3. Experimental Section
IR spectra were registered on a Fourier spectrometer Infralum FT-801 in KBr pellets (ISP SB RAS, Novosibirsk, Russia). 1H and 13C-NMR spectra were acquired on a JEOL JNM–ECA 600 spectrometer (JEOL Ltd., Tokyo, Japan) (with operating frequencies of 600 and 150 MHz, respectively) in CDCl3 and DMSO-d6 solution at 23 °C. Signal of the residual protons of the solvent (7.26 ppm for CHCl3) was used as the reference in 1H-NMR spectra, while solvents signals (77.2 ppm for CDCl3, 39.4 ppm for DMSO-d6) were used as the reference in 13C-NMR spectra. Mass spectra were recorded with LCMS-8040 Triple quadrupole liquid chromatograph mass-spectrometer from Shimadzu (Shimadzu Corporation, Tokyo, Japan). Elemental analysis was performed on a EuroVector EA-3000 elemental Analyzer (Eurovector, S.p.A., Milan, Italy). Melting points were determined by the open capillary method on a Stuart SMP10 apparatus (Bibby Sterilin Ltd., Stone, UK). X-ray diffraction data were obtained on an automatic three-circle Bruker APEX-II CCD diffractometer (Bruker AXS GmbH, Karlsruhe, Germany). Sorbfil PTX-AF-A-UV plates (Imid Ltd., Krasnodar, Russia) were used for thin-layer chromatography and visualization with the iodine vapor. Column chromatography was performed on silica gel 40–60 µm, 60 Å. DMAD, methyl propiolate, acetylacetylene (Acros Organics, Geel, Belgium) and trifluoroethanol (SIA “P&M-Invest” Ltd., Moscow, Russia) were used without further purification. All solvents were distilled before use.
3.1. Synthesis of 1-Tetrazolyl-Substituted Isoquinolines 1a–c (General Method)
Sodium azide (0.72 g, 11 mmol) was added to a solution of cotarnine chloride (2.12 g, 8.3 mmol) or 6,7-dimethoxy-2-methyl-3,4-dihydroisoquinolinium iodide (2.77 g, 8.3 mmol) or 2-methyl-3,4-dihydroisoquinolinium iodide (2.27 g, 8.3 mmol) and p-methoxyphenyl isonitrile (1.49 g, 11 mmol) in methanol (25 mL) at 20 °C. Stirring was continued at room temperature, monitoring of the reaction progress was performed by TLC (EtOAc-hexane, 1:2). Cotarnine chloride and dimethoxy isoquinolinium iodide react for 1 day; unsubstituted aromatic fragment isoquinolinium iodide reacts for 2 days. The methanol was removed in vacuo, the residue was crystallized from a mixture of EtOAc-hexane (1:2) to afford 1-tetrazolyl-substituted isoquinolines 1a–c.
6,7-Dimethoxy-1-[1-(4-methoxyphenyl)-1H-tetrazol-5-yl]-2-methyl-1,2,3,4-tetrahydroisoquinoline (1a), Yield 1.96 g (62%); beige solid; m.p. = 108–110 °С; Rf = 0.48 (EtOAc-hexane, 1:1); 1H-NMR (600 MHz, CDCl3): δ 6.95 (d, J = 7.8 Hz, 2H, H-Ar), 6.75 (d, J = 7.8 Hz, 2H, H-Ar), 6.42 (s, 1H, H-8), 6.15 (s, 1H, H-5), 5.05 (s, 1H, H-1), 3.79 (s, 6H, OCH3), 3.64 (s, 3H, OCH3), 2.94–2.91 (m, 1H, 3-CH2), 2.53–2.49 (m, 1H, 3-CH2), 2.46–2.43 (m, 2H, 4-CH2), 2.25 (s, 3H, N–CH3); 13C-NMR (150 MHz, CDCl3): δ 160.3, 156.6, 148.2, 147.7, 127.3, 127.1 (3C), 123.9, 113.5 (2C), 110.9, 108.9, 60.1, 55.9, 55.8, 55.5, 51.1, 43.7, 28.1; LCMS m/z: 382 [M + H]+. Elemental analysis: calcd. for C20H23N5O3 C 62.98, H 6.08, N 18.36%, found C 62.72, H 5.85, N 18.47%.
