Use of Red Wine Polyphenols as a Natural Preservative in Health-Promoting Omega-3 Fatty Acids-Enriched Lamb Patties
Abstract
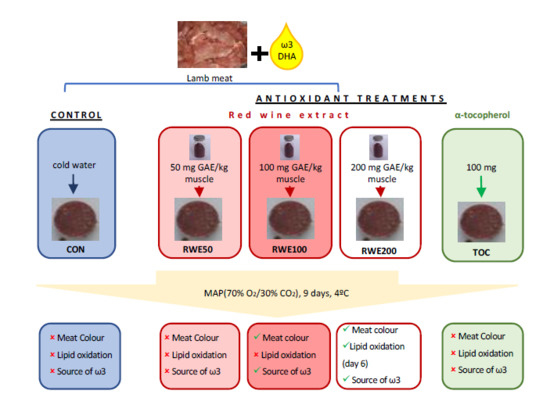
:1. Introduction
2. Results
2.1. Instrumental Colour Parameters
2.2. Lipid and Protein Oxidation
2.3. Long Chain (LC) n-3 PUFA Content
2.4. Sensory Evaluation
3. Discussion
4. Materials and Methods
4.1. Preparation, Packaging, and Storage of Lamb Patties
4.2. Instrumental Colour Measurements
4.3. Measurement of Lipid And Protein Oxidation
4.4. Fatty Acid Analysis
4.5. Sensory Analysis of Cooked Patties
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vranken, L.; Avermaete, T.; Petalios, D.; Mathijs, E. Curbing global meat consumption: Emerging evidence of a second nutrition transition. Environ. Sci. Policy 2014, 39, 95–106. [Google Scholar] [CrossRef]
- Klurfeld, D.M. Research gaps in evaluating the relationship of meat and health. Meat Sci. 2015, 109, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Olmedilla-Alonso, B.; Jiménez-Colmenero, F.; Sánchez-Muniz, F.J. Development and assessment of healthy properties of meat and meat products designed as functional foods. Meat Sci. 2013, 95, 919–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA (European Food Safety Authority). Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA Journal 2010, 8, 1461. [Google Scholar] [CrossRef] [Green Version]
- Echeverría, F.; Valenzuela, R.; Hernandez-Rodas, M.C. Docosahexaenoic acid (DHA), a fundamental fatty acid for the brain: New dietary sources. Prostaglandins Leukot Essent Fatty Acids. 2017, 124, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, D.M.; Bertol, T.M.; Pflanzer, S.B.; Sgarbieri, V.C.; Pollonio, M.A. n-3 in meat products: Benefits and effects on lipid oxidative stability. J. Sci. Food Agric. 2016, 96, 2620–2634. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Naczk, M. Antioxidant properties of food phenolics. In Phenolics in Food and Nutraceuticals; CRC Press: New York, NY, USA, 2004; pp. 397–438. ISBN 9781587161384. [Google Scholar]
- Muíño, I.; Apeleo, E.; de la Fuente, J.; Pérez-Santaescolástica, C.; Rivas-Cañedo, A.; Pérez, C.; Díaz, M.T.; Cañeque, V.; Lauzurica, S. Effect of dietary supplementation with red wine extract or vitamin E, in combination with linseed and fish oil, on lamb meat quality. Meat Sci. 2014, 98, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Decker, E.A.; Faustman, C.; Mancini, R.A. The effects of antioxidant combinations on color and lipid oxidation in n-3 oil fortified ground beef patties. Meat Sci. 2005, 70, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, D.B. Changes in the colour and opacity of meat. Food Chem. 1982, 9, 75–88. [Google Scholar] [CrossRef]
- Resconi, V.C.; Escudero, A.; Beltrán, J.A.; Olleta, J.L.; Sañudo, C.; Campo, M.M. Color, lipid oxidation, sensory quality, and aroma compounds of beef steaks displayed under different levels of oxygen in a modified atmosphere package. J. Food Sci. 2012, 71, S10–S18. [Google Scholar] [CrossRef] [PubMed]
- Özvural, E.B.; Vural, H. Grape seed flour is a viable ingredient to improve the nutritional profile and reduce lipid oxidation of frankfurters. Meat Sci. 2011, 88, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Shirahigue, L.D.; Contreras-Castillo, C.J.; Selani, M.M.; Nadai, A.P.; Mourão, G.B.; Gallo, C.R. Winery grape-residue extract: Effects on quality and sensory attributes of cooked chicken meat. Food Sci. Biotechnol. 2011, 20, 1257–1264. [Google Scholar] [CrossRef]
- Carpenter, C.E.; Cornforth, D.P.; Whittier, D. Consumer preferences for beef color and packaging did not affect eating satisfaction. Meat Sci. 2001, 57, 359–363. [Google Scholar] [CrossRef]
- AMSA (American Meat Science Association). Meat color measurement guidelines; American Meat Science Association and National Livestock and Meat Board: Champaign, IL, USA, 2012; Available online: http://meatscience.org/docs/default-source/publications-resources/Hot-Topics/2012_12_meat_clr_guide.pdf?sfvrsn=d818b8b3_0.
