Synthesis of Pyridazine Derivatives by Suzuki-Miyaura Cross-Coupling Reaction and Evaluation of Their Optical and Electronic Properties through Experimental and Theoretical Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. Study of the Optical (Linear and Nonlinear) Properties
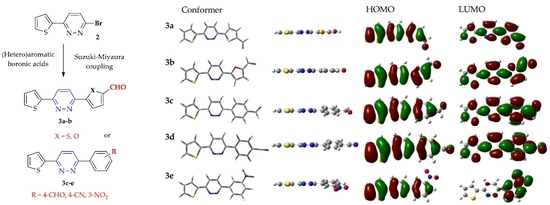
2.3. Theoretical Calculations
3. Experimental
3.1. Materials and Methods
3.2. Synthesis and Characterization
3.2.1. Procedure for the Synthesis of Thienylpyridazine Precursor 2
3.2.2. General procedure for the Synthesis of Thienylpyridazines 3a–e through Suzuki-Miyaura Cross Coupling
3.2.3. Nonlinear Optical Measurements
3.2.4. Theoretical Calculations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd metal catalysts for cross-couplings and related reactions in the 21st century: A critical review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Uozumi, Y. Recent advances in palladium-catalyzed cross-coupling reactions at ppm to ppb molar catalyst loadings. Adv. Synth. Catal. 2018, 360, 602–625. [Google Scholar] [CrossRef]
- Maluenda, I.; Navarro, O. Recent developments in the Suzuki-Miyaura reaction: 2010–2014. Molecules 2015, 20, 7528–7557. [Google Scholar] [CrossRef] [PubMed]
- Lennox, A.J.J.; Lloyd-Jones, G.C. Selection of boron reagents for Suzuki-Miyaura coupling. Chem. Soc. Rev. 2014, 43, 412–443. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A. Cross-coupling reactions of organoboranes: an easy way to construct C-C bonds. Angew. Chem. Int. Ed. Engl. 2011, 50, 6722–6737. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Jaballah, M.Y.; Serya, R.T.; Abouzid, K. Pyridazine based scaffolds as privileged structures in anti-cancer therapy. Drug. Res. 2017, 67, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Coates, W.J. Pyridazines and their benzo derivatives. In Comprehensive Heterocyclic Chemistry II; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon Press: Oxford, UK, 1996; Volume 6, pp. 1–91. ISBN 0-08-042072-9. [Google Scholar]
- Lincker, F.; Kreher, D.; Attias, A.-J.; Do, J.; Kim, E.; Hapiot, P.; Lemaître, N.; Geffroy, B.; Ulrich, G.; Ziessel, R. Rodlike fluorescent π-conjugated 3,3′-bipyridazine ligand: optical, electronic, and complexation properties. Inorg. Chem. 2010, 49, 3991–4001. [Google Scholar] [CrossRef] [PubMed]
- Achelle, S.; Plé, N.; Kreher, D.; Mathevet, F.; Turck, A.; Attias, A.-J. Oligomers containing ethynylpyridazine moieties: synthesis, fluorescence and liquid crystalline properties. Diazines 50. Heterocycles 2008, 75, 357–374. [Google Scholar]
- Cheng, Y.; Ma, B.; Wudl, F. Synthesis and optical properties of a series of pyrrolopyridazine derivatives: Deep blue organic luminophors for electroluminescent devices. J. Mater. Chem. 1999, 9, 2183–2188. [Google Scholar] [CrossRef]
- Figueiredo, H.; Silva, B.; Raposo, M.M.M.; Fonseca, A.M.; Neves, I.C.; Quintelas, C.; Tavares, T. Immobilization of Fe(III) complexes of pyridazine derivatives prepared from biosorbents supported on zeolites. Microporous Mesoporous Mater. 2008, 109, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, H.; Silva, B.; Quintelas, C.; Raposo, M.M.M.; Parpot, P.; Fonseca, A.M.; Lewandowska, A.E.; Bañares, M.A.; Neves, I.C.; Tavares, T. Immobilization of chromium complexes in zeolite Y obtained from biosorbents: synthesis, characterization and catalytic behaviour. Appl. Catal. B Environ. 2010, 94, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Brooker, S.; Davidson, T.C.; Hay, S.J.; Kelly, R.J.; Kennepohl, D.K.; Plieger, P.G.; Moubaraki, B.; Murray, K.S.; Bill, E.; Bothe, E. Doubly pyridazine-bridged macrocyclic complexes of copper in +1, +2 and mixed valent oxidation states. Coordin. Chem. Rev. 2001, 216, 3–30. [Google Scholar] [CrossRef]