4-Methoxy-5-[1-(4-methoxyphenyl)-1H-tetrazol-5-yl]-6-methyl-5,6,7,8-tetrahydro[1,3]dioxolo[4,5-g]isoquinoline (1b), Yield 3.09 g (94%); beige solid; m.p. = 143–145 °С; Rf = 0.41 (EtOAc-hexane, 2:1); 1H-NMR (600 MHz, CDCl3): δ 7.55–7.52 (m, 2H, H-Ar), 7.06–7.03 (m, 2H, H-Ar), 6.34 (s, 1H, H-9), 5.85 (d, J = 1.4 Hz, 1H, 2-CH2), 5.81 (d, J = 1.4 Hz, 1H, 2-CH2), 5.04 (s, 1H, H-5), 3.89 (s, 3H, OCH3), 3.62 (s, 3H, OCH3), 3.24–3.19 (m, 1H, 7-CH2), 2.90–2.85 (m, 1H, 8-CH2), 2.68–2.65 (m, 1H, 7-CH2), 2.63–2.59 (m, 1H, 8-CH2), 2.22 (s, 3H, N–CH3); 13C-NMR (150 MHz, CDCl3): δ 160.9, 155.9, 149.0, 139.9, 134.1, 129.3, 127.4, 127.1 (2C), 117.2, 114.7 (2C), 103.2, 100.9, 58.9, 55.8, 52.7, 46.3, 42.1, 25.6; LCMS m/z: 396 [M + H]+. Elemental analysis: calcd. for C20H21N5O4 C 60.75, H 5.35, N 17.71%, found C 60.51, H 5.01, N 17.60%.
1-[1-(4-Methoxyphenyl)-1H-tetrazol-5-yl]-2-methyl-1,2,3,4-tetrahydroisoquinoline (1c), Yield 1.84 g (69%); yellow solid; m.p. = 90–92 °С; Rf = 0.59 (EtOAc–hexane, 1:2); 1H-NMR (600 MHz, CDCl3): δ 7.09 (t, J = 7.7 Hz, 1H, H-Ar), 7.03 (t, J = 7.4 Hz, 1H, H-Ar), 6.96 (d, J = 7.4 Hz, 1H, H-Ar), 6.93–6.91 (m, 2H, H-Ar), 6.77–6.75 (m, 2H, H-Ar), 6.72 (d, J = 7.7 Hz, 1H, H-Ar), 5.15 (s, 1H, H-1), 3.81 (s, 3H, OCH3), 3.00–2.95 (m, 1H, 3-CH2), 2.59–2.53 (m, 3H, 3,4-CH2), 2.29 (s, 3H, N–CH3); 13C-NMR (150 MHz, CDCl3): δ 160.4, 156.6, 134.8, 132.4, 128.6, 127.3 (2C), 127.1, 126.6 (2C), 126.2, 113.5 (2C), 60.3, 55.5, 51.1, 43.7, 28.5; LCMS m/z: 322 [M + H]+. Elemental analysis: calcd. for C18H19N5O C 67.27, H 5.96, N 21.79%, found C 66.95, H 6.20, N 21.91%.
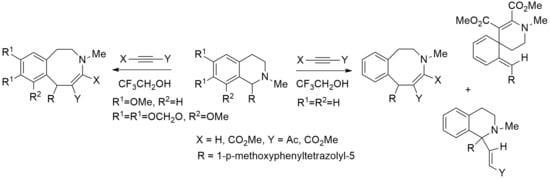
3.2. The Interaction of Isoquinolines 1a, 1b with Activated Alkynes (General Method)
Alkynes (DMAD, methyl propiolate or acetylacetylene) (4 mmol) was added to a solution of isoquinolines 1a or 1b (2 mmol) in trifluoroethanol (10 mL). The reaction mixture was kept at 20 °C, isoquinolines 1a, 1b reacted with methyl propiolate for 1 day, with acetylacetylene for 5 h and 10 days, respectively, with DMAD for 5 and 12 days, respectively. The reaction progress was monitored by TLC (sorbphil, EtOAc-hexane, 1:2). The solvent was evaporated in vacuum and the residue was recrystallized from EtOAc-hexane mixture.