- Greene, B.E.; Hsin, I.; Zipser, M.Y. Retardation of oxidative color changes in raw ground beef. J. Food Sci. 1971, 36, 940–942. [Google Scholar] [CrossRef]
- Jongberg, S.; Skov, S.H.; Tørngren, M.A.; Skibsted, L.H.; Lund, M.N. Effect of white grape extract and modified atmosphere packaging on lipid and protein oxidation in chill stored beef patties. Food Chem. 2011, 128, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Gómez, I.; Beriain, M.J.; Mendizabal, J.A.; Realini, C.; Purroy, A. Shelf life of ground beef enriched with omega-3 and/or conjugated linoleic acid and use of grape seed extract to inhibit lipid oxidation. Food Sci. Nutr. 2016, 4, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Campo, M.M.; Nute, G.R.; Hughes, S.I.; Enser, M.; Wood, J.D.; Richardson, R.I. Flavour perception of oxidation in beef. Meat Sci. 2006, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Azizkhani, M.; Tooryan, F. Antioxidant and antimicrobial activities of rosemary extract, mint extract and a mixture of tocopherols in beef sausage during storage at 4 °C. J. Food Saf. 2015, 35, 128–136. [Google Scholar] [CrossRef]
- Niki, E.; Traber, M.G. A history of vitamin E. Ann. Nutr. MeTable 2012, 61, 207–212. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, M.N.; Monahan, F.J.; Burke, R.M.; Allen, P. The effect of oxygen level and exogenous α-tocopherol on the oxidative stability of minced beef in modified atmosphere packs. Meat Sci. 2000, 55, 39–45. [Google Scholar] [CrossRef]
- Commission Regulation of European Union. Commission Regulation (EU) Nº 116/2010, amending Regulation (EC) Nº 1924/2006 of the European Parliament and of the Council with regard to the list of nutrition claims. Off. J. Eur. Union 2010, L 37, 10/02/10, 16–18. [Google Scholar]
- Bañón, S.; Díaz, P.; Rodríguez, M.; Garrido, M.D.; Price, A. Ascorbate, green tea and grape seed extracts increase the shelf life of low sulphite beef patties. Meat Sci. 2007, 77, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, B.; Brothersen, C.; McMahon, D.J. Fortification of foods with omega-3 polyunsaturated fatty acids. Crit. Rev. Food Sci. Nutr. 2014, 54, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estévez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Siró, I.; Kápolna, E.; Kápolna, B.; Lugasi, A. Functional food. Product development, marketing and consumer acceptance – A review. Appetite 2008, 51, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, A.; Miranda, F.J.; Rubio, S.; Valero, V. Innovations and trends in meat consumption: An application of the Delphi method in Spain. Meat Sci. 2012, 92, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.; Kallas, Z.; Costa-Font, M.; Gil, J.M.; Realini, C.E. Impact of hedonic evaluation on consumers’ preferences for beef attributes including its enrichment with n-3 and CLA fatty acids. Meat Sci. 2016, 111, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Global Organisation for EPA and DHA (GOED). Global recommendations for EPA and DHA intake (Rev. 19 November 2014). 2014. Available online: http://www.issfal.org/GlobalRecommendationsSummary19Nov2014Landscape_-3-.pdf.