- Cheng, T.R.; Huang, C.H.; Gan, L.B.; Luo, C.P.; Yu, A.C.; Zhao, X.S. The investigation of second harmonic generation from novel molecules [(E)-N-alkyl-4-{2-[4-(dialkylamino)phenyl]ethenyl}pyridazinium iodide]. J. Mater. Chem. 1998, 8, 931–935. [Google Scholar] [CrossRef]
- Ortiz, R.P.; Casado, J.; Hernández, V.; Navarrete, J.T.L.; Letizia, J.A.; Ratner, M.A.; Facchetti, A.; Marks, T.J. Thiophene–diazine molecular semiconductors: synthesis, structural, electrochemical, optical, and electronic structural properties; implementation in organic field-effect transistors. Chem. Eur. J. 2009, 15, 5023–5039. [Google Scholar] [CrossRef] [PubMed]
- Achelle, S.; Plé, N.; Turck, A. Incorporation of pyridazine rings in the structure of functionalized π-conjugated materials. RSC Adv. 2011, 1, 364–388. [Google Scholar] [CrossRef]
- Dhas, S.S.J.; Das, S.J.; Dhas, S.M.B. Linear and nonlinear optical studies on 3,6-bis-(2-pyridyl)pyridazine. Optik 2013, 124, 5968–5971. [Google Scholar] [CrossRef]
- Mei, Q.-B.; Weng, J.-N.; Tong, B.-H.; Tian, R.-Q.; Jiang, Y.-Z.; Hua, Q.-F.; Huang, W. Progress in the application of diazine compounds in optoelectronic functional materials. Acta Phys. Chim. Sin. 2014, 30, 595–607. [Google Scholar]
- Marques, S.; Castro, M.A.; Leão, S.A.; Fonseca, T.L. Second hyperpolarizability of the calcium-doped lithium salt of pyridazine Li–H3C4N2⋯Ca. Chem. Phys. Lett. 2016, 659, 76–79. [Google Scholar] [CrossRef]
- Klikar, M.; Le Poul, P.; Růžička, A.; Pytela, O.; Barsella, A.; Dorkenoo, K.D.; Robin-le Guen, F.; Bureš, F.; Achelle, S. Dipolar NLO chromophores bearing diazine rings as π-conjugated linkers. J. Org. Chem. 2017, 82, 9435–9451. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-H.; Shen, J.-Y.; Pu, S.-C.; Wen, Y.-S.; Lin, J.T.; Chou, P.-T.; Yeh, M.-C.P. Synthesis and characterization of new fluorescent two-photon absorption chromophores. J. Mater. Chem. 2006, 16, 850–857. [Google Scholar] [CrossRef]
- Achelle, S.; Barsella, A.; Baudequin, C.; Caro, B.; Robin-le Guen, F. Synthesis and photophysical investigation of a series of push−pull arylvinyldiazine chromophores. J. Org. Chem. 2012, 77, 4087–4096. [Google Scholar] [CrossRef] [PubMed]
- Veronese, L.; Procopio, E.Q.; De Rossi, F.; Brown, T.M.; Mercandelli, P.; Mussini, P.; D’Alfonso, G.; Panigati, M. New dinuclear hydrido-carbonyl rhenium complexes designed as photosensitizers in dye-sensitized solar cells. New J. Chem. 2016, 40, 2910–2919. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, F.W.; Brust, A.; Cuny, E. Sugar-derived building blocks. Part 26. Hydrophilic pyrroles, pyridazines and diazepinones from fructose and isomaltulose. Green Chem. 2001, 3, 201–209. [Google Scholar] [CrossRef]
- Brulé, C.; Bouillon, J.-P.; Nicolaï, E.; Portella, C. Fluorinated ketene dithioacetals. Part 10. Synthesis of new perfluorinated (2H)-pyridazin-3-ones and 3-(alkyl- or arylamino) substituted pyridazines. Synthesis 2003, 2003, 436–442. [Google Scholar] [CrossRef]