Methyl (4E)-8,9-dimethoxy-6-[1-(4-methoxyphenyl)-1H-tetrazol-5-yl]-3-methyl-1,2,3,6-tetrahydro-3-benzazocin-5-carboxylate (2a), Yield 0.90 g (97%); white solid; m.p. = 152–154 °С; Rf = 0.41 (EtOAc-hexane, 2:1); IR (KBr) ν 1635 cm−1 (С=O); 1H-NMR (600 MHz, CDCl3): δ 7.43 (s, 1H, H-4), 7.08 (d, J = 8.7 Hz, 2H, H-Ar), 6.85 (d, J = 8.7 Hz, 2H, H-Ar), 6.57 (s, 1H, H-7), 6.32 (s, 1H, H-10), 6.00 (s, 1H, H-6), 3.98–3.93 (m, 1H, 2-CH2), 3.81 (s, 6H, OCH3), 3.66 (s, 3H, OCH3), 3.54 (s, 3H, OCH3), 3.06–2.96 (m, 3H, 1,2-CH2), 2.93 (s, 3H, N–CH3); 13C-NMR (150 MHz, CDCl3): δ 169.4, 160.8, 159.6, 153.6, 147.5, 147.3, 128.7, 127.9, 127.7 (2C), 126.6, 115.3, 114.6, 114.1 (2C), 94.0, 55.8, 55.6, 55.5, 51.4, 51.2, 44.2, 40.8, 35.1; LCMS m/z: 466 [M + H]+. Elemental analysis: calcd. for C24H27N5O5 C 61.92, H 5.85, N 15.04%, found C 61.70, H 6.01, N 14.90%.
1-{(4E)-8,9-Dimethoxy-6-[1-(4-methoxyphenyl)-1H-tetrazol-5-yl]-3-methyl-1,2,3,6-tetrahydro-3-benzazocine-5-yl}ethanone (2b), Yield 0.78 g (87%); beige solid; m.p. = 194–195 °С; Rf = 0.26 (EtOAc- hexane, 2:1); IR (KBr) ν 1620 cm−1 (С=O); 1H-NMR (600 MHz, CDCl3): δ 7.32 (s, 1H, H-4), 7.10 (d, J = 9.0 Hz, 2H, H-Ar), 6.84 (d, J = 9.0 Hz, 2H, H-Ar,), 6.53 (s, 1H, H-7), 6.40 (s, 1H, H-10), 6.25 (s, 1H, H-6), 4.24–4.19 (m, 1H, 2-CH2), 3.81 (s, 6H, OCH3), 3.63 (s, 3H, OCH3), 3.10 (ddd, J = 15.5, 6.6, 2.7 Hz, 1H, 2-CH2), 3.01 (s, 3H, N–CH3), 2.98–2.93 (m, 2H, 1-CH2), 2.07 (s, 3H, COCH3); 13C-NMR (150 MHz, CDCl3): δ 193.3; 160.7, 159.5, 155.8, 147.4, 147.3, 128.4, 128.0, 127.6 (2C), 126.6, 115.5, 114.5, 114.0 (2C), 108.5, 55.8, 55.54, 55.51, 51.2, 44.4, 38.0, 34.7, 24.7; LCMS m/z: 450 [M + H]+. Elemental analysis: calcd. for C24H27N5O4 C 64.13, H 6.05, N 15.58%, found C 63.92, H 6.25, N 15.41%.
