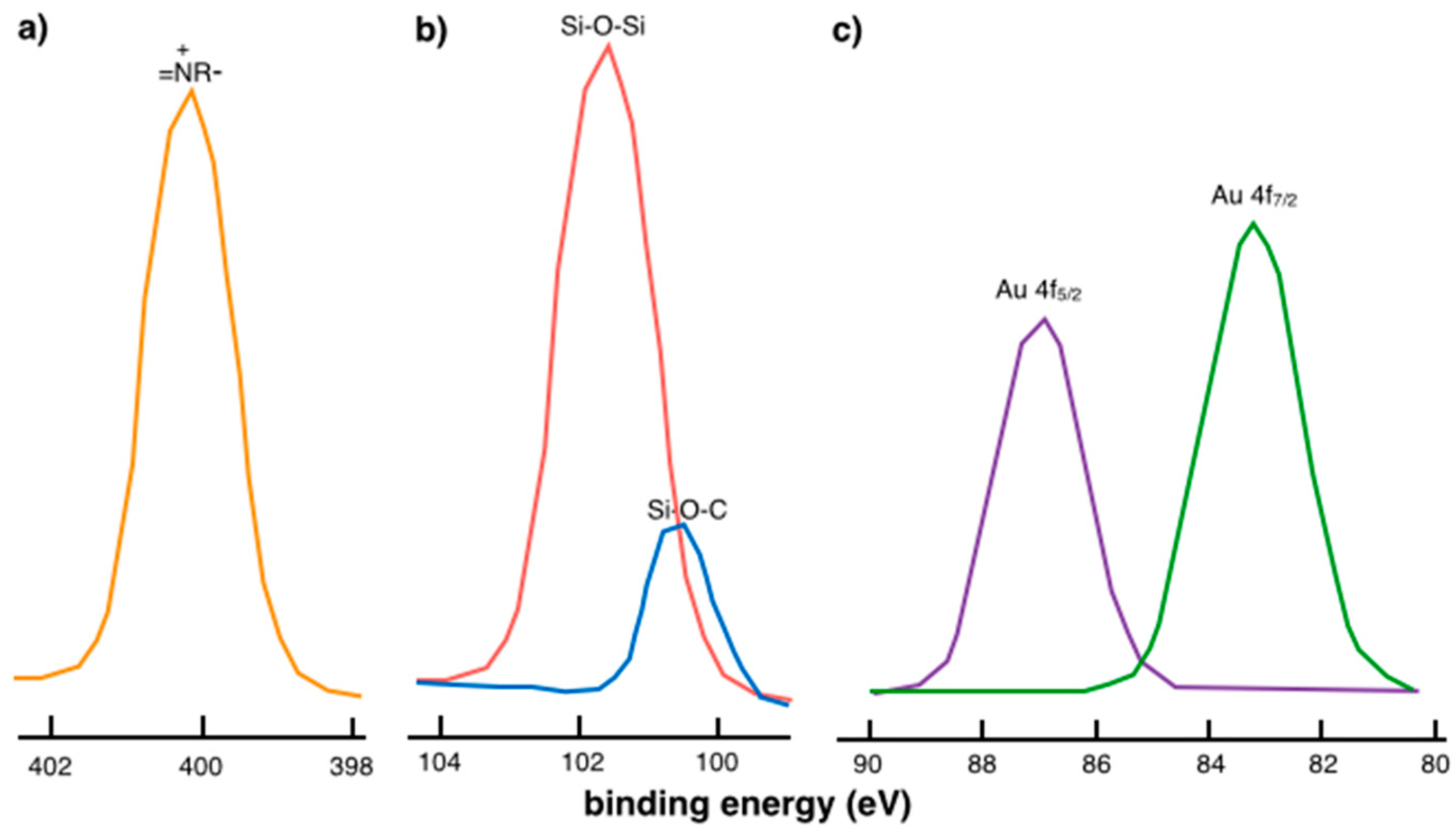
2.1. Physical Confinement of the Ionic Liquid and Complex 1 on the Silica Surface
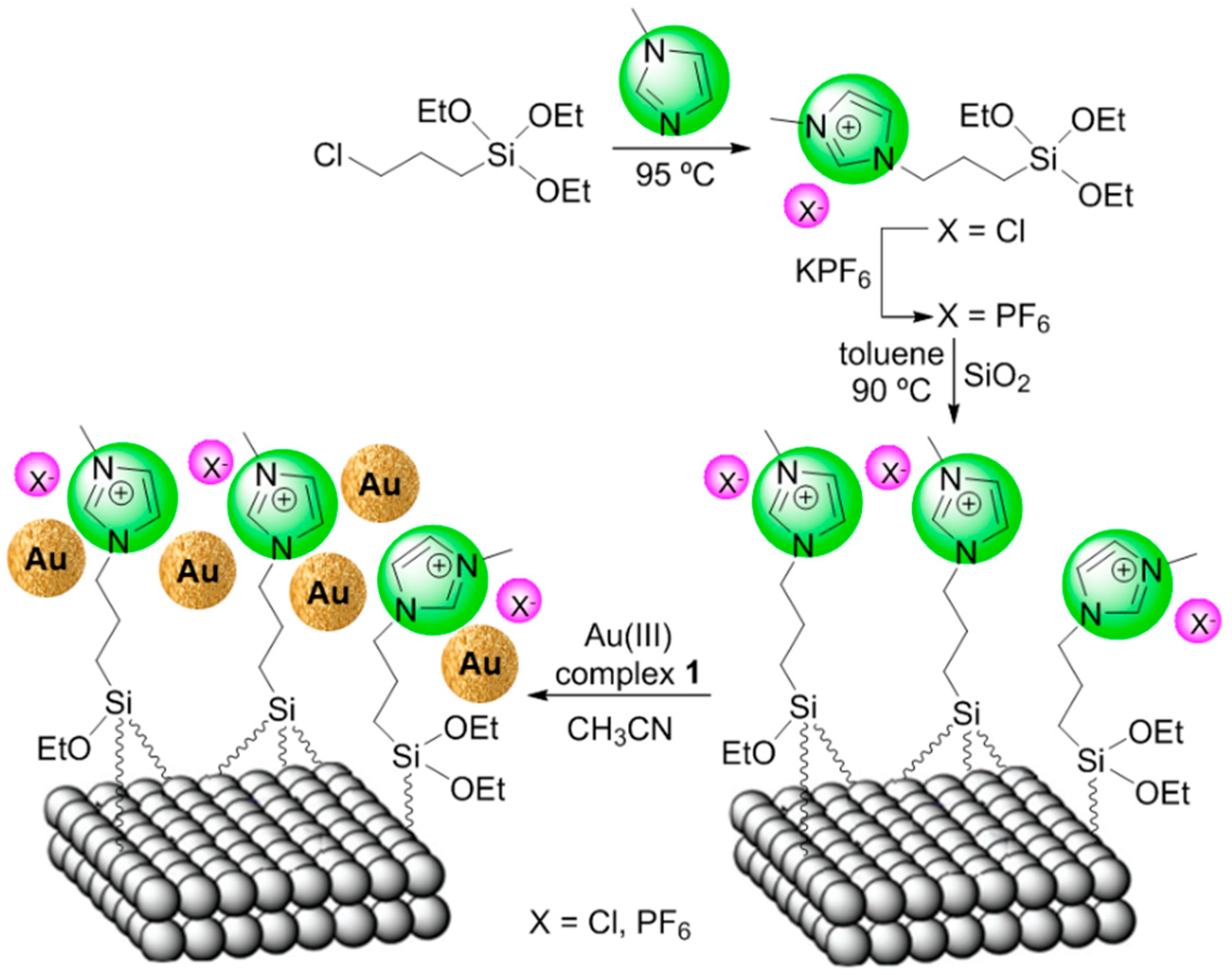
2.2. Covalent Bonding Via Ionic Liquid Fragments Attached to the Support
3. Conclusions
4. Materials and Methods
4.1. TEM and XPS Measurements
4.2. Scanning Electron Microscopy
4.3. Representative Procedure for Catalyst Preparation
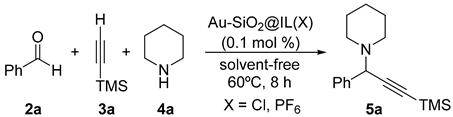
4.4. General Procedure for the Gold-Catalyzed, Three-Component Coupling
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peshkov, V.A.; Pereshivko, O.P.; Nechaev, A.A.; Peshkov, A.A.; Van der Eycken, E.V. Reactions of Secondary Propargylamines with Heteroallenes for the Synthesis of Diverse Heterocycles. Chem. Soc. Rev. 2018, 47, 3861–3898. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, S.; Vessally, E.; Edjlali, L.; Hosseinzadeh-Khanmiri, R.; Ghorbani-Kalhor, E. N-Propargylamines: Versatile Building Blocks in the Construction of Thiazole cores. Beilstein J. Org. Chem. 2017, 13, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Amit, T.; Bar-Am, O.; Mechlovich, D.; Kupershmidt, L.; Youdim, M.B.H.; Weinreb, O. The Novel Multitarget Iron Chelating and Propargylamine Drug M30 Affects APP Regulation and Processing Activities in Alzheimer’s Disease Models. Neuropharmacology 2017, 123, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Vessally, E.; Hosseinian, A.; Edjlali, L.; Bekhradnia, A.; Esrafili, M.D. New Page to Access Pyrazines and their Ring Fused Analogues: Synthesis from N-Propargylamines. Curr. Org. Synth. 2017, 14, 557–567. [Google Scholar] [CrossRef]
- Vessally, E.; Soleimani-Amiri, S.; Hosseinian, A.; Edjlali, L.; Bekhradnia, A. New Protocols to Access Imidazoles and their Ring Fused Analogues: Synthesis from N-propargylamines. RSC Adv. 2017, 7, 7079–7091. [Google Scholar] [CrossRef]
- Lauder, K.; Toscani, A.; Scalacci, N.; Castagnolo, D. Synthesis and Reactivity of Propargylamines in Organic Chemistry. Chem. Rev. 2017, 117, 14091–14200. [Google Scholar] [CrossRef] [PubMed]
- Peshkov, V.A.; Pereshivko, O.P.; Van der Eycken, E.V. A walk around the A3-coupling. Chem. Soc. Rev. 2012, 41, 3790–3807. [Google Scholar] [CrossRef] [PubMed]
- Cammarata, J.R.; Rivera, R.; Fuentes, F.; Otero, Y.; Ocando-Mavrez, E.; Arce, A.; Garcia, J.M. Single and Double A3-coupling (Aldehyde-Amine-Alkyne) Reaction Catalyzed by an Air Stable Copper(I)-phosphole Complex. Tetrahedron Lett. 2017, 58, 4078–4081. [Google Scholar] [CrossRef]
- Shi, L.; Tu, Y.-Q.; Wang, M.; Zhang, F.-M.; Fan, C.-A. Microwave-Promoted Three-Component Coupling of Aldehyde, Alkyne, and Amine via C−H Activation Catalyzed by Copper in Water. Org. Lett. 2004, 6, 1001–1003. [Google Scholar] [CrossRef] [PubMed]
- Huma, H.Z.S.; Halder, R.; Kalra, S.S.; Das, J.; Iqbal, J. Cu(I)-Catalyzed Three Component Coupling Protocol for the Synthesis of Quinoline Derivatives. Tetrahedron Lett. 2002, 43, 6485–6488. [Google Scholar] [CrossRef]