- Lee, M.-S.; Kim, E.-S.; Moon, A.R.; Park, M.S. Synthesis of novel allylthio heterocyclo(or aryl)alkylaminopyridazines and their anticancer activity against SK-Hep-1 cells. Bull. Korean Chem. Soc. 2009, 30, 83–91. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, Y.; Bi, F.; Wang, Q. Design, synthesis, and bioactivity study of novel benzoylpyridazyl ureas. J. Agric. Food. Chem. 2009, 57, 6356–6361. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhu, Y.; Lian, M.; Liu, M.; Yuan, J.; Yin, G.; Wu, A. Unexpected C–C bond cleavage: a route to 3,6-diarylpyridazines and 6-arylpyridazin-3-ones from 1,3-dicarbonyl compounds and methyl ketones. J. Org. Chem. 2012, 77, 9865–9870. [Google Scholar] [CrossRef] [PubMed]
- Lenden, P.; Entwistle, D.A.; Willis, M.C. An alkyne hydroacylation route to highly substituted furans. Angew. Chem. Int. Ed. 2011, 50, 10657–10660. [Google Scholar] [CrossRef] [PubMed]
- Stetter, H.; Jonas, F. Addition von aldehyden an aktivierte doppelbindungen XXV synthesen und reaktionen von verzweigten tricarbonyl-verbindungen. Chem Ber. 1981, 114, 564–580. [Google Scholar] [CrossRef]
- Cecchi, M.; Micoli, A.; Giomi, D. Nucleophilic aromatic substitutions on 4,5-dicyanopyridazine. Pyrrole and indole systems as carbon nucleophiles. Tetrahedron 2006, 62, 12281–12287. [Google Scholar] [CrossRef]
- Shin, M.S.; Kang, Y.J.; Chung, H.A.; Park, J.W.; Kweon, D.H.; Lee, W.S.; Yoon, Y.J.; Kim, S.K. Synthesis of some 3,6-disubstituted pyridazines. J. Heterocycl. Chem. 1999, 36, 1135–1142. [Google Scholar] [CrossRef]
- Coad, P.; Coad, R.A.; Clough, S.; Hyepock, J.; Salisbury, R.; Wilkins, C. Nucleophilic substitution at the pyridazine ring carbons. I. Synthesis of iodopyridazines. J. Org. Chem. 1963, 28, 218. [Google Scholar] [CrossRef]
- Coad, P.; Coad, R.A. Nucleophilic substitution at the pyridazine ring carbons. II. Synthesis of pyridazinonyl- and bispyridazinonylpyridazines. J. Org. Chem. 1963, 28, 1919. [Google Scholar] [CrossRef]
- Coad, P.; Coad, R.A.; Hyepock, J. Nucleophilic substitution at the pyridazine ring carbons. III. Alkoxide exchange. J. Org. Chem. 1964, 29, 1751. [Google Scholar] [CrossRef]
- Vieira, L.M.C.; Fonseca, A.M.; Raposo, M.M.M.; Kirsch, G. Electrochemical and spectroscopic studies of pyridazine derivatives. Port. Electrochim. Acta 2004, 22, 11–18. [Google Scholar] [CrossRef]
- Turck, A.; Plé, N.; Mongin, F.; Queguiner, G. Advances in the directed metallation of azines and diazines (pyridines, pyrimidines, pyrazines, pyridazines, quinolines, benzodiazines and carbolines). Part 2: Metallation of pyrimidines, pyrazines, pyridazines and benzodiazines. Tetrahedron 2001, 57, 4489–4506. [Google Scholar] [CrossRef]
- Chevallier, F.; Mongin, F. Functionalization of diazines and benzo derivatives through deprotonated intermediates. Chem. Soc. Rev. 2008, 37, 595–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turck, A.P.; Plé, N.; Mojovic, L.; Quéguiner, G. Diazines VII. Nouvelle voie de synthèse d’un antidepresseur en serie pyridazinique, la minaprine, utilisant les reactions de metallation ortho dirigée et de couplage croisé de Suzuki. Bull. Soc. Chim. Fr. 1993, 130, 488. [Google Scholar]
- Draper, T.L.; Bailey, T.R. Synthesis of unsymmetrical 3,6-disubstituted pyridazines. A palladium-catalyzed approach from 3-iodopyridazines. J. Org. Chem. 1995, 60, 748–750. [Google Scholar] [CrossRef]