Dimethyl (4E)-8,9-dimethoxy-6-[1-(4-methoxyphenyl)-1H-tetrazol-5-yl]-3-methyl-1,2,3,6-tetrahydro-3-benzazocin-4,5-dicarboxylate (2c), Yield 0.97 g (93%); beige solid; m.p. = 108–110 °С; Rf = 0.47 (EtOAc-hexane, 1:1); IR (KBr) ν 1736, 1683 cm−1 (С=O); 1H-NMR (600 MHz, CDCl3): δ 6.98 (d, J = 8.7 Hz, 2H, H-Ar), 6.81 (d, J = 8.7 Hz, 2H, H-Ar), 6.48 (s, 1H, H-10), 6.14 (s, 1H, H-7), 5.88 (s, 1H, H-6), 4.65–4.62 (m, 1H, 2-CH2), 3.80 (s, 3H, OCH3), 3.79 (s, 3H, OCH3), 3.72 (s, 3H, OCH3), 3.61 (s, 3H, OCH3), 3.60 (s, 3H, OCH3), 3.35 (ddd, J = 15.1, 8.7, 1.8 Hz, 1H, 1-CH2), 2.86 (ddd, J = 8.7, 11.4, 16.2 Hz, 1H, 2-CH2), 2.56–2.53 (m, 1H, 1-CH2), 2.52 (s, 3H, N–CH3); 13C-NMR (150 MHz, CDCl3): δ 168.2, 166.2, 160.8, 157.8, 156.7, 147.9, 147.4, 129.6, 127.4 (2C), 126.4, 126.2, 115.8, 114.2 (2C), 113.8, 99.8, 55.9, 55.6, 55.5, 55.2, 52.5, 51.8, 43.2, 38.3, 32.8; LCMS m/z: 524 [M + H]+. Elemental analysis: calcd. for C26H29N5O7 C 59.65, H 5.58, N 13.38%, found C 59.83, H 5.71, N 13.45%.
Methyl (8E)-10-[1-(4-methoxyphenyl)-1H-tetrazol-5-yl]-7,11-dimethyl-5,6,7,10-tetrahydro[1,3]dioxolo[4,5-i][3]benzazocine-9-carboxylate (2d), Yield 0.93 g (97%); white solid; m.p. = 167–169 °С; Rf = 0.35 (EtOAc-hexane, 2:1); IR (KBr) ν 1681 cm−1 (С=O); 1H-NMR (600 MHz, CDCl3): δ 7.42 (m, 1H, H-8), 7.16 (d, J = 8.8 Hz, 2H, H-Ar), 6.90 (d, J = 8.8 Hz, 2H, H-Ar), 6.85 (s, 1H, H-10), 6.30 (s, 1H, H-4), 5.88 (d, J = 1.5 Hz, 1H, 2-CH2), 5.83 (d, J = 1.5 Hz, 1H, 2-CH2), 4.03 (ddd, J = 15.3, 9.8, 4.8 Hz, 1H, 6-CH2), 3.84 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 3.55 (s, 3H, OCH3), 3.08 (dt, J = 15.3, 5.3 Hz, 1H, 6-CH2), 2.92 (s, 3H, N–CH3), 2.90–2.89 (m, 1H, 5-CH2), 2.80 (dt, J = 16.3, 5.3 Hz, 1H, 5-CH2); 13C-NMR (150 MHz, CDCl3): δ 167.0, 160.6, 160.0, 153.5, 147.8, 141.2, 135.6, 132.3, 127.1 (2C), 126.9, 121.6, 113.9 (2C), 105.3, 101.0, 99.3, 59.9, 55.6, 52.4, 51.1, 45.0, 34.9, 30.0; LCMS m/z: 480 [M + H]+. Elemental analysis: calcd. for C24H25N5O6 C 60.12, H 5.26, N 14.61%, found C 59.89, H 5.46, N 14.52%.
1-{(8E)-11-Methoxy-10-[1-(4-methoxyphenyl)-1H-tetrazol-5-yl]-7-methyl-5,6,7,10-tetrahydro[1,3]dioxolo[4,5-i][3]benzazocin-9-yl}ethanone (2e), Yield 0.88 g (95%); white solid; m.p. = 225–227 °С; Rf = 0.42 (EtOAc-hexane, 1:1); IR (KBr) ν 1620 cm−1 (С=O); 1H-NMR (600 MHz, CDCl3): δ 7.27 (s, 1H, H-8); 7.18 (br.s, 1H, H-10), 7.15–7.12 (m, 2H, H-Ar), 6.87–6.85 (m, 2H, H-Ar), 6.28 (s, 1H, H-4), 5.87 (d, J = 1.4 Hz, 1H, 2-CH2), 5.81 (d, J = 1.4 Hz, 1H, 2-CH2), 4.15–4.10 (m, 1H, 6-CH2), 3.83 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 3.10 (ddd, J = 15.6, 6.4, 4.6 Hz, 1H, 6-CH2), 2.96 (s, H, N–CH3), 2.87 (ddd, J = 16.6, 10.2, 6.4 Hz, 1H, 5-CH2), 2.67 (dt, J = 16.6, 4.6 Hz, 1H, 5-CH2), 2.06 (s, 3H, CH3); 13C-NMR (150 MHz, CDCl3): δ 192.7; 160.3, 159.7, 155.3, 147.6, 141.1, 135.4, 131.6, 126.7, 126.7 (2C), 121.4, 113.7 (2C), 107.7, 104.9, 100.7, 59.6, 55.4, 52.1, 44.7, 34.5, 27.9, 24.5; LCMS m/z: 464 [M + H]+. Elemental analysis: calcd. for C24H25N5O5 C 62.19, H 5.44, N 15.11%, found C 61.93, H 5.63, N 15.26%.