- Ohno, H.; Ohta, Y.; Oishi, S.; Fujii, N. Direct Synthesis of 2-(Aminomethyl)indoles through Copper(I)-Catalyzed Domino Three-Component Coupling and Cyclization Reactions. Angew. Chem. Int. Ed. 2007, 46, 2295–2298. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Ji, J.-X. A Novel and Convenient Copper-Catalyzed Three-Component Coupling of Aldehydes, Alkynes, and Hydroxylamines leading to Propargylamines. Tetrahedron Lett. 2012, 53, 4797–4801. [Google Scholar] [CrossRef]
- Wei, C.; Li, Z.; Li, C.-J. The First Silver-Catalyzed Three-Component Coupling of Aldehyde, Alkyne, and Amine. Org. Lett. 2003, 5, 4473–4475. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.M.; Babu, N.S.; Suryanarayana, I.; Prasad, P.S.S.; Lingaiah, N. The Silver Salt of 12-Tungstophosphoric Acid: An Efficient Catalyst for the Three-Component Coupling of an Aldehyde, an Amine and an Alkyne. Tetrahedron Lett. 2006, 47, 7563–7566. [Google Scholar] [CrossRef]
- Zhang, Y.; Santos, A.M.; Herdtweck, E.; Mink, J.; Kuhn, F.E. Organonitrile Ligated Silver Complexes with Perfluorinated Weakly Coordinating Anions and their Catalytic Application for Coupling Reactions. New J. Chem. 2005, 29, 366–370. [Google Scholar] [CrossRef]
- Li, Z.; Wei, C.; Chen, L.; Varma, R.S.; Li, C.-J. Three-Component Coupling of Aldehyde, Alkyne, and Amine Catalyzed by Silver in Ionic Liquid. Tetrahedron Lett. 2004, 45, 2443–2446. [Google Scholar] [CrossRef]
- Li, C.J.; Wei, C. Highly Efficient Grignard-type Imine Additions via C-H Activation in Water and under Solvent-Free Conditions. Chem. Commun. 2002, 268–269. [Google Scholar] [CrossRef]
- Lo, V.K.Y.; Liu, Y.; Wong, M.K.; Che, C.M. Gold(III) Salen Complex-Catalyzed Synthesis of Propargylamines via a Three-Component Coupling Reaction. Org. Lett. 2006, 8, 1529–1532. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Lee, C.G.; Na, J.E.; Kim, J.N. Alkynylation of N-Tosylimines with Aryl Acetylenes Promoted by ZnBr2 and N,N-diisopropylethylamine in Acetonitrile. Tetrahedron Lett. 2005, 46, 69–74. [Google Scholar] [CrossRef]
- Ramu, E.; Varala, R.; Sreelatha, N.; Adapa, S.R. Zn(OAc)2·2H2O: A Versatile Catalyst for the One-Pot Synthesis of Propargylamines. Tetrahedron Lett. 2007, 48, 7184–7190. [Google Scholar] [CrossRef]
- Kantam, M.L.; Balasubrahmanyam, V.; Kumar, K.S.; Venkanna, G.T. Efficient One-Pot Synthesis of Propargylamines using Zinc Dust. Tetrahedron Lett. 2007, 48, 7332–7334. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Wang, L. Iron-Catalyzed Ligand-Free Three-Component Coupling Reactions of Aldehydes, Terminal Alkynes, and Amines. Chem. Eur. J. 2009, 15, 2045–2049. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; Nauyen, R.V.; Li, C.J. Iron-Catalyzed Three-Component Coupling of Aldehyde, Alkyne, and Amine under Neat Conditions in Air. Tetrahedron Lett. 2009, 50, 2895–2898. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Wang, M.; Wang, L. Indium-Catalyzed Highly Efficient Three-Component Coupling of Aldehyde, Alkyne, and Amine via C−H Bond Activation. J. Org. Chem. 2009, 74, 4364–4367. [Google Scholar] [CrossRef] [PubMed]
- Jadav, J.S.; Reddy, B.V.S.; Gopal, A.V.H.; Patil, K.S. InBr3-Catalyzed Three-Component Reaction: A Facile Synthesis of Propargylamines. Tetrahedron Lett. 2009, 50, 3493–3496. [Google Scholar] [CrossRef]
- Fischer, C.; Carreira, E.M. Direct Addition of TMS-acetylene to Aldimines Catalyzed by a Simple, Commercially Available Ir(I) Complex. Org. Lett. 2001, 3, 4319–4321. [Google Scholar] [CrossRef] [PubMed]