- Parrot, I.; Rival, Y.; Wermuth, C.G. Synthesis of substituted 3-amino-6-arylpyridazines via Suzuki reaction. Synthesis 1999, 1999, 1163–1168. [Google Scholar] [CrossRef]
- Maes, B.U.; Lemier, G.L.; Dommisse, R.; Augustyns, K.; Haemers, A. A new approach towards the synthesis of 3-amino-6-(hetero)arylpyridazines based on palladium catalyzed cross-coupling reaction. Tetrahedron 2000, 56, 1777–1781. [Google Scholar] [CrossRef]
- Guery, S.; Parrot, I.; Rival, Y.; Wermuth, C.G. Efficient one-step synthesis of 3-amino-6-arylpyridazines. Tetrahedron Lett. 2001, 42, 2115–2117. [Google Scholar] [CrossRef]
- Littke, A.F.; Fu, G.C. Palladium-catalyzed coupling reactions of aryl chlorides. Angew. Chem. Int. Ed. 2002, 41, 4176–4211. [Google Scholar] [CrossRef]
- Maes, B.U.W.; Kosmrly, J.; Lemier, G.L. Palladium catalyzed reactions on chloropyridazines. J. Heterocycl. Chem. 2002, 39, 535–543. [Google Scholar] [CrossRef]
- Maes, B.U.W.; Tapolcsányi, P.; Meyers, C.; Mátyus, P. Palladium-catalyzed reactions on 1,2-diazines. Curr. Org. Chem. 2006, 10, 377–417. [Google Scholar] [CrossRef]
- Nara, S.; Martinez, J.; Wermuth, C.-G.; Parrot, I. Palladium-catalysed cross-coupling reactions on pyridazine moieties. Synlett 2006, 2006, 3185–3204. [Google Scholar]
- Organ, M.G.; Avola, S.; Dubovyk, I.; Hadei, N.; Kantchev, E.A.B.; O’Brien, C.J.; Valente, C. A user-friendly, all-purpose Pd–NHC (NHC=N-Heterocyclic carbene) precatalyst for the Negishi reaction: A step towards a universal cross-coupling catalyst. Chem. Eur. J. 2006, 12, 4749–4755. [Google Scholar] [CrossRef] [PubMed]
- Sosabowski, M.; Powell, P. Coupling of organotin reagents with aryl, acyl and heteroaryl halides: Synthesis of pyridazine and quinoxalone derivatives. J. Chem. Res. 1995, 10, 402–403. [Google Scholar] [CrossRef]
- Costa, S.P.; Batista, R.M.; Cardoso, P.; Belsley, M.; Raposo, M.M.M. 2-arylthienyl-substituted 1,3-benzothiazoles as new nonlinear optical chromophores. Eur. J. Org. Chem. 2006, 2006, 3938–3946. [Google Scholar] [CrossRef] [Green Version]
- Batista, R.M.; Costa, S.P.; Malheiro, E.L.; Belsley, M.; Raposo, M.M.M. Synthesis and characterization of new thienylpyrrolyl-benzothiazoles as efficient and thermally stable nonlinear optical chromophores. Tetrahedron 2007, 63, 4258–4265. [Google Scholar] [CrossRef] [Green Version]
- Batista, R.M.; Costa, S.P.; Belsley, M.; Raposo, M.M.M. Synthesis and second-order nonlinear optical properties of new chromophores containing benzimidazole, thiophene, and pyrrole heterocycles. Tetrahedron 2007, 63, 9842–9849. [Google Scholar] [CrossRef] [Green Version]
- Batista, R.M.; Costa, S.P.; Belsley, M.; Lodeiro, C.; Raposo, M.M.M. Synthesis and characterization of novel (oligo)thienyl-imidazo-phenanthrolines as versatile π-conjugated systems for several optical applications. Tetrahedron 2008, 64, 9230–9238. [Google Scholar] [CrossRef]
- Batista, R.M.; Costa, S.P.; Belsley, M.; Raposo, M.M.M. Synthesis and optical properties of novel, thermally stable phenanthrolines bearing an arylthienyl-imidazo conjugation pathway. Dyes Pigments 2009, 80, 329–336. [Google Scholar] [CrossRef]
- Raposo, M.M.M.; Castro, M.C.R.; Belsley, M.; Fonseca, A.M.C. Push–pull bithiophene azo-chromophores bearing thiazole and benzothiazole acceptor moieties: Synthesis and evaluation of their redox and nonlinear optical properties. Dyes Pigments 2011, 91, 454–465. [Google Scholar] [CrossRef] [Green Version]