Dimethyl (8E)-11-methoxy-10-[1-(4-methoxyphenyl)-1H-tetrazol-5-yl]-7-methyl-5,6,7,10-tetrahydro[1,3]dioxolo[4,5-i][3]benzazocin-8,9-dicarboxylate (2f), Yield 1.03 g (96%); white solid; m.p. = 212–214 °С; Rf = 0.47 (EtOAc-hexane, 1:1); IR (KBr) ν 1732, 1691 cm−1 (С=O); 1H-NMR (600 MHz, CDCl3): δ 7.13–7.12 (m, 2H, H-Ar), 6.86–6.84 (m, 2H, H-Ar), 6.72 (s, 1H, H-10), 6.22 (s, 1H, H-4), 5.86 (d, J = 1.4 Hz, 1H, 2-CH2), 5.78 (d, J = 1.4 Hz, 1H, 2-CH2), 4.35 (ddd, J = 15.2, 10.0, 6.0 Hz, 1H, 6-CH2), 3.81 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 3.72 (s, 3H, OCH3), 3.58 (s, 3H, OCH3), 3.30 (ddd, J = 15.2, 6.7, 4.0 Hz, 1H, 6-CH2), 2.87 (ddd, J = 16.7, 10.0, 6.7 Hz, 1H, 5-CH2), 2.53 (s, 3H, N–CH3), 2.45–2.41 (m, 1H, 5-CH2); 13C-NMR (150 MHz, CDCl3): δ 167.5; 166.4, 160.6, 158.3, 155.6, 148.1, 141.1, 135.7, 132.6, 126.8 (2C), 126.5, 120.4, 114.0 (2C), 105.1, 101.1, 99.7, 60.1, 55.9, 55.6, 52.4, 51.7, 38.8, 32.62, 32.60; LCMS m/z: 538 [M + H]+. Elemental analysis: calcd. for C26H27N5O8 C 58.10, H 5.06, N 13.03%, found C 57.83, H 5.21, N 12.90%.
3.3. The Interaction of Isoquinoline 1c with DMAD
DMAD (0.44 g, 3.1 mmol) was added to a solution of isoquinoline 1c (0.5 g, 1.56 mmol) in trifluoroethanol (5 mL). The mixture was kept at 20 °C for 2 days. The reaction progress was monitored by TLC (sorbfil, EtOAc-hexane, 1:3). The solvent was evaporated in vacuum, and the residue was chromatographed on a silica gel column. Azocine 2g and spiro compound 3 were eluted with EtOAc-hexane, 1:2, and recrystallized from EtOAc-hexane mixture.