- Afraj, N.S.; Chinpiao, C.; Lee, G.H. Manganese(II) Chloride Catalyzed Highly Efficient One-Pot Synthesis of Propargylamines and Fused Triazoles via Three-component Coupling Reaction under Solvent-free Conditions. RSC Adv. 2014, 4, 26301–26308. [Google Scholar] [CrossRef]
- Hua, L.P.; Lei, W. Mercurous Chloride Catalyzed Mannich Condensation of Terminal Alkynes with Secondary Amines and Aldehydes. Chin. J. Chem. 2005, 23, 1076–1080. [Google Scholar] [CrossRef]
- Samai, S.; Nandi, G.C.; Singh, M.S. An Efficient and Facile One-Pot Synthesis of Propargylamines by Three-Component Coupling of Aldehydes, Amines, and Alkynes via C–H Activation Catalyzed by NiCl2. Tetrahedron Lett. 2010, 51, 5555–5558. [Google Scholar] [CrossRef]
- Bonfield, E.R.; Li, C.J. Efficient Preparation of the Isoindoline Framework via a Six Component, Tandem Double A3-Coupling and [2+2+2] Cycloaddition Reaction. Adv. Synth. Catal. 2008, 350, 370–374. [Google Scholar] [CrossRef]
- Chen, W.W.; Bi, H.P.; Li, C.J. The First Cobalt-Catalyzed Transformation of Alkynyl C-H Bond: Aldehyde-Alkyne-Amine (A3) Coupling. Synlett 2010, 475–479. [Google Scholar] [CrossRef]
- Teimouri, A.; Chermahini, A.N.; Narimani, M. A Simple and Efficient One-Pot Three-Component Synthesis of Propargylamines Using Bismuth (III) Chloride. Bull. Korean Chem. Soc. 2012, 33, 1556–1560. [Google Scholar] [CrossRef] [Green Version]
- Raghuvanshi, D.S.; Singh, K.N. Highly Efficient Cadmium-Catalyzed Three-Component Coupling of an Aldehyde, Alkyne, and Amine via C-H Activation under Microwave Conditions. Synlett 2011, 373–377. [Google Scholar] [CrossRef]
- Ramu, V.G.; Bordoloi, A.; Nagaiah, T.C.; Schuhmann, W.; Muhler, M.; Cabrele, C. Copper Nanoparticles Stabilized on Nitrogen-doped Carbon Nanotubes as Efficient and Recyclable Catalysts for Alkyne/Aldehyde/Cyclic Amine A3-type Coupling Reactions. Appl. Catal. A 2012, 431, 88–94. [Google Scholar] [CrossRef]
- Zhang, F.; Lai, Q.; Shi, X.; Song, Z. Triazole-Gold (TAAu) Catalyzed Three-Component Coupling (A3 Reaction) towards the Synthesis of 2,4-Disubstituted Quinoline Derivatives. Chin. Chem. Lett. 2018. [Google Scholar] [CrossRef]
- Price, G.A.; Brisdon, A.K.; Randall, S.; Lewis, E.; Whittaker, D.M.; Pritchard, R.G.; Muryn, C.A.; Flower, K.R.; Quayle, P. Some Insights into the Gold-Catalyzed A3-Coupling Reaction. J. Organomet. Chem. 2017, 846, 251–262. [Google Scholar] [CrossRef]
- Grirrane, A.; Alvarez, E.; Garcia, H.; Corma, A. Preparation of Tremorine and Gemini Surfactant Precursors with Cationic Ethynyl-Bridged Digold Catalysts. Chem. Eur. J. 2017, 23, 2792–2801. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rudolph, M.; Rominger, F.; Xie, J.; Hashmi, A.; Stephen, K. A Gold-Catalyzed A3 Coupling/Cyclization/Elimination Sequence as Versatile Tool for the Synthesis of Furfuryl Alcohol Derivatives from Glyceraldehyde and Alkynes. Adv. Synth. Catal. 2016, 358, 207–211. [Google Scholar] [CrossRef]
- Corma, A. Attempts to Fill the Gap between Enzymatic, Homogeneous, and Heterogeneous Catalysis. Catal. Rev. Sci. Eng. 2004, 46, 369–417. [Google Scholar] [CrossRef]