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effect – A review. J. Func. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Kanner, J.; Gorelik, S.; Roman, S.; Kohen, R. Protection by polyphenols of postprandial human plasma and low-density lipoprotein modification: The stomach as a bioreactor. J. Agric. Food Chem. 2012, 60, 8790–8796. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.; Pazos, M.; Torres, J.L.; Medina, I. Antioxidant mechanism of grape procyanidins in muscle tissues: Redox interactions with endogenous ascorbic acid and α-tocopherol. Food Chem. 2012, 134, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Krzywicki, K. Assessment of relative content of myoglobin, oxymyoglobin and metmyoglobin at the surface of beef. Meat Sci. 1979, 3, 1–10. [Google Scholar] [CrossRef]
- De la Fuente, J.; Álvarez, I.; Díaz, M.T.; Pérez, C.; Cañeque, V. Determinación de los pigmentos de la carne por espectrofotometría. In Estandarización de las metodologías para evaluar la calidad del producto (animal vivo, canal, carne y grasa) en los ruminates; Cañeque, V., Sañudo, C., Eds.; INIA: Madrid, Spain, 2005; Volume 3, pp. 226–236. ISBN 84-7498-509-9. [Google Scholar]
- Maraschiello, C.; Sárraga, C.; García Regueiro, J.A. Glutathione peroxidase activity, TBARS, and α-tocopherol in meat from chickens fed different diets. J. Agric. Food Chem. 1999, 47, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Ganhão, R.; Morcuende, D.; Estévez, M. Protein oxidation in emulsified cooked burger patties with added fruit extracts: Influence on colour and texture deterioration during chill storage. Meat Sci. 2010, 85, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.F.; Tweed, J.K.S.; Kim, E.J.; Scollan, N.D. Beef, chicken and lamb fatty acid analysis – a simplified direct bimethylation procedure using freeze-dried material. Meat Sci. 2012, 92, 863–866. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| SP | Treatment (T) | SEM | Significance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (days) | CON | RWE50 | RWE100 | RWE200 | TOC | T | SP | TxSP | ||
| L* | 2.14 | *** | ns | * | ||||||
| 0 | 45.3 | 45.8 | 45.4 | 43.6 | 45.4 | |||||
| 3 | 45.4 wx | 41.8x | 44.1 wx | 41.9x | 48.3 w | |||||
| 6 | 48.3w | 45.5 w | 43.2 wx | 39.0x | 46.6 w | |||||
| 9 | 47.1 | 47.1 | 43.2 | 42.1 | 46.3 | |||||
| a* | 1.02 | *** | *** | *** | ||||||
| 0 | 16.6 a,w | 14.1 a,wx | 13.3 a,xy | 12.5 a,xy | 10.9 a,y | |||||
| 3 | 11.6 b | 10.6b | 10.4 b | 10.2 ab | 9.53 ab | |||||