Dimethyl 6-[1-(4-methoxyphenyl)-1H-tetrazol-5-yl]-3-methyl-1,2,3,6-tetrahydro-3-benzazocin-4,5-dicarboxylate (2g), Yield 0.36 g (50%); white solid; m.p. = 125–127 °С; Rf = 0.70 (EtOAc-hexane, 1:2); IR (KBr) ν 1734, 1681 cm−1 (С=O); 1H-NMR (600 MHz, CDCl3): δ 7.10–7.08 (m, 1H, H-Ar); 7.02–6.97 (m, 4H, H-Ar), 6.81–6.80 (m, 3H, H-Ar), 6.03 (s, 1H, H-6), 4.60–4.53 (m, 1H, 2-CH2), 3.82 (s, 3H, OCH3), 3.73 (s, 3H, OCH3), 3.63 (s, 3H, OCH3), 3.39 (dd, J = 13.8, 8.1 Hz, 1H, 2-CH2,), 2.99–2.93 (m, 1H, 1-CH2), 2.62 (dd, J = 15.9, 6.8 Hz, 1H, 1-CH2,), 2.49 (s, 3H, N–CH3); 13C-NMR (150 MHz, DMSO-d6): δ 167.4, 165.6, 160.3, 157.6, 155.8, 137.2, 134.2, 132.3, 130.9, 127.5, 127.1 (2С), 127.0, 126.0, 114.3 (2С), 99.4, 55.6, 54.0, 52.4, 51.6, 42.6, 38.2, 32.6; LCMS m/z: 464 [M + H]+. Elemental analysis: calcd. for C24H25N5O5 C 62.19, H 5.44, N 15.11%, found C 61.93, H 5.69, N 15.28%.
Dimethyl (11E)-11-{[1-(4-methoxyphenyl)-1H-tetrazol-5-yl]methylidene}-3-methyl-3-azaspiro[5.5]undeca-1,7,9-triene-1,2-dicarboxylate (3), Yield 0.12 g (17%); white solid; m.p. = 126–127 °С; Rf = 0.50 (EtOAc-hexane, 1:1); IR (KBr) ν 1736, 1680 cm−1 (С=O); 1H-NMR (600 MHz, CDCl3): δ 7.73 (d, J = 9.7 Hz, 1H, H-cyclohexadiene); 7.34 (d, J = 8.7 Hz, 2H, H-Ar), 7.04 (d, J = 8.7 Hz, 2H, H-Ar), 6.31 (dd, J = 9.7, 5.5 Hz, 1H, H-cyclohexadiene), 6.03–5.99 (m, 2H, H-cyclohexadiene), 5.91 (s, 1H, =CH-Ar), 3.88 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 3.54 (s, 3H, OCH3), 3.22 (ddd, J = 13.1, 9.6, 3.9 Hz, 1H, 4-CH2), 2.97 (ddd, J = 13.1, 4.8, 4.6 Hz, 1H, 4-CH2), 2.75 (s, 3H, N–CH3), 1.87 (ddd, J = 13.6, 9.6, 3.9 Hz, 1H, 5-CH2,), 1.81–1.77 (m, 1H, 5-CH2); 13C-NMR (150 MHz, CDCl3): δ 166.6, 165.6, 160.5, 156.4, 151.2, 150.7, 128.5, 142.0, 126.7, 126.2 (2С), 122.0, 119.4, 114.8 (2С), 105.3, 97.4, 55.6, 52.6, 50.9, 42.5 (2С), 39.5, 33.7, LCMS m/z: 464 [M + H]+. Elemental analysis: calcd. for C24H25N5O5 C 62.19, H 5.44, N 15.11%, found C 61.43, H 5.72, N 15.21%.
3.4. X-ray Structure Determination of Compound 3
The structures of product
3 were unambiguously established by X-ray diffraction study and are shown in
Figure 1 along with the atomic numbering schemes. The tetrahydropyridine ring of the spiro compound
3 assumes a slightly distorted “sofa” conformation with the carbon atom C(16) extending from the plane formed by the remaining atoms of the ring by 0.658 Å. Carbonyl fragment C(19)–O(3) of the ester group is practically coplanar with the basal plane of the tetrahydropyridine ring C(15)–C(18)=C(21)–N(5)–C(17) (the dihedral angle is equal to 6.40°) due to the conjugation of bonds. The nitrogen atom N(5) has a trigonal planar configuration (the sum of the valence angles is equal to 359.7°). The cyclohexadiene ring of the spiro compound
3 assumes a slightly distorted “sofa” conformation with the carbon atom C(15) extending from the plane formed by the remaining atoms of the ring by 0.444 Å. The 4-methoxyphenyl substituent in molecule
3 is twisted with a tetrazole ring at an angle of 34.20°. The molecule of spiro compound
3 contains the asymmetric carbon atom C(15). The molecules of compound
3 in the crystal form centrosymmetric dimers due to two intermolecular hydrogen bonds C(5)–H(5)···O(5)*.