- Widenhoefer, R.A.; Han, X. Gold-Catalyzed Hydroamination of C–C Multiple Bonds. Eur. J. Org. Chem. 2006, 4555–4563. [Google Scholar] [CrossRef]
- Zhang, X.; Corma, A. Efficient Addition of Alcohols, Amines and Phenol to Unactivated Alkenes by AuIII or PdII Stabilized by CuCl2. Dalton Trans. 2008, 3, 397–403. [Google Scholar] [CrossRef]
- Haruta, M. When Gold Is Not Noble: Catalysis by Nanoparticles. Chem. Rec. 2003, 3, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Stratakis, M.; García, H. Catalysis by Supported Gold Nanoparticles: Beyond Aerobic Oxidative Processes. Chem. Rev. 2012, 112, 4469–4506. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Serna, P. Chemoselective Hydrogenation of Nitro Compounds with Supported Gold Catalysts. Science 2006, 313, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Kantam, M.L.; Prakash, B.V.; Reddy, C.R.V.; Sreedhar, B. Layered Double Hydroxide-Supported Gold Catalyst for Three-Component Aldehyde-Amine-Alkyne Coupling. Synlett 2005, 2329–2332. [Google Scholar] [CrossRef]
- Zhang, X.; Corma, A. Supported Gold(III) Catalysts for Highly Efficient Three-Component Coupling Reactions. Angew. Chem. Int. Ed. 2008, 47, 4358–4361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Jin, R. Catalysis by Gold Nanoparticles. Carbon-carbon Coupling Reactions. Nanotechnol. Rev. 2013, 2, 529–545. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Ghorbannezhad, F.; Sajadi, S.M. A Review on Recent Advances in the Application of Nanocatalysts in A3 Coupling Reactions. Chem. Rec. 2018. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.K.; Das, R. Progress in Synthesis of Propargylamine and Its Derivatives by Nanoparticle Catalysis via A3 coupling: A Decade Update. ChemistrySelect 2018, 3, 147–169. [Google Scholar] [CrossRef]
- Borah, B.J.; Borah, S.J.; Saikia, K.; Dutta, D.K. Efficient One-pot Synthesis of Propargylamines Catalysed by Gold Nanocrystals Stabilized on Montmorillonite. Catal. Sci. Technol. 2014, 14, 4001–4009. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Pourkaveh, R. Efficient Synthesis of Propargylamines in Aqueous Media Catalyzed by Au Nanoparticles under Ambient Temperature. ChemistrySelect 2018, 3, 2053–2058. [Google Scholar] [CrossRef]
- Gholinejad, M.; Bonyasi, R.; Najera, C.; Saadati, F.; Bahrami, M.; Dasvarz, N. Gold Nanoparticles Supported on Imidazole-Modified Bentonite: Environmentally Benign Heterogeneous Catalyst for the Three-Component Synthesis of Propargylamines in Water. ChemPlusChem 2018, 83, 431–438. [Google Scholar] [CrossRef]
- Feiz, A.; Bazgir, A. Gold Nanoparticles Supported on Mercaptoethanol Directly Bonded to MCM-41: An Efficient Catalyst for the Synthesis of Propargylamines. Catal. Commun. 2016, 73, 88–92. [Google Scholar] [CrossRef]
- Loni, M.; Yazdani, H.; Bazgir, A. Gold Nanoparticles-Decorated Dithiocarbamate Nanocomposite: An Efficient Heterogeneous Catalyst for the Green A3-Coupling Synthesis of Propargylamines. Catal. Lett. 2018. [Google Scholar] [CrossRef]
- Hussain, N.; Das, M.R. Magnetically Recoverable Graphene-Based Nanocomposite Material as an Efficient Catalyst for the Synthesis of Propargylamines via A3 Coupling Reaction. New J. Chem. 2017, 41, 12756–12766. [Google Scholar] [CrossRef]