- Raposo, M.M.M.; Fonseca, A.M.C.; Castro, M.C.R.; Belsley, M.; Cardoso, M.F.S.; Carvalho, L.M.; Coelho, P.J. Synthesis and characterization of novel diazenes bearing pyrrole, thiophene and thiazole heterocycles as efficient photochromic and nonlinear optical (NLO) materials. Dyes Pigments 2011, 91, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Raposo, M.M.M.; Castro, M.C.R.; Fonseca, A.M.C.; Schellenberg, P.; Belsley, M. Design, synthesis, and characterization of the electrochemical, nonlinear optical properties, and theoretical studies of novel thienylpyrrole azo dyes bearing benzothiazole acceptor groups. Tetrahedron 2011, 67, 5189–5198. [Google Scholar] [CrossRef] [Green Version]
- Castro, M.C.R.; Schellenberg, P.; Belsley, M.; Fonseca, A.M.C.; Fernandes, S.S.; Raposo, M.M.M. Design, synthesis and evaluation of redox, second order nonlinear optical properties and theoretical DFT studies of novel bithiophene azo dyes functionalized with thiadiazole acceptor groups. Dyes Pigments 2012, 95, 392–399. [Google Scholar] [CrossRef] [Green Version]
- Batista, R.M.; Isakov, D.; Raposo, M.M.M.; Belsley, M.; Bdikin, I.; Kholkin, A.L.; Costa, S.P.; Gomes, E. Ferroelectric nanofibers with an embedded optically nonlinear benzothiazole derivative. J. Nanopart. Res. 2014, 16, 2502. [Google Scholar] [CrossRef] [Green Version]
- Castro, M.C.R.; Belsley, M.; Raposo, M.M.M. Push–pull second harmonic generation chromophores bearing pyrrole and thiazole heterocycles functionalized with several acceptor moieties: syntheses and characterization. Dyes Pigments 2016, 128, 89–95. [Google Scholar] [CrossRef]
- Fernandes, S.S.; Belsley, M.; Ciarrocchi, C.; Licchelli, M.; Raposo, M.M.M. Terpyridine derivatives functionalized with (hetero)aromatic groups and the corresponding Ru complexes: Synthesis and characterization as SHG chromophores. Dyes Pigments 2018, 150, 49–58. [Google Scholar] [CrossRef]
- Steck, E.A.; Brundage, R.P. Pyridazine derivatives. V. Some ethers and thioethers derived from 3,6-dichloropyridazine 1959, 81, 6511–6514. [CrossRef]
- Raposo, M.M.M.; Kirsch, G. A combination of the Friedel-Crafts and Lawesson reactions to 5-substituted 2,2-bithiophenes. Heterocycles 2001, 8, 1487–1497. [Google Scholar] [CrossRef]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. (Eds.) Handbook of Photochemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006; ISBN 97808247237742006. [Google Scholar]
- Demas, J.N.; Crosby, G.A. Measurement of photoluminescence quantum yields. Review. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence: Principles and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2012; ISBN 978-3-527-32837-6. [Google Scholar]
- Clays, K.; Persoons, A. Hyper-Rayleigh scattering in solution. Rev. Sci. Instrum. 1992, 63, 3285–3289. [Google Scholar] [CrossRef]
- Clays, K.; Persoons, A. Hyper-Rayleigh scattering in solution. Phys. Rev. Lett. 1991, 66, 2980. [Google Scholar] [CrossRef] [PubMed]
- Kaatz, P.; Shelton, D.P. Polarized hyper-Rayleigh light scattering measurements of nonlinear optical chromophores. J. Chem. Phys. 1996, 105, 3918–3929. [Google Scholar] [CrossRef]