The crystals of compound 3 (C24H25N5O5, M 463.49) are triclinic, space group P-1 at 120 K: a = 8.2773(9), b = 11.6273(13), c = 11.9759(12) Å; β = 94.980(2)°; V 1097.7(2) Å3; Z 2; dcalc 1.402 g cm−3; F(000) 488.0; μ 0.101 mm−1. The unit cell parameters and intensity of 8547 reflections (4994 independent reflections, Rint 0.0235) were measured on an automatic three-circle diffractometer Bruker SMART APEX-II CCD (MoKa radiation (λ = 0.71073 Å), graphite-monochromator, ω-scanning, 3.57° ≤ 2Θ ≤ 54.968°).
The structure was determined by the direct method and refined by the least-squares technique in the full-matrix anisotropic approximation for non-hydrogen atoms based on
F2. The positions of hydrogen atoms were calculated geometrically and were included in the refinement with fixed positional parameters (the “rider” model) and with isotropic displacement parameters (
Uiso(H) = 1.5 U
eq(C) for CH
3 groups and 1.2
Ueq(C) for other groups). The final probability factors were
R1 = 0.044 (
I ≥ 2
σ (
I)) and
wR2 = 0.106 for all independent reflections. All calculations were carried out using the programs OLEX-2 [
20] and SHELXTL [
21] software package. Tables of the coordinates of atoms, bond lengths, valence and torsion angles, and anisotropic temperature parameters of compound
3 were deposited at the Cambridge Crystallographic Data Center (deposit CCDC 1848342).
3.5. The Interaction of Isoquinoline 1c with Methyl Propiolate
Methyl propiolate (0.52 g, 6.2 mmol) was added to a solution of isoquinoline 1c (0.5 g, 1.56 mmol) in trifluoroethanol (7 mL). The reaction mixture was heated under reflux for 120 h. The reaction progress was monitored by TLC (sorbfil, EtOAc-hexane, 1:3). The solvent was evaporated in vacuum. The residue was chromatographed on a silica gel column. Vinylisoquinoline 4a and benzazocine 2h were eluted with EtOAc-hexane, 1:2, and recrystallized from EtOAc-hexane mixture.
Methyl (4E)-6-[1-(4-methoxyphenyl)-1H-tetrazol-5-yl]-3-methyl-1,2,3,6-tetrahydro-3-benzazocin-5-carboxylate (2h), Yiled 0.14 g (22%); white solid; m.p. = 139–140 °С; Rf = 0.37 (EtOAc-hexane, 1:1); IR (KBr) ν 1679 cm−1 (С=O); 1H-NMR (600 MHz, CDCl3): δ 7.37 (s, 1H, H-4), 7.13–7.10 (m, 4H, H-Ar), 7.05–7.02 (m, 1H, H-Ar), 6.96 (d, J = 7.8 Hz, 1H, H-Ar), 6.86 (d, J = 8.7 Hz, 2H, H-Ar), 6.13 (s, 1H, H-6), 3.88–3.84 (m, 1H, 2-CH2), 3.83 (s, 3H, OCH3), 3.53 (s, 3H, OCH3), 3.13–3.03 (m, 3H, 1,2-CH2), 2.91 (s, 3H, N–CH3); 13C-NMR (150 MHz, CDCl3): δ 169.3, 160.8, 159.4, 153.6, 136.6, 136.1, 132.4, 131.8, 127.7 (2С), 127.32, 127.26, 126.7, 114.1 (2С), 94.3, 55.6, 51.32, 51.25, 44.2, 41.3, 35.7; LCMS m/z: 406 [M + H]+. Elemental analysis: calcd. for C22H23N5O3 C 65.17, H 5.72, N 17.27%, found C 65.36, H 5.95, N 17.03%.