- Lili, L.; Xishi, T.; Guanglin, Y.; Huanmei, G.; Qingguo, M. Gold and Silver Nanoparticles Supported on Metal-organic Frameworks: A Highly Active Catalyst for Three-component Coupling Reaction. Chem. Res. Chin. Univ. 2016, 32, 443–450. [Google Scholar] [CrossRef]
- Liu, L.; Tai, X.; Zhou, X. Au3+/Au0 Supported on Chromium(III) Terephthalate Metal Organic Framework (MIL-101) as an Efficient Heterogeneous Catalyst for Three-Component Coupling Synthesis of Propargylamines. Materials 2017, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tai, X.; Zhang, N.; Meng, Q.; Xin, C. Supported Au/MIL-53(Al): A Reusable Green Solid Catalyst for the Three-component Coupling Reaction of Aldehyde, Alkyne, and Amine. Reac. Kinet. Mech. Catal. 2016, 119, 335–348. [Google Scholar] [CrossRef]
- Lili, L.; Xin, Z.; Shumin, R.; Ying, Y.; Xiaoping, D.; Jinsen, G.; Chunming, X.; Jing, H. Catalysis by Metal–organic Frameworks: Proline and Gold Functionalized MOFs for the Aldol and Three-component Coupling Reactions. RSC Adv. 2014, 4, 13093–13107. [Google Scholar] [CrossRef]
- Zhao, X.-B.; Ha, W.; Jiang, K.; Chen, J.; Yang, J.-L.; Shi, Y.-P. Efficient Synthesis of Camptothecin Propargylamine Derivatives in Water Catalyzed by Macroporous Adsorption Resin-supported Gold Nanoparticles. Green Chem. 2017, 19, 1399–1406. [Google Scholar] [CrossRef]
- Gholinejad, M.; Hamed, F.; Nájera, C. Gold Nanoparticles Supported on Polyacrylamide Containing a Phosphorus Ligand as an Efficient Heterogeneous Catalyst for Three-Component Synthesis of Propargylamines in Water. Synlett 2016, 27, 1193–1201. [Google Scholar] [CrossRef] [Green Version]
- Gholinejad, M.; Zareh, F.; Najera, C. Iron Oxide Modified with Pyridyl-triazole Ligand for Stabilization of Gold Nanoparticles: An Efficient Heterogeneous Catalyst for A3 Coupling Reaction in Water. Appl. Organomet. Chem. 2018. [Google Scholar] [CrossRef]
- Zohreh, N.; Hassan, S.H.; Jahani, M.; Xaba, M.S.; Meijboom, R. Stabilization of Au NPs on Symmetrical Tridentate NNN-Pincer Ligand Grafted on Magnetic Support as Water Dispersible and Recyclable Catalyst for Coupling Raction of Terminal Alkyne. J. Catal. 2017, 356, 255–268. [Google Scholar] [CrossRef]
- Aghahosseini, H.; Rezaei, S.J.T.; Tadayyon, M.; Ramazani, A.; Amani, V.; Ahmadi, R.; Abdolahnjadian, D. Highly Efficient Aqueous Synthesis of Propargylamines through C–H Activation Catalyzed by Magnetic Organosilica-Supported Gold Nanoparticles as an Artificial Metalloenzyme. Eur. J. Inorg. Chem. 2018, 22, 2589–2598. [Google Scholar] [CrossRef]
- Mallampati, R.; Valiyaveettil, S. Eggshell Membrane-Supported Recyclable Catalytic Noble Metal Nanoparticles for Organic Reactions. ACS Sustain. Chem. Eng. 2014, 2, 855–859. [Google Scholar] [CrossRef]
- Bhargavaa, A.; Jaina, N.; Gangopadhyayb, S.; Panwar, J. Development of Gold Nanoparticle-Fungal Hybrid Based Heterogeneous Interface for Catalytic Applications. Process Biochem. 2015, 50, 1293–1300. [Google Scholar] [CrossRef]
- Claus, J.; Sommer, F.O.; Kragl, U. Ionic liquids in Biotechnology and Beyond. Solid State Ionics 2018, 314, 119–128. [Google Scholar] [CrossRef]