- Reis, H. Problems in the comparison of theoretical and experimental hyperpolarizabilities revisited. J. Chem. Phys. 2006, 125, 014506. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.S.; Herbivo, C.; Aires-de-Sousa, J.; Comel, A.; Belsley, M.; Raposo, M.M.M. Theoretical and experimental studies of aryl-bithiophene based push-pull π-conjugated heterocyclic systems bearing cyanoacetic or rhodanine-3-acetic acid acceptors for SHG nonlinear optical applications. Dyes Pigments 2018, 149, 566–573. [Google Scholar] [CrossRef]
- Oudar, J.L. Optical nonlinearities of conjugated molecules: stilbene derivatives and highly polar aromatic compounds. J. Chem. Phys. 1977, 67, 446–457. [Google Scholar] [CrossRef]
- Oudar, J.L.; Chemla, D.S. Hyperpolarizabilities of the nitroanilines and their relations to the excited state dipole moment. J. Chem. Phys. 1977, 66, 2664–2668. [Google Scholar] [CrossRef]
- Oudar, J.L.; Zyss, J. Structural dependence of nonlinear-optical properties of methyl-(2,4-dinitrophenyl)-aminopropanoate crystals. Phys. Rev. A 1982, 26, 2016. [Google Scholar] [CrossRef]
- Pyatt, R.D.; Shelton, D.P. Hyper-rayleigh scattering from CH4, CD4, CF4, and CCl4. J. Chem. Phys. 2001, 114, 9938–9946. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision B.01 Edition; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
Sample Availability: Samples of the compounds 1–3 are available from the authors. |



| Cpds. | Yield | Absorption 1 | Emission 2 | |||
|---|---|---|---|---|---|---|
| λmax (nm) | ε (M−1·cm−1) | λem (nm) | Stokes’ Shift (cm−1) | ΦF | ||
| 3a | 28 | 357 | 29,800 | 428 | 4650 | 0.006 |
| 3b | 14 | 354 | 27,800 | 432 | 5100 | 0.006 |
| 3c | 15 | 332 | 26,000 | 405 | 5430 | 0.003 |
| 3d | 28 | 323 | 26,100 | 404 | 6210 | 0.004 |
| 3e | 25 | 314 | 24,100 | 408 | 7340 | 0.005 |
| Cpds. | Absorption 1 | β 2 (10−30 esu) | β0 3 (10−30 esu) | |
|---|---|---|---|---|
| λmax (nm) | ε (M−1·cm−1) | |||
| 3a | 348 | 24,100 | 155 | 75 |
| 3b | 348 | 31,100 | 100 | 50 |
| 3c | 326 | 24,900 | 54 | 30 |
| 3d | 323 | 26,100 | 175 | 100 |
| 3e | 314 | 24,100 | - | - |
| pNA | 370 | - | 62 | 28 |
| Cpds. | μ (D) 1 | βII (10−30 esu) 2 | βtot (10−30 esu) 3 | EHOMO (eV) 4 | ELUMO (eV) 5 | Eg (eV) 6 |
|---|---|---|---|---|---|---|
| 3a | 8.98 | 53.17 | 88.61 | −6.27 | −2.73 | 3.54 |
| 3b | 5.56 | 42.63 | 70.97 | −6.24 | −2.63 | 3.61 |
| 3c | 5.33 | 56.38 | 93.89 | −6.35 | −2.56 | 3.79 |
| 3d | 8.04 | 41.48 | 69.06 | −6.40 | −2.50 | 3.90 |
| 3e | 4.34 | 29.07 | 48.33 | −6.41 | −2.79 | 3.62 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, S.S.M.; Aires-de-Sousa, J.; Belsley, M.; Raposo, M.M.M. Synthesis of Pyridazine Derivatives by Suzuki-Miyaura Cross-Coupling Reaction and Evaluation of Their Optical and Electronic Properties through Experimental and Theoretical Studies. Molecules 2018, 23, 3014. https://doi.org/10.3390/molecules23113014
Fernandes SSM, Aires-de-Sousa J, Belsley M, Raposo MMM. Synthesis of Pyridazine Derivatives by Suzuki-Miyaura Cross-Coupling Reaction and Evaluation of Their Optical and Electronic Properties through Experimental and Theoretical Studies. Molecules. 2018; 23(11):3014. https://doi.org/10.3390/molecules23113014
Chicago/Turabian StyleFernandes, Sara S. M., João Aires-de-Sousa, Michael Belsley, and M. Manuela M. Raposo. 2018. "Synthesis of Pyridazine Derivatives by Suzuki-Miyaura Cross-Coupling Reaction and Evaluation of Their Optical and Electronic Properties through Experimental and Theoretical Studies" Molecules 23, no. 11: 3014. https://doi.org/10.3390/molecules23113014