Methyl (2E)-3-{1-[1-(4-methoxyphenyl)-1H-tetrazol-5-yl]-2-methyl-1,2,3,4-tetrahydroisoquinolin-1-yl}prop-2-enoate (4а), Yield 0.063 g (10%); white solid; m.p. = 132–134 °С; Rf = 0.54 (EtOAc-hexane, 1:2); IR (KBr) ν 1681 cm−1 (С=O); 1H-NMR (600 MHz, CDCl3): δ 7.84 (d, J = 16.0 Hz, 1H, CH=CHCO2CH3), 7.11–7.09 (m, 1H, H-Ar), 7.01 (t, J = 7.5 Hz, 1H, H-Ar), 6.90 (d, J = 7.5 Hz, 1H, H-Ar), 6.66 (d, J = 8.7 Hz, 2H, H-Ar), 6.58–6.55 (m, 3H, H-Ar), 5.36 (d, J = 16.0 Hz, 1H, CH=CHCO2CH3), 3.77 (s, 3H, OCH3), 3.73 (s, 3H, OCH3), 2.64–2.59 (m, 1H, 3-CH2), 2.52 (dd, J = 12.6, 5.1 Hz, 1H, 4-CH2), 2.42–2.40 (m, 1H, 3-CH2), 2.24–2.20 (m, 1H, 4-CH2), 2.18 (s, 3H, N–CH3); 13C-NMR (150 MHz, CDCl3): δ 165.8, 160.3, 158.5, 144.5, 135.5, 133.3, 128.7 (2C), 127.8, 127.51 (2C), 127.46, 126.6, 126.2, 113.2 (2C), 63.4, 55.5, 51.7, 45.5, 38.8, 28.5; LCMS m/z: 406 [M + H]+. Elemental analysis: calcd. for C22H23N5O3 C 65.17, H 5.72, N 17.27%, found C 64.42, H 5.89, N 17.41%.
3.6. The Interaction of Isoquinoline 1c with Acetylacetylene
Acetylacetylene (0.54 g, 7.9 mmol) was added to a solution of isoquinoline 1c (0.25 g, 0.78 mmol) in trifluoroethanol (5 mL). The reaction mixture was refluxed for 180 hours. The reaction progress was monitored by TLC (sorbfil, EtOAc-hexane, 1:3). The solvent was evaporated in vacuum. The residue was purified by column chromatography on silica gel. Isoquinoline 4b was eluted with EtOAc-hexane, 1:3.
(3E)-4-{1-[1-(4-Methoxyphenyl)-1H-tetrazol-5-yl]-2-methyl-1,2,3,4-tetrahydroisoquinolin-1-yl}but-3-ene-2-one (4b), Yiled 0.052 g (17%); white solid; m.p. = 132–134 °С; Rf = 0.40 (EtOAc-hexane, 1:2); IR (KBr) ν 1630 cm−1 (С=O); 1H-NMR (600 MHz, CDCl3): δ 7.73 (d, J = 16.5 Hz, 1H, CH=CH-Ac), 7.13 (t, J = 7.5 Hz, 1H, H-Ar), 7.03 (t, J = 7.9 Hz, 1H, H-Ar), 6.92 (d, J = 7.5 Hz, 1H, H-Ar), 6.69 (d, J = 9.1 Hz, 2H, H-Ar), 6.63 (d, J = 9.1 Hz, 2H, H-Ar), 6.59 (d, J = 7.9 Hz, 1H, H-Ar), 5.58 (d, J = 16.5 Hz, 1H, CH=CH-Ac), 3.80 (s, 3H, OCH3), 2.73–2.64 (m, 1H, 3-CH2), 2.60–2.54 (m, 1H, 4-CH2), 2.49–2.46 (m, 1H, 3-CH2), 2.39 (m, 3H, N–CH3), 2.31–2.20 (m, 4H, COCH3 and 4-CH2); 13C-NMR (150 MHz, CDCl3): δ 198.3, 160.5, 158.2, 143.7, 136.2, 135.5, 133.2, 128.9, 128.8, 128.1, 127.7 (2C), 127.5, 126.5, 113.4 (2C), 63.7, 55.7, 45.8, 39.0, 28.5, 27.1; LCMS m/z: 390 [M + H]+. Elemental analysis: calcd. for C22H23N5O2 C 67.85, H 5.95, N 17.98%, found C 67.98, H 6.21, N 18.10%.