- Karimi, B.; Tavakolian, M.; Akbari, M.; Mansouri, F. Ionic Liquids in Asymmetric Synthesis: An Overall View from Reaction Media to Supported Ionic Liquid Catalysis. ChemCatChem 2018, 10, 3173–3205. [Google Scholar] [CrossRef]
- Li, J.; Yang, S.; Wu, W.; Jiang, H. Recent Advances in Pd-Catalyzed CrossCoupling Reaction in Ionic Liquids. Eur. J. Org. Chem. 2018, 11, 1284–1306. [Google Scholar] [CrossRef]
- Riisager, A.; Fehrmann, R. Imidazolium-based Ionic Liquids Grafted on Solid Surfaces. Chem. Soc. Rev. 2014, 43, 7171–7187. [Google Scholar] [CrossRef]
- He, Z.; Alexandridis, P. Ionic liquid and Nanoparticle Hybrid Systems: Emerging applications. Adv. Colloid Interface Sci. 2017, 244, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Wu, Y.P.; Zheng, K.B.; Hu, Y.L. Applications of Supported Ionic Liquids. Curr. Org. Chem. 2018, 22, 462–484. [Google Scholar] [CrossRef]
- Haumann, M.; Wasserscheid, P. SILP and SCILL Catalysis. In Catalysis in Ionic Liquids: From Catalyst Synthesis to Application; Hardacre, C., Parvulescu, V., Eds.; Royal Society of Chemistry: Cambridge, UK, 2014; pp. 410–432. [Google Scholar]
- Moghaddam, F.M.; Ayati, S.E.; Hosseinib, S.H.; Pourjavadi, A. Gold Immobilized onto Poly(ionic liquid) Functionalized Magnetic Nanoparticles: A Robust Magnetically Recoverable Catalyst for the Synthesis of Propargylamine in Water. RSC Adv. 2015, 5, 34502–34510. [Google Scholar] [CrossRef]
- Karimi, B.; Gholinejad, M.; Khorasani, M. Highly Efficient Three-component Coupling Reaction Catalyzed by Gold Nanoparticles Supported on Periodic Mesoporous Organosilica with Ionic Liquid Framework. Chem. Commun. 2012, 48, 8961–8963. [Google Scholar] [CrossRef] [PubMed]
- Dhondge, A.P.; Afraj, S.N.; Nuzlia, C.; Chen, C.; Lee, G.-H. A Facile Synthesis of 4,6,7,8,8a,9-Hexahydropyrrolo[1,2a][1,2,3]triazolo[1,5-d]pyrazines by a Three-Component Coupling Reaction Followed by Intramolecular 1,3-Dipolar Cycloaddition. Eur. J. Org. Chem. 2013, 19, 4119–4130. [Google Scholar] [CrossRef]
- Ananda, N.; Ramudua, P.; Reddya, K.H.P.; Raoa, K.S.R.; Jagadeeshb, B.; Babub, V.S.P.; Burri, D.R. Gold Nanoparticles Immobilized on Lipoic Acid Functionalized SBA-15: Synthesis, Characterization and Catalytic Applications. Appl. Catal. A 2013, 454, 119–126. [Google Scholar] [CrossRef]
- Cheng, M.; Zhang, Q.; Hu, X.-Y.; Li, B.-G.; Ji, J.-X.; Chan, A.S.C. Gold-Catalyzed Direct Intermolecular Coupling of Ketones, Secondary Amines, and Alkynes: A Facile and Versatile Access to Propargylic Amines Containing a Quaternary Carbon Center. Adv. Synth. Catal. 2011, 353, 1274–1278. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.-L.; Gray, D.G.; Li, C.-J. A3-Coupling Catalyzed by Robust Au Nanoparticles Covalently Bonded to HS-Functionalized Cellulose Nanocrystalline Films. Beilstein J. Org. Chem. 2013, 9, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Bobadilla, L.F.; Blasco, T.; Odriozola, J.A. Gold(III) Stabilized over Ionic Liquids Grafted on MCM-41 for Highly Efficient Three-component Coupling Reactions. Phys. Chem. Chem. Phys. 2013, 15, 16927–16934. [Google Scholar] [CrossRef] [PubMed]