| 6 | 6.64 c,x | 8.88bc,wx | 9.78 b,w | 9.17 ab,w | 7.32 bc,wx | |||||
| 9 | 6.33 c | 6.90 c | 7.65 b | 7.82 b | 5.35 c | |||||
| Hue angle | 2.38 | *** | *** | *** | ||||||
| 0 | 52.5 b | 54.9 b | 55.9 b | 57.1 a | 58.4 b | |||||
| 3 | 56.6 b | 60.1 ab | 57.5 ab | 56.8 a | 60.4 ab | |||||
| 6 | 67.1 a,w | 62.9 a,wx | 57.0 ab,x | 59.4 a,x | 66.9 a,w | |||||
| 9 | 71.0 a,w | 66.1 a,wx | 63.0 a,x | 60.9 a,x | 71.4 a,w | |||||
| MetMb | 2.31 | *** | *** | *** | ||||||
| (%) | 0 | 16.5 d | 16.8 b | 18.0 c | 19.4 c | 15.6 d | ||||
| 3 | 26.7 c,x | 34.1 a,w | 26.2 b,x | 25.9 b,x | 26.7 c,x | |||||
| 6 | 42.5 b,w | 30.5 a,x | 31.1 ab,x | 30.6 ab,x | 34.4 b,x | |||||
| 9 | 49.4 a,x | 36.5 ab,y | 34.8 a,y | 34.5 a,y | 59.1 a,w | |||||
| SP | Treatment (T) | SEM | Significance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (days) | CON | RWE50 | RWE100 | RWE200 | TOC | T | SP | TxSP | ||
| EPA+DHA | 157.3 | ns | *** | ** | ||||||
| (mg/100 g | 0 | 95.2 a | 99.8 a | 89.7 a | 95.7 a | 77.8 a | ||||
| meat) | 3 | 91.8 a | 95.6 a | 110.0 a | 80.5 ab | 82.7 a | ||||
| 6 | 72.6 a | 76.4 a | 57.3 b | 69.6 ab | 63.3 a | |||||
| 9 | 21.5 b,w | 39.6 b,wx | 43.6 b,wx | 62.5 b,x | 31.0 b,wx | |||||
| SP | Treatment (T) | SEM | Significance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (days) | CON | RWE50 | RWE100 | RWE200 | TOC | Mean | T | SP | TxSP | ||
| Lamb odour | 15.5 | ** | *** | ns | |||||||
| 0 | 35.1 | 33.0 | 24.7 | 18.8 | 34.4 | 29.2 | |||||
| 3 | 23.1 | 21.9 | 19.6 | 16.9 | 20.9 | 20.5 | |||||
| Mean | 29.1w | 27.4w | 22.1wx | 17.8x | 27.7w | ||||||
| Rancid odour | 3.28 | ns | * | ns | |||||||
| 0 | 0.39 | 0.51 | 1.05 | 0.64 | 0.52 | 0.62 | |||||
| 3 | 1.68 | 0.65 | 0.77 | 2.92 | 2.37 | 1.68 | |||||
| Mean | 1.04 | 0.58 | 0.91 | 1.78 | 1.44 | ||||||
| Fish odour | 17.0 | ns | *** | ns | |||||||
| 0 | 1.00 | 0.00 | 4.19 | 2.28 | 4.58 | 2.39 | |||||
| 3 | 28.7 | 25.3 | 11.3 | 18.5 | 23.8 | 21.5 | |||||
| Mean | 14.9 | 12.6 | 7.74 | 10.4 | 14.2 | ||||||
| Odd odour | 15.1 | *** | * | ns | |||||||
| 0 | 8.07 | 11.3 | 20.5 | 28.9 | 13.4 | 16.4 | |||||
| 3 | 12.9 | 7.94 | 15.7 | 20.6 | 7.50 | 12.9 | |||||
| Mean | 10.5x | 9.61x | 18.1wx | 24.8w | 10.4x | ||||||
| Lamb flavour | 14.9 | ns | *** | ns | |||||||
| 0 | 37.0 | 33.0 | 31.2 | 25.2 | 36.8 | 32.6 | |||||
| 3 | 18.1 | 18.6 | 22.8 | 21.1 | 24.2 | 21.0 | |||||
| Mean | 27.5 | 25.8 | 27.0 | 23.2 | 30.5 | ||||||
| Fatty flavour | 5.68 | ns | ns | ns | |||||||