- Belmonte-Sánchez, E.; Iglesias, M.J.; El Hajjouji, H.; Roces, L.; García-Granda, S.; Villuendas, P.; Urriolabeitia, E.P.; López-Ortiz, F. Cycloaurated Phosphinothioic Amide Complex as a Precursor of Gold(I) Nanoparticles: Efficient Catalysts for A3 Synthesis of Propargylamines under Solvent-Free Conditions. Organometallics 2017, 36, 1962–1973. [Google Scholar] [CrossRef]
- Hagiwara, H.; Sugawara, Y.; Isobe, K.; Hoshi, T.; Suzuki, T. Immobilization of Pd(OAc)2 in Ionic Liquid on Silica: Application to Sustainable Mizoroki-Heck Reaction. Org. Lett. 2004, 6, 2325–2328. [Google Scholar] [CrossRef] [PubMed]
- Brenna, S.; Posset, T.; Furrer, J.; Blümel, J. 14N NMR and Two-Dimensional Suspension 1H and 13C HRMAS NMR Spectroscopy of Ionic Liquids Immobilized on Silica. Chem. Eur. J. 2006, 12, 2880–2888. [Google Scholar] [CrossRef] [PubMed]
- Hayamizu, K.; Tsuzuki, S.; Seki, S. Molecular Motions and Ion Diffusions of the Room-Temperature Ionic Liquid 1,2-Dimethyl-3-propylimidazolium Bis(trifluoromethylsulfonyl)amide (DMPImTFSA) Studied by 1H, 13C, and 19F NMR. J. Phys. Chem. A 2008, 112, 12027–12036. [Google Scholar] [CrossRef] [PubMed]
- Shylesh, S.; Hanna, D.; Werner, S.; Bell, A.T. Factors Influencing the Activity, Selectivity, and Stability of Rh-Based Supported Ionic Liquid Phase (SILP) Catalysts for Hydroformylation of Propene. ACS Catal. 2012, 2, 487–493. [Google Scholar] [CrossRef]
- Shah, F.U.; Holmgren, A.; Rutland, M.W.; Glavatskih, S.; Antzutkin, O.N. Interfacial Behavior of Orthoborate Ionic Liquids at Inorganic Oxide Surfaces Probed by NMR, IR, and Raman Spectroscopy. J. Phys. Chem. C 2018, 122, 19687–19698. [Google Scholar] [CrossRef]
- Movahed, S.K.; Esmatpoursalmani, R.; Bazgir, A. N-Heterocyclic Carbene Palladium Complex Supported on Ionic Liquid-Modified Graphene Oxide as an Efficient and Recyclable Catalyst for Suzuki Reaction. RSC Adv. 2014, 4, 14586–14591. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Q.; Ji, J.; Zhang, G.; Zhang, F.; Fan, X. Cooperative Catalysis by Acid–Base Bifunctional Graphene. RSC Adv. 2013, 3, 13655–13658. [Google Scholar] [CrossRef]
- Mehnert, C.P.; Cook, R.A.; Dispenziere, N.C.; Afeworki, M. Supported Ionic Liquid Catalysis—A New Concept for Homogeneous Hydroformylation Catalysis. J. Am. Chem. Soc. 2002, 124, 12932–12933. [Google Scholar] [CrossRef] [PubMed]
- Brauer, U.G.; de La Hoz, A.T.; Miller, K.M. The effect of counteranion on the physicochemical and thermal properties of 4-methyl-1-propyl-1,2,4-triazolium ionic liquids. J. Mol. Liq. B 2015, 210, 286–292. [Google Scholar] [CrossRef]
- Alvim, H.G.O.; Pinheiro, D.L.J.; Carvalho-Silva, V.H.; Fioramonte, M.; Gozzo, F.C.; da Silva, W.A.; Amarante, G.W.; Neto, B.A.D. Combined Role of the Asymmetric Counteranion-Directed Catalysis (ACDC) and Ionic Liquid Effect for the Enantioselective Biginelli Multicomponent Reaction. J. Org. Chem. 2018, 83, 12143–12153. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, G.; Corma, A.; Iglesias, M.; Sánchez, F. Heterogenized Gold Complexes: Recoverable Catalysts for Multicomponent Reactions of Aldehydes, Terminal Alkynes, and Amines. ACS Catal. 2012, 2, 399–406. [Google Scholar] [CrossRef]
- Wei, C.; Li, C.-L. A Highly Efficient Three-Component Coupling of Aldehyde, Alkyne, and Amines via C−H Activation Catalyzed by Gold in Water. J. Am. Chem. Soc. 2003, 125, 9584–9585. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.L.; Yang, J.; Wei, Y.; Yinga, J.Y. Transition Metal-Catalysed, Direct and Site-Selective N1-, C2- or C3-Arylation of the Indole Nucleus: 20 Years of Improvements. Adv. Synth. Catal. 2009, 351, 2887–2896. [Google Scholar] [CrossRef]
- Chen, H.-B.; Zhao, Y.; Liao, Y. Aldehyde–Alkyne–Amine (A3) Coupling Catalyzed by a Highly Efficient Dicopper Complex. RSC Adv. 2015, 5, 37737–37741. [Google Scholar] [CrossRef]
- Cruz, P.; Pérez, Y.; del Hierro, I.; Fernández-Galán, R.; Fajardo, M. ε-Caprolactone Polymerization Using Titanium Complexes Immobilized onto Silica Based Materials Functionalized with Ionic Liquids: Insights into Steric, Electronic and Support Effects. RSC Adv. 2016, 6, 19723–19733. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 5 are available from the authors. |





| Entry | Catalyst | n (mol%) | Time (h) | Conv. (%) a |
|---|---|---|---|---|
| 1 | (dppta)AuCl2–SILP | 1 | 6 | 99 |
| 2 | (dppta)AuCl2–SILP | 0.5 | 6 | 98 |
| 3 | (dppta)AuCl2–SILP | 0.1 | 8 | 97 |
| 4 | (dppta)AuCl2 | 1 | 6 | 97 |
| 5 | (dppta)AuCl2 | 0.1 | 8 | 99 |

| Entry | R1 | R2 | R3 | X | m | Time (h) | 5 | Conv. (%) a |
|---|---|---|---|---|---|---|---|---|
| 1 | Ph | TMS | H | CH2 | 1 | 8 | a | 97 |
| 2 | 4-ClC6H4 | TMS | H | CH2 | 1 | 8 | b | 94 |
| 3 | 4-OMeC6H4 | TMS | H | CH2 | 1 | 14 | c | 83 |
| 4 | Ph | Ph | H | CH2 | 1 | 8 | d | 94 |
| 5 | Ph | 4-FC6H4 | H | CH2 | 1 | 14 | e | 86 |
| 6 | Ph | 2-OMeC6H4 | H | CH2 | 1 | 14 | f | 90 |
| 7 | Ph | 3-MeC6H4 | H | CH2 | 1 | 8 | g | 93 |
| 8 | Ph | 3(C≡C)C6H4 | H | CH2 | 1 | 8 | h | 83 |
| 9 | Ph | TMS | H | O | 1 | 8 | i | 97 |
| 10 | Ph | Ph | H | O | 1 | 8 | j | 98 |
| 11 | Ph | TMS | CH3 | CH2 | 0 | 8 | k | 98(97) b |
| 12 | Ph | Ph | CH3 | CH2 | 0 | 8 | l | 87(98) b |
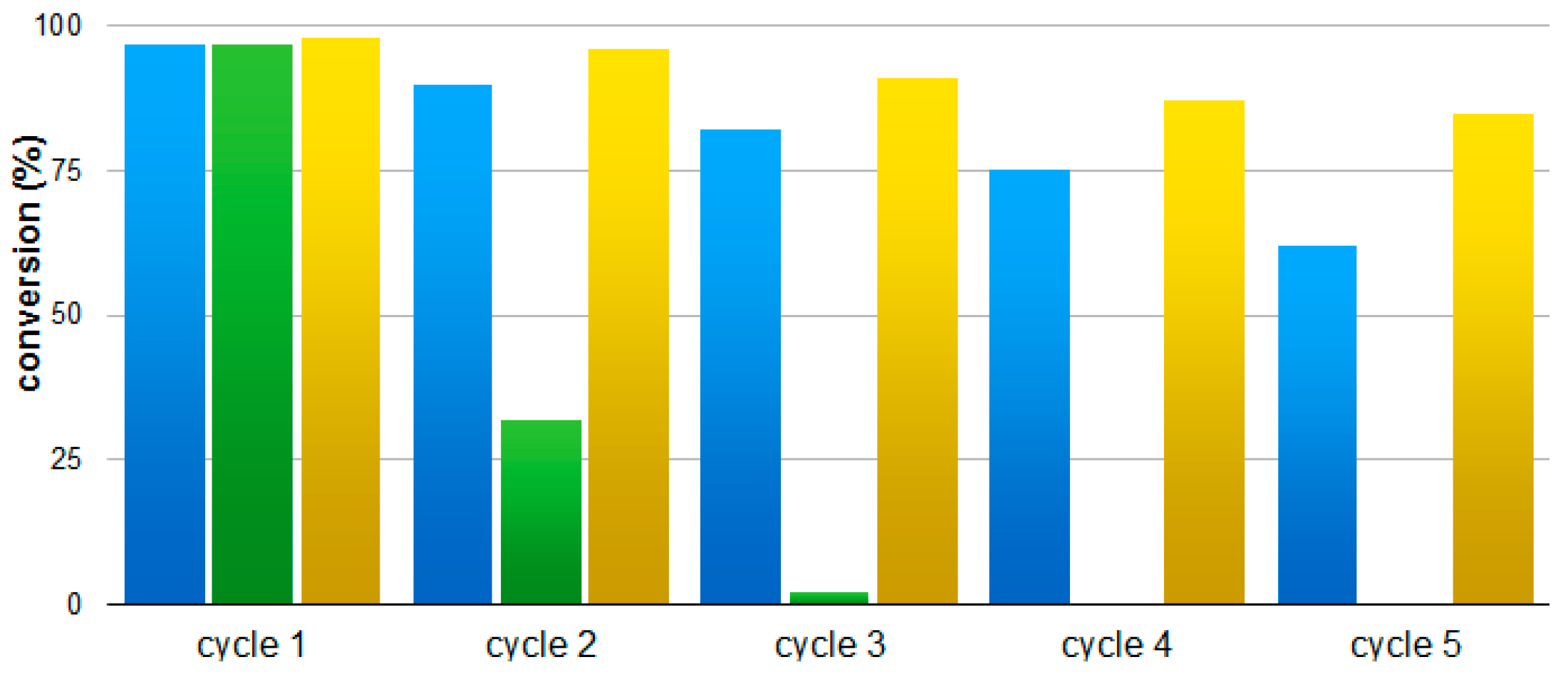
| Entry | Cycle | Conv. (%) a |
|---|---|---|
| 1 | 1 | 97 |
| 2 | 2 | 90 |
| 3 | 3 | 81 |
| 4 | 4 | 75 |
| 5 | 5 | 62 |
| Entry | Catalyst | Conversion (%) a | ||||
|---|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Cycle 5 | ||
| 1 | Au–SiO2@IL(Cl) | 97 | 32 | 0 | - | - |
| 2 | Au–SiO2@IL(PF6) | 98 | 96 | 91 | 86 | 85 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soengas, R.; Navarro, Y.; Iglesias, M.J.; López-Ortiz, F. Immobilized Gold Nanoparticles Prepared from Gold(III)-Containing Ionic Liquids on Silica: Application to the Sustainable Synthesis of Propargylamines. Molecules 2018, 23, 2975. https://doi.org/10.3390/molecules23112975
Soengas R, Navarro Y, Iglesias MJ, López-Ortiz F. Immobilized Gold Nanoparticles Prepared from Gold(III)-Containing Ionic Liquids on Silica: Application to the Sustainable Synthesis of Propargylamines. Molecules. 2018; 23(11):2975. https://doi.org/10.3390/molecules23112975
Chicago/Turabian StyleSoengas, Raquel, Yolanda Navarro, María José Iglesias, and Fernando López-Ortiz. 2018. "Immobilized Gold Nanoparticles Prepared from Gold(III)-Containing Ionic Liquids on Silica: Application to the Sustainable Synthesis of Propargylamines" Molecules 23, no. 11: 2975. https://doi.org/10.3390/molecules23112975