| 0 | 4.16 | 3.85 | 2.13 | 1.38 | 0.00 | 2.30 | |||||
| 3 | 3.80 | 1.59 | 0.32 | 4.26 | 4.13 | 2.82 | |||||
| Mean | 3.98 | 2.72 | 1.22 | 2.82 | 2.05 | ||||||
| Rancid flavour | 6.79 | ns | ns | ns | |||||||
| 0 | 0.00 | 3.22 | 2.00 | 0.23 | 0.00 | 0.98 | |||||
| 3 | 2.09 | 1.46 | 0.00 | 5.74 | 2.82 | 2.28 | |||||
| Mean | 0.81 | 2.34 | 0.64 | 2.99 | 1.36 | ||||||
| Fish flavour | 18.5 | ns | *** | ns | |||||||
| 0 | 2.24 | 0.00 | 5.35 | 0.43 | 5.58 | 2.57 | |||||
| 3 | 31.0 | 31.3 | 18.5 | 26.1 | 29.6 | 27.3 | |||||
| Mean | 16.6 | 15.3 | 11.9 | 13.3 | 17.6 | ||||||
| Odd flavour | 15.7 | ns | *** | ns | |||||||
| 0 | 16.4 | 31.5 | 23.3 | 32.2 | 21.9 | 25.1 | |||||
| 3 | 17.5 | 16.0 | 14.3 | 16.2 | 8.98 | 14.6 | |||||
| Mean | 17.0 | 23.7 | 18.8 | 24.2 | 15.4 | ||||||
| Juiciness | 17.5 | * | *** | ns | |||||||
| 0 | 47.5 | 41.3 | 49.6 | 59.7 | 47.1 | 49.0 | |||||
| 3 | 34.8 | 36.8 | 38.9 | 41.2 | 34.5 | 37.2 | |||||
| Mean | 41.2wx | 39.1x | 44.2wx | 50.4w | 40.8wx | ||||||
| Chewiness | 15.7 | ns | ** | ns | |||||||
| 0 | 26.1 | 24.6 | 21.7 | 15.6 | 20.7 | 21.8 | |||||
| 3 | 30.4 | 22.8 | 28.1 | 26.4 | 27.4 | 27.0 | |||||
| Mean | 28.3 | 23.7 | 24.9 | 21.0 | 24.1 | ||||||
| Overall liking | 18.2 | ns | *** | ns | |||||||
| 0 | 51.0 | 47.6 | 55.0 | 48.1 | 49.2 | 50.2 | |||||
| 3 | 30.3 | 28.2 | 29.5 | 32.1 | 30.8 | 30.2 | |||||
| Mean | 40.7 | 37.9 | 42.3 | 40.1 | 40.0 | ||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muíño, I.; Fuente, J.d.l.; Pérez, C.; Apeleo, E.; Pérez-Santaescolástica, C.; Cañeque, V.; Lauzurica, S.; Bermejo-Poza, R.; Díaz, M.T. Use of Red Wine Polyphenols as a Natural Preservative in Health-Promoting Omega-3 Fatty Acids-Enriched Lamb Patties. Molecules 2018, 23, 3080. https://doi.org/10.3390/molecules23123080
Muíño I, Fuente Jdl, Pérez C, Apeleo E, Pérez-Santaescolástica C, Cañeque V, Lauzurica S, Bermejo-Poza R, Díaz MT. Use of Red Wine Polyphenols as a Natural Preservative in Health-Promoting Omega-3 Fatty Acids-Enriched Lamb Patties. Molecules. 2018; 23(12):3080. https://doi.org/10.3390/molecules23123080
Chicago/Turabian StyleMuíño, Iria, Jesús de la Fuente, Concepción Pérez, Elizabeth Apeleo, Cristina Pérez-Santaescolástica, Vicente Cañeque, Sara Lauzurica, Rubén Bermejo-Poza, and María Teresa Díaz. 2018. "Use of Red Wine Polyphenols as a Natural Preservative in Health-Promoting Omega-3 Fatty Acids-Enriched Lamb Patties" Molecules 23, no. 12: 3080. https://doi.org/10.3390/molecules23